Abstract
In the aim to estimate the protective role of calcium (Ca) and ethylene glycol tetraacetic acid (EGTA) against cadmium (Cd)–induced damage, chickpea (Cicer arietinum L.) seeds were exposed to 200 μM Cd stress for 6 days or 3 days then subjected to co-treatment of the metal with either 100 mM CaCl2 or 100 μM EGTA for 3 additional days. The addition of Ca and EGTA improved seedling growth. This protecting effect was correlated to the alleviation of the metal-induced oxidative stress, exemplified by the reduction of hydrogen peroxide (H2O2) contents. Besides, Ca and EGTA stimulated thioredoxin (Trx) and thioredoxin reductase (NTR) activities (2.75- and 1.75-fold increase when compared to Cd-stressed, respectively) protecting, thereby, protein –SH groups from the Cd-mediated oxidation, and modulated ferredoxin (Fdx) activity to a control level. Moreover, Ca and EGTA reinstated the glutathione redox steady state, mainly via preserving a high level of glutathione reduced form (GSH). This effect coincided with the maintaining of the Cd-stimulated glutathione reductase (GR) activity and the decline of glutathione peroxidase (GPX, 43% lower than Cd-stressed shoots) activity. Ca and EGTA counteracted the inhibitory effect of Cd on the activity and gene expression of Cu/Zn-superoxide dismutase (Cu/Zn-SOD) isoenzyme and modulated the activities of catalase (CAT) and ascorbate peroxidase (APX). Overall, our results provided evidence that Ca and EGTA supplement could be a promising approach in the remediation of Cd-contaminated environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is classified as a highly toxic heavy metal (HM), mainly resulting from anthropogenic activities (Cheng et al. 2014). Thus, contaminated soil remediation is an increasingly urgent necessity. In this aim, chemical and physiological technologies were carried out to improve plant tolerance to environmental constraints. In this context, germination, being a crucial stage in plant development and highly sensitive to the stress imposed by HM, is considered as a tool of choice to appreciate the changes in the immediate environment of the seed (Bewley 1997). The germinating metabolism and related processes, for instance imbibition capacity, resumption of the respiration, and mobilization of reserves, are affected by metal stress resulting in a reduced germination capacity of seeds and growth delay of the embryonic axis (Nouairi et al. 2019).
Oxidative stress induction is one of the early aspects of HM toxicity (Kharbech et al. 2020). Cadmium, a non-redox element, generates reactive oxygen species (ROS) mainly through indirect mechanisms (Benavides et al. 2005). Owing to its high affinity for thiol, carboxyl, and amine groups of proteins and its ability to substitute essential ions in protein functional sites, Cd inhibits the suitable functioning of proteins in electron transport chains or antioxidant defense (Štolfa et al. 2015). This dysfunction, in turn, leads to an increased electron leakage and, consequently, an excessive ROS production or/and a decreased cellular capacities of ROS neutralization (Heyno et al. 2008; Srivastava et al. 2018). To cope with Cd-induced oxidative stress, plants trigger “specific” mechanisms involving a variety of molecules to control the levels of ROS present in the cells (Štolfa et al. 2015). Glutathione (GSH) is involved in various biological processes such as cell division and differentiation, senescence, regulation of sulfide transport, signal transduction, metabolite conjugation, and protein and nucleic acid synthesis (Zagorchev et al. 2013). Because of its reducing capacity, GSH is a preponderant player in maintaining redox homeostasis. It is also a precursor of phytochelatins that play a key role in HM sequestration in plants (Yadav 2010). Furthermore, GSH is involved in the recycling of the ascorbate, achieved by the Asada-Halliwell cycle (Potters et al. 2004). On the other hand, plant cells contain numerous protein-disulfide reductases, such as thioredoxin (Trx), NADP-dependent thioredoxin reductase (NTR), glutaredoxin, and peroxiredoxin, implicated, through cellular redox state control, in stress resistance (Zaffagnini et al. 2019). Ferredoxin (Fdx) and Trx systems complement GSH in maintaining the thiol homeostasis by reducing the disulfide bridge of target proteins, protecting, thereby, sulfhydryl (−SH) groups, highly sensitive to ROS-mediated oxidation (Zaffagnini et al. 2019).
Enzymatic and non-enzymatic antioxidative systems act in synergy to reduce the intracellular level of ROS. However, controversial responses of antioxidant enzymes to stressful conditions were noticed. Antioxidative enzymes can be stimulated as they can be, under other conditions, inhibited following post-transcriptional modifications (oxidation, S-thiolation) or even following a down-regulation of involved genes (Rahoui et al. 2016).
Several approaches have been undertaken to reduce the impairment caused by HM pollution. Among these strategies, the exogenous application of certain compounds, including calcium (Ca) and organic and inorganic acids, has proven its effectiveness in ameliorating HM stress (Rafiq et al. 2018; Ben Massoud et al. 2019; Valivand and Amooaghaie 2020).
Calcium is considered as a second messenger involved in plant responses to biotic and abiotic environmental cues and plant cell metabolism regulation (Yang and Poovaiah 2003; Dayod et al. 2010). Recently, the results of Nouairi et al. (2019) showed that pre-treatment of faba bean seeds with CaCl2 induced a reduction in Cd accumulation, competing for Ca2+ channels, improved cell membrane stability, and increased antioxidant defense systems. Moreover, Ca amendment of Cd-polluted soil ameliorated photosynthetic attributes in Brassica napus plants and ameliorated flavonoid and saponin yield in Panax notoginseng roots (Wan et al. 2011; Zu et al. 2020). Controversial effects of chelators on HM bioavailability and antioxidant defense system have been reported. Inorganic, i.e., ethylenediaminetetraacetic acid (EDTA), sodium dodecyl sulfate (SDS), and ethylene glycol tetraacetic acid (EGTA), and organic, i.e., malic, oxalic, and citric acids, induced variable responses in plants confronted with HM stress, depending on the chelator and metal concentrations, the duration of exposure to stress, and the plant species (Han et al. 2018; Song et al. 2018; Alzahrani et al. 2020; Soyingbe et al. 2020).
The present investigation aimed to gain insight into the processes by which Ca and EGTA confer Cd tolerance to chickpea seedlings through the study of thiol redox homeostasis and the related enzymes. Also, the antioxidant system was examined.
Materials and methods
Germination and treatment conditions
Seeds of chickpea (Cicer arietinum L., cv. Beja 1) were sterilized, rinsed with distilled water, and then germinated for 3 days at 25 °C in the dark over two sheets of Whatman filter paper moistened with 20 mL distilled water (first batch) or aqueous solutions of 200 μM CdCl2 (second batch). Seeds from the first batch continued the germination in distilled water (control condition) or in the presence of 100 mM CaCl2 or 100 μM EGTA. The seeds of the second batch continued to germinate in the presence of 200 μM CdCl2 (stress condition) or were transferred in distilled water (stress abruption) or the combination of 200 μM CdCl2 with 100 mM Ca (Cd + Ca treatment) or 100 μM EGTA (Cd + EGTA treatment). Applied concentrations were selected based on preliminary studies showing that 200 μM Cd caused almost 50% reduction of seedling growth and 100 mM Ca and 100 μM EGTA caused maximum growth recovery.
For each treatment, 6-day-old shoots were harvested, weighed, and frozen in liquid nitrogen for biochemical analyses or dried at 70 °C until weight stabilization for dry weight (DW) estimation. Freshly harvested shoots were used for gene expression studies.
Hydrogen peroxide content determination
Hydrogen peroxide (H2O2) content was determined according to Sergiev et al. (1997). Shoots were homogenized in trichloroacetic acid (TCA, 0.1% w/v). The homogenate was then centrifuged at 10,000×g for 15 min at 4 °C. The resulting supernatant was added to potassium phosphate buffer (25 mM, pH 7.0) and KI (1 M). The absorbance was measured at 390 nm and H2O2 concentration was calculated using a standard curve.
Determination of the antiradical capacity
The aqueous sample extracts were prepared according to the Dudonné et al. (2009) method and the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging capacity of each sample was determined according to the method of Parejo et al. (2003). DPPH radicals have an absorption maximum at 517 nm, which decreases when DPPH• is reduced by a radical scavenging compound. The radical scavenging activity was calculated using the following equation:
where AB = absorbance of DPPH• solution (blank sample), and AE= absorbance of the shoot extract.
The IC50 (effective concentration corresponding to 50% inhibition) values, expressed in mg/mL, were calculated for each extract from a curve representing the variation of the free radical scavenging activity depending on the concentration of shoot extracts. The lowest IC50 value corresponds to the highest antiradical activity.
Protein extraction and total thiol determination
Shoot samples were homogenized in 50 mM potassium phosphate buffer (pH 7.0) containing 5 mM Na ascorbate and 0.2 mM ethylenediamine tetraacetic acid in an ice bath. The resulting supernatant, after centrifugation at 10,000×g for 15 min at 4 °C, was used for the determination of protein and total thiol contents, and enzyme activities. Proteins were quantified according to Bradford (1976) and total thiol groups were evaluated according to Ellman’s (1959) method.
Enzyme assays
Superoxide dismutase (SOD; EC. 1.15.1.1) activity was determined by using the protocol of Misra and Fridovich (1972) based on the ability of SOD to inhibit the autoxidation of epinephrine, thus reducing the formation of the adrenochrome. The autoxidation of epinephrine was assessed by the rise in the absorbance at 480 nm of sodium carbonate/bicarbonate buffer (62.5 mM, pH 10.4) containing 1.88 U/mL catalase, 125 μM EDTA, and 10 μL epinephrine (11 mg epinephrine in 2 mL 0.1 N HCl). One unit of SOD was defined as the amount of protein extract that causes a 50% loss in the rate of adrenochrome formation. The addition of 1 mM KCN and 5 mM H2O2 to the reaction medium inhibits Cu/Zn-SOD and Fe-SOD, respectively (Salin and Lyon 1983).
The activity of catalase (CAT; EC 1.11.1.6) was assessed by the decrease in absorbance at 240 nm (ε = 0.036 mM−1 cm−1) due to the consumption of H2O2 following the Aebi (1984) method. The reaction medium consisted of 10 mM H2O2 in 25 mM K-phosphate buffer, pH 7.0, and the reaction was triggered by the addition of the protein extract. Glutathione peroxidase (GPX; EC 1.11.1.9) activity was measured according to the method described by Nagalakshmi and Prasad (2001) based on the continuous regeneration of glutathione oxidized form (GSSG), which is produced by the action of GPX and catalyzed by glutathione reductase (GR) to the detriment of the NADPH form. Reaction medium contained K-phosphate buffer (50 mM, pH 8.0), 100 mM NaCl, 1 mM GSH, 2.5 mM H2O2, 0.5 mM NADPH, and 1 U GR. The oxidation of NADPH was monitored by measuring the decrease in absorbance at 340 nm (ε = 6.22 mM−1 cm−1). Glutathione reductase (GR; EC 1.6.4.2) was monitored in K-phosphate buffer (50 mM, pH 7.0) containing 0.2 mM NADPH and 0.5 mM GSSG. The oxidation of NADPH was determined by the decrease in the absorbance at 340 nm (ε = 6.22 mM−1 cm−1; Foyer and Halliwell 1976). Ascorbate peroxidase (APX; EC 1.11.1.11) activity was determined according to the protocol of Nakano and Asada (1981). The reaction medium consisted of K-phosphate buffer (25 mM, pH 7.0) containing 0.5 mM Na ascorbate, 5 mM H2O2, and 0.1 mM EDTA. The decrease of the absorbance at 290 nm, due to the consumption of ascorbate, was monitored (ε = 2.8 mM−1 cm−1). The Trx activity has been evaluated by the NAD-MDH test in Tris-HCl buffer (50 mM, pH 8.0), containing 1 mM oxaloacetate, 0.2 mM NADH, 10 μM MDH, and 1 mM DTT. The oxidation of NADH was followed by a decrease of the absorbance at 340 nm (ε = 6.22 M−1 cm−1; Jacquot et al. 1994). The NTR (EC 1.8.1.9) activity was measured in the following reaction medium: 50 mM Tris-HCl (pH 8), 100 μM DTNB (5,5-dithiobis-(2-nitrobenzoic acid)), 0.2 mM NADPH, and 30 μg/mL Trx (reduced form). The reduction of DTNB was determined by the increase in the absorbance at 412 nm (ε = 13600 M−1 cm−1; Jacquot et al. 1994). The activity of Fdx was assessed by the increase of the absorbance at 550 nm of the reaction mixture containing 50 mM Tris-HCl (pH 7.8), 250 μM NADPH, 40 μM oxidized cytochrome C, 0.1 μM ferredoxin-NADP reductase (FNR), and the protein extract (ε = 19.1 mM−1 cm−1; Green et al. 1991).
Quantification of glutathione contents
Thiols were extracted according to the method of Garcia et al. (2008). Briefly, lyophilized shoot tissue was homogenized in 15% TCA at 4 °C. The derivation reaction was realized using 1 mM 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB prepared in 0.5 M K-phosphate buffer, pH 8; Katrusiak et al. 2001). After 5 min of incubation in an ice bath, the reaction was stopped with H3PO4 (7 M); then, the homogenate was centrifuged at 12,000×g for 10 min at 4 °C. For total GSH determination, oxidized (GSSG) forms were reduced with dithiothreitol (DTT, 10 mM) before the derivation reaction.
The chromatographic separation was carried out using a reverse-phase column (Agilent, 1100 Series, USA) connected to a UV-visible detector set at 330 nm (Katrusiak et al. 2001). Extracts (20 μL) were injected into a Zorbax Eclipse Plus C18 column (5 μm, 4.6 × 250 mm; Agilent, USA). The mobile phase consisted of acetonitrile as mobile phase A and acidified water (pH 3.5) as mobile phase B at a flow rate of 1.2 mL min−1. The elution profile was as follows: 0–15 min, 10% A; 15–16 min, 100% A; 16–20 min, 10% A. The comparison of the retention time of the samples with those obtained with corresponding standards permitted to identify the different peaks.
The total (GSH + GSSG) and reduced (GSH) forms of glutathione are quantified using calibration curves made from DTNB-derived glutathione (Sigma) solutions. Concentrations were determined based on the area of the peaks and GSSG contents were calculated as the difference between the total and reduced forms.
Determination of antioxidant gene expression
RNA extraction and assay
The total RNA was extracted according to the protocol of Jacquemond et al. (2009) using the Trizol LS Reagent (Trizol RNA stabilization solution, Invitrogen, Life Technologies). One hundred milligrams of fresh plant tissue were ground directly, with tissue lyser II (QIAGEN), in 500 μL of extraction buffer (0.5 M sodium trisodium citrate; 0.1% thioglycolic acid). The ground material was homogenized with 500 μL of Trizol, then vortexed and incubated for 5 min at room temperature before the addition of 100 μL of cold chloroform. The mixture is then incubated for 15 min at room temperature and centrifuged at 14,000 rpm for 15 min at 4 °C. The mixture separates into a red lower phase containing a mixture of phenol and chloroform, an interphase containing the proteins, and a clear aqueous upper phase containing the nucleic acids. The aqueous phase was collected and the nucleic acids were precipitated in the presence of 250 μL of isopropanol for 20 min at − 20 °C. The nucleic acids were recuperated by centrifugation at 14,000 rpm for 15 min at 4 °C. The obtained pellet was washed with 250 μL of cold 70% ethanol and centrifuged for 10 min at 14,000 rpm at 4 °C, then dried immediately in a speed-vac for 1 to 2 min before being taken up in 30 μL of RNase-free water.
The quantification of the RNA was carried out by using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA) with multiple optical sources.
Synthesis of cDNA: reverse transcription (RT)
For the synthesis of the cDNA, 2 μg of RNA was incubated at 95 °C for 2 min in the presence of 50 ng of reverse primer in a final volume of 10 μL. The mixture was immediately cooled in ice and then added with 4 μL of 5× buffer (250 mM Tris-HCl, 375 mM KCl, 15 mM MgCl), 1 μL 0.1 M DTT, 10 mM dNTP, 0.5 μL RNAsin (RNase inhibitor; 40 U/μL), and 40 U of M-MLV reverse transcriptase (200 U/μL). The reaction occurred at 37 °C for 60 min.
Real-time quantitative PCR
Quantitative real-time PCR (qPCR) was performed with a sequence detection system (Applied Biosystems, ABI 7000) in a final volume of 25 μL including 10 μL Igreen qPCR master MixRox (BIOMATIK, USA), 50 ng of cDNA sample as a template, and 400 nM forward and reverse primers. PCR reactions were realized in 96-well optical reaction plates (Applied Biosystems, USA) under the following cycle conditions: 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 1 min at 60 °C. Genes and the corresponding primers were designed from Chickpea Transcriptome Database (CTBD TC17160) (http://www.nipgr.res.in/ctdb.html): CAT (AJ 131046), forward (F) -5′-TCCACAGGGATGAAGAGGTCAATTAC-3′ and reverse (R) -5′-AACGATCAGCCTGAGACCAGTATG-3′; APX (AB0 24991), F-5′-TCTATCAGTTGGGTGGTGTTGTTG-3′ and R-5′-TTGCCAAACACATCCCTCAAATGG-3′; SOD (AJ 012691), F-5′-ACAAATGGCTGCATATCAACCGGAC-3′ and R-5′-TTCCTATGATGGAGTTTGGTCCAG-3′; The actin Cicer arietinum gene (ACT) was used as the internal control gene (AJ 012685): F-5′-TCTTGAGTGGTGGTTCTACTATGTTCC-3′ and R-5′-GCTCTCAGTGCTCCATGATTTGAAC-3′.
The relative quantification of the transcripts of each gene was determined by applying the 2−ΔΔCt method (Livak and Schmittgen 2001):
Statistical analysis
Germination was carried out three times in succession to have three independent replications. In each replication, the seeds of 3 crystallizers were subjected to the same treatment. Overall differences were tested by one-way analysis of variance (ANOVA test) and pairwise comparisons between treatments were performed using the Tukey HSD multiple comparisons test. Pearson’s correlations were determined to visualize the relationship between the different studied parameters. Also, a factorial analysis was conducted by principal component analysis (PCA). A probability of p < 0.05 was considered significant in all statistical analyses performed using XLSTAT software.
Results
Effects on growth
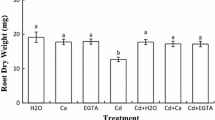
Cadmium reduced chickpea shoot growth by 41% when compared to the control (p < 0.05; Fig. 1). However, Ca and EGTA addition to the germinating medium contaminated with Cd suppressed the detrimental effect of the metal on shoot DW (Fig. 1). Interestingly, this protective effect was similar to that of Cd + H2O treatment.
Effects on H2O2 content
Cadmium exposure led to H2O2 over-accumulation in chickpea shoots by almost 100% over the control (Fig. 2). The application of Ca and EGTA counteracted the Cd-mediated H2O2 accumulation (− 46% of the Cd-stressed shoots; Fig. 2). Besides, in the absence of Cd, Ca and EGTA did not influence the accrual of H2O2 in shoot cells. Similar response was observed when the seedlings were transferred to the control condition upon 3-day Cd exposure (Fig. 2).
H2O2 content in chickpea shoots after seed imbibition in H2O or 200 μM CdCl2 in the presence and absence of 10 mM CaCl2 and 100 μM EGTA. Each measurement was performed in an extract obtained from several seedlings. Values (± SE, n = 3) followed by a common letter are not different at the 0.05 level of significance according to Tukey’s test
Effects on antiradical capacity, thiol content, and thioredoxin system
As illustrated in Fig. 3, Cd resulted in a substantial increase (2.5-fold increase compared to the control; Fig. 3) of the IC50 in shoot cells. This finding reflected the loss of the antiradical capacity under metal distress. The combinations Cd + Ca and Cd + EGTA improved the cell performance to reduce free radicals by approximately 80% and 50% as compared to the Cd-stressed and the control, respectively (Fig. 3). Unlike Ca and EGTA treatments, Cd and the combination Cd + effectors increased –SH groups content with an increment rate of 43% and 27% of control, respectively (Table 1). Besides, the thioredoxin system displayed a treatment-dependent response profile. Cd declined markedly Trx and NTR activities (3.8- and 1.8-fold lesser than the control, respectively; Fig. 4a, b) and enhanced the Fdx one by 1.4-fold of control (Fig. 4c). The effectors improved Trx activity by 175% over the Cd-treated samples but without reaching the control level (Fig. 4a). Nevertheless, the co-application of Cd and effectors corrected NTR and Fdx activities (Fig. 4b, c). In the absence of Cd, Ca and EGTA did not influence the antiradical capacity nor the –SH group content and the thioredoxin system (Figs. 3 and 4; Table 1).
Antiradical capacity, evaluated by the determination of the concentration corresponding to 50% inhibition of DPPH free radical (IC50, mg mL−1), of aqueous extracts obtained from chickpea shoots after seed imbibition in H2O or 200 μM CdCl2 in the presence and absence of 10 mM CaCl2 and 100 μM EGTA. Values (± SE, n = 3) followed by a common letter are not different at the 0.05 level of significance according to Tukey’s test
Effects on glutathione homeostasis and related enzymes
Cadmium significantly (p < 0.05) raised the glutathione redox status (GSH to GSSG ratio) by 36% over the control (Table 1). Concomitantly, a decreased content of the reduced form (GSH; 37% decrease versus control; Table 1) and unchanged level of the oxidized form (GSSG) were observed. Likewise, GR and GPX activities were enhanced by 75% and 71% of control, respectively (Fig. 5). Calcium and EGTA maintained the up-regulated GR activity and reduced the GPX one to a control level (Fig. 5). Thus, the effectors completely suppressed the disrupting effect of Cd on the GSH/GSSG ratio and the GSH content, and kept the level of GSSG unchanged (Table 1).
Effects on antioxidative enzymatic and transcriptional systems
As illustrated in Fig. 6a, activities of SOD isoforms exhibited various responses under Cd stress. The Mn-SOD activity was enhanced by 56%, whereas the Cu/Zn-SOD activity was reduced by 85% of control, and theFe-SOD activity was maintained at the control level (Fig. 6a). Furthermore, the investigation of the transcriptional system revealed a down-regulation of the Cu/Zn-SOD gene by 1.73-fold of the control (Fig. 6b). Besides, Cd stimulated APX and CAT activities by 127% and 355% over the control, respectively (Fig. 6c, e), but declined the expression of APX gene by 2.6-fold compared to the unstressed samples and did not significantly (p > 0.05) affect CAT gene transcripts (Fig. 6d, f). The exogenous effectors counteracted the stimulating effect of Cd on APX activity (Fig. 6c) and partially impeded the impact on CAT activity (Fig. 6e). Simultaneously, Ca mitigated the Cd-elicited repression on APX gene expression (Fig. 6d), but EGTA was the most effective with a 160% increase versus Cd treatment (Fig. 6d). The sensitivity profile of the CAT transcriptional system revealed significant variability (Fig. 6f). Calcium did not affect CAT gene expression, while EGTA, Cd + Ca, and Cd + EGTA induced its up-regulation by more than 3.6-, 2.4-, and 1.9-fold, respectively (Fig. 6f).
Activities of SOD (a), APX (c), and CAT (e) enzymes and expression of Cu/Zn-SOD (b), APX (d), and CAT (f) genes, estimated in chickpea shoots after seed imbibition in H2O or 200 μM CdCl2 in the presence and absence of 10 mM CaCl2 and 100 μM EGTA. Cicer arietinum actin gene was used as reference. Values (± SE, n = 3) followed by a common letter are not different at the 0.05 level of significance according to Tukey’s test
Pearson’s correlation and principal component analysis
The statistical analysis (Table 2) revealed that the redox stress biomarker, H2O2 content, was negatively correlated with shoot DW (r = − 0.973, p < 0.01), Trx (r = − 0.878, p < 0.01), NTR (r = − 0.815, p < 0.05), GSH content (r = − 0.939, p < 0.01), GSH/GSSG ratio (r = − 0.887, p < 0.01), and APX and Cu/Zn-SOD gene expressions (r = − 0.762 and − 0.756, respectively, p < 0.05). Contrariwise, a positive correlation associated the contents of H2O2 to –SH groups (r = 0.790, p < 0.05), IC50 (r = 0.893, p < 0.01), Fdx (r = 0.785, p < 0.05), GPX (r = 0.957, p < 0.01), APX (r = 0.980, p < 0.01), and CAT (r = 0.904, p < 0.01) activities (Table 2).
These findings were further confirmed by PCA illustration. As shown in Fig. 7a, H2O2 and –SH contents and the activities of Fdx, GPX, APX, and CAT were loaded in the positive side of the first component (PC1, 65% of the variance). By contrast, GSH, GSH/GSSG ratio, Trx, NTR, and the gene expressions of APX and Cu/Zn-SOD were loaded in the opposite direction (Fig. 7a). As judged by Pearson’s correlation analysis, there was no significant correlation (at p < 0.05) between enzymatic activities (Cu/Zn-SOD, APX, and CAT) and the expression of related genes (r values were 0.120, − 0.702, and 0.072, respectively; Table 2).
Principal component analysis (PCA) of various parameters (a) and treatments (b) applied to chickpea shoots after seed imbibition in H2O or 200 μM CdCl2 in the presence and absence of 10 mM CaCl2 and 100 μM EGTA. The first component (PC1) represents 65.03% of the inertia and the second component (PC2) explains 15.39% of the inertia
Applied treatments were mapped in the space spanned by the first two principal components, PC1 versus PC2 (15.39% of inertia; Fig. 7b). This score plot illustrated a visible clustering depending on germination conditions. The PC1 separated the Cd treatment from the control (H2O), Ca and EGTA treatments that exhibited a distinct cluster. On the other hand, Cd stress abruption (Cd + H2O) and co-treatments (Cd + effectors) had low PC1 scores (Fig. 7b).
Overall, the biochemical results were confirmed by the statistical analysis and provided further clues of the effectiveness of Ca and EGTA in alleviating Cd stress in chickpea shoots.
Discussion
Ca and EGTA protect growth
Stunted growth represents the early symptom of Cd toxicity (Ci et al. 2009), as illustrated in Fig. 1. The adverse effect of Cd on biomass production was attributed to the failure of the reserve mobilization-related processes (Rahoui et al. 2015). In addition to the induction of apoptosis, inhibition of cell elongation, peroxidation of membrane lipid, protein carbonylation, and binding of the metal ions to the DNA were reported (Romero-Puertas et al. 2002; Štolfa et al. 2015). Interestingly, Ca and EGTA addition to the Cd-contaminated medium induced growth recovery evidenced by DW correction (Fig. 1). The protective impact of Ca and chelators against HM-induced damage on growth has been largely reviewed (Huang et al. 2017). It was suggested that exogenous Ca competes with Cd ions against Ca channels, whereas EGTA acts as an exogenous chelator, leading in both cases to the restriction of Cd accumulation in plant cells (Sakouhi et al. 2016). In concordance with our results, recently, Li et al. (2021) reported that Ca(OH)2 and CaCl2 treatments decreased Cd availability in soils and Cd contents in all parts of Panax notoginseng plant. The authors noticed that ATPase activity correlated positively with the Ca to Cd content ratio, suggesting the involvement of ATPase in the uptake of Ca2+ and Cd2+ by root cells. Furthermore, Ca application decreased the frequency of the Cd-mediated chromosomal aberration, ameliorated the mitotic index, and modulated plant growth regulators, such as auxin (El-Ashry and Mohamed 2012; Li et al. 2016).
Ca and EGTA ameliorate antiradical capacity and maintain redox homeostasis
Oxidative burst, evidenced by ROS overproduction, particularly the H2O2 signaling molecule, is among the early aspects of the toxicity induced by environmental constraints (Cuypers et al. 2010). Our results showed the same trend of response, as testified by the H2O2 over-accumulation in chickpea shoots (Fig. 2), the negative correlation between H2O2 content and seedling growth (Table 2), and the opposite direction of these two parameters in the PCA illustration (Fig. 7a). The enhanced production of ROS can be the result of the disruption of the balance between prooxidant and antioxidant systems, the oxidation of proteins, and the disruption of mitochondrial respiration (Gratão et al. 2015).
As predicted, the tested effectors (Ca and EGTA) annihilated, completely, the Cd-imposed rise in H2O2 content, indicating the recovery of the cellular redox state (Fig. 2). This protective role should be assigned, at least in part, to the restriction of the accumulation of Cd ions in cell tissues as judged by our previous investigation (Sakouhi et al. 2016). Furthermore, growing evidence suggests that competitive (Ca) and chelating (EDTA) agents are effective in reducing ROS levels in plant tissues by improving ROS-scavenging activities (Rafiq et al. 2018).
The application of Ca and EGTA reversed the substantial depressive effect of Cd on the antiradical capacity of chickpea shoot cells (Fig. 3). Thus, the growth recovery implemented by Ca and EGTA (Fig. 1) could be the achievement of the improved cellular antioxidant capacity, resulting from the interaction between the effectors and the metal.
Since thiols play a central role in plant adaptation to environmental stress, we focused on the examination of thiol homeostasis. Herein, the increased content of –SH groups may suggest protein protection against Cd-induced oxidation, which could be the result of the activation of thiol compound synthesis and/or disulfide group reduction involved systems. Given the above considerations, the thioredoxin system, involved in thiol redox state, was examined with interest.
Thioredoxins are small proteins with an extremely reactive site able to reduce the disulfide bridges of the target proteins (Zaffagnini et al. 2019). Besides, they modulate plant cell oxidative stress response and are involved in signaling pathways (Zaffagnini et al. 2012). The depressive impact of Cd on Trx should be the result of protein oxidation as well as the decreased NTR activity (Fig. 4b), known for its recycling role of the Trx reduced forms. Exogenous Ca and EGTA counteracted the adverse effect of Cd on both Trx and NTR activities (Fig. 4a, b) and restored control Fdx activity (Fig. 4c). We suppose that effectors alleviated the Cd-inhibiting effect on the Trx system, which, in turn, catalyzed the reduction of H2O2 (Fig. 2), preventing, thereby, oxidative stress and apoptosis occurring, as previously reported under the same conditions (Sakouhi et al. 2016). This assumption is strengthened by the negative correlation between Trx and H2O2 content (Table 2) and the factorial analysis (Fig. 7a). Glutathione, owing to its sulfhydryl group and its high intracellular content level, is considered the main redox buffer maintaining cellular redox homeostasis (Foyer and Noctor 2011). Current results give evidence that Cd critically perturbs the redox status of glutathione (Table 1) sustaining, thereby, previous studies (Singh et al. 2016). The shift of the glutathione redox balance in favor of GSSG is caused by a depletion of the reduced forms of glutathione (Table 1) without altering the content of GSSG, despite the drastic increase of GR activity (Fig. 5a). The negative correlation between GSH, GPX, and H2O2 (Table 2) suggested that the noted depletion of GSH in Cd-stressed seedlings resulted from the enhancement of GPX activity, using GSH as a reducing agent for H2O2. This fact was further confirmed by PCA results since PC1 opposed GPX and H2O2 to GSH and the GSH/GSSG ratio (Fig. 7a). Moreover, the negative correlation between glutathione redox ratio (GSH/GSSG) and IC50, which is inversely proportional to the antiradical capacity, evinced the important role of glutathione homeostasis in the cell ability to reduce free radicals (Table 2).
Exogenous Ca and EGTA reversed the disruptive effect of Cd on GSH redox state as in the case of stress abruption (Cd + H2O treatment), mainly through the restoration of a steady-state level of GSH (Table 1). The efficiency of Ca and EGTA in the restoration of glutathione redox homeostasis coincides with the up-regulated GR activity (Fig. 5a) and the declined consumption of GSH by GPX activity (Fig. 5b). These data are in agreement with the assumption that Ca efficiency in Cd stress alleviation is mainly related to its ability to maintain GSH optimal level and, thereby, increasing phytochelatin biosynthesis, as suggested by López-Climent et al. (2014).
Ca and EGTA modulate the antioxidant system
Under Cd stress, divergent responses of the enzymatic and transcriptional systems have been reported (Gratão et al. 2019). This effect was explained by metal ion interference with transcription mechanisms by the displacement of Zn ions by Cd in transcription factors, owing to the chemical similarity of the two ions (Di Toppi and Gabbrielli 1999). Interestingly, the current results corroborated our previous study, suggesting a differential response to Cd stress according to SOD isoform (Fig. 6a) as well as to the seedling part (Sakouhi et al. 2018). The substitution of Zn by Cd might cause Cu/Zn-SOD enzyme inactivation and protein denaturation by oxidation (Cuypers et al. 2010; Semane et al. 2010). However, no direct link was revealed by the results of Pearson’s correlation between the response of this activity and H2O2 contents (Table 2). On the other hand, Cd exposure caused a gene expression inhibition, stated by the decreased Cu/Zn-SOD gene transcript accumulation (Fig. 6b). Khraiwesh et al. (2010) suggested that the post-transcriptional regulation of this isoform might be attributed to the binding of the microRNAs to the enzyme mRNA, resulting in either its degradation or protein biosynthesis inhibition.
The modulating effect of Ca on the antioxidative system was connected to its role in signal transduction, in which Ca acts as a second messenger (Dayod et al. 2010). Exogenous Ca corrected the Cd-induced perturbation of the cytosolic Ca content (Farzadfar et al. 2013). This fact is perceived by Ca2+ sensors, such as calmodulin, calmodulin-like protein, calcineurin B–like protein, and Ca-dependent protein kinase, which trigger downstream signaling responses, resulting in modification of antioxidant activities and target protein-related gene expression at an appropriate threshold (Hashimoto and Kudla 2011). For instance, Ca-calmodulin complex occurrence regulated gene expression and metabolism involved in plant tolerance to Cd stress (Yang and Poovaiah 2003). This finding could explain the noticed up-regulation of APX and CAT gene expressions following co-treatment Cd + Ca (Fig. 6d, f), whereas Ca, when applied in the absence of Cd, caused no significant change in the investigated gene expression (Fig. 6b, d, e). Besides, emerging concepts suggest the involvement of crosstalk between Ca and H2O2 signaling pathways in antioxidant system regulation to improve plant resistance to oxidative stress (Steinhorst and Kudla 2014).
The effects of the chelating agents on the transcriptional system are still lacking. Our results suggested an indirect effect, through the reduction of H2O2 accumulation (Fig. 2) that, in turn, controls the level of target gene expression. However, the enhancing impact of EGTA, when added alone, on Cu/Zn-SOD and CAT gene expressions (Fig. 6b, f) confirmed the occurrence of a regulating pathway mediated by EGTA.
The absence of a significant correlation between enzymatic activities and the expression pattern of related genes (Table 2) sustains the hypothesis that the noted modulation of enzyme activities was mainly resulting from post-translational modifications.
Conclusions
The present investigation provided new insight that Ca and EGTA addition, even after the initiation of the germination, could be a promising alternative in Cd stress alleviation. Exogenous Ca and EGTA counteracted the detrimental effect of Cd on seedling growth and prevented oxidative stress via the restoration of thiol homeostasis and the modulation of antioxidative enzymes at both transcriptional and post-transcriptional levels. However, further physiological and molecular studies are needed to determine the most appropriate treatment condition and to elucidate the entire mechanism by which Ca and EGTA act to improve plant Cd tolerance.
References
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Alzahrani Y, Alharby HF, Hakeem KR, Alsamadany H (2020) Modulating effect of EDTA and SDS on growth, biochemical parameters and antioxidant defense system of Dahlia variabilis grown under cadmium and lead-induced stress. Not Bot Horti Agrobot Cluj Napoca 48:906–923. https://doi.org/10.15835/nbha48211909
Ben Massoud M, Sakouhi L, Chaoui A (2019) Effect of plant growth regulators, calcium and citric acid on copper toxicity in pea seedlings. J Plant Nutr 42:1230–1242. https://doi.org/10.1080/01904167.2019.1609506
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34. https://doi.org/10.1590/S1677-04202005000100003
Bewley J (1997) Seed germination and dormancy. Plant Cell 9:1055–1066. https://doi.org/10.1105/tpc.9.7.1055
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cheng K, Tian HZ, Zhao D et al (2014) Atmospheric emission inventory of cadmium from anthropogenic sources. Int J Environ Sci Technol 11:605–616. https://doi.org/10.1007/s13762-013-0206-3
Ci D, Jiang D, Dai T et al (2009) Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 77:1620–1625. https://doi.org/10.1016/j.chemosphere.2009.08.062
Cuypers A, Plusquin M, Remans T et al (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940. https://doi.org/10.1007/s10534-010-9329-x
Dayod M, Tyerman SD, Leigh RA, Gilliham M (2010) Calcium storage in plants and the implications for calcium biofortification. Protoplasma 247:215–231. https://doi.org/10.1007/s00709-010-0182-0
Di Toppi LS, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exper Bot 41:105–130. https://doi.org/10.1016/S0098-8472(98)00058-6
Dudonné S, Vitrac X, Coutière P et al (2009) Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem 57:1768–1774. https://doi.org/10.1021/jf803011r
El-Ashry ZM, Mohamed FI (2012) Protective effects of some antioxidant metals against chromosomal damage induced by cadmium in Vicia faba plants. Int J Agric Res 7:376–387. https://doi.org/10.3923/ijar.2012.376.387
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M (2013) Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. plants. Environ Sci Pollut Res Int 20:1413–1422. https://doi.org/10.1007/s11356-012-1181-9
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. https://doi.org/10.1007/BF00386001
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18. https://doi.org/10.1104/pp.110.167569
Garcia SC, Schott K, Charão M et al (2008) Quantification of reduced glutathione by HPLC-UV in erythrocytes of hemodialysis patients. Biomed Chromatogr 22:460–468. https://doi.org/10.1002/bmc.954
Gratão PL, Monteiro CC, Tezotto T et al (2015) Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals 28:803–816. https://doi.org/10.1007/s10534-015-9867-3
Gratão PL, Alves LR, Lima LW (2019) Heavy metal toxicity and plant productivity: role of metal scavengers. In: Srivastava S, Srivastava AK, Suprasanna P (eds) Plant-metal interactions. Springer International Publishing, Cham, pp 49–60. https://doi.org/10.1007/978-3-030-20732-8_3
Green LS, Yee BC, Buchanan BB et al (1991) Ferredoxin and ferredoxin-NADP reductase from photosynthetic and nonphotosynthetic tissues of tomato. Plant Physiol 96:1207–1213. https://doi.org/10.1104/pp.96.4.1207
Han Y, Zhang L, Gu J et al (2018) Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int Biodeter Biodegr 128:15–21. https://doi.org/10.1016/j.ibiod.2016.05.011
Hashimoto K, Kudla J (2011) Calcium decoding mechanisms in plants. Biochimie 93:2054–2059. https://doi.org/10.1016/j.biochi.2011.05.019
Heyno E, Klose C, Krieger-Liszkay A (2008) Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol 179:687–699. https://doi.org/10.1111/j.1469-8137.2008.02512.x
Huang D, Gong X, Liu Y et al (2017) Effects of calcium at toxic concentrations of cadmium in plants. Planta 5:863–873. https://doi.org/10.1007/s00425-017-2664-1
Jacquemond M, Verdin E, Dalmon A et al (2009) Serological and molecular detection of tomato chlorosis virus and tomato infectious chlorosis virus in tomato. Plant Pathology 58:210–220. https://doi.org/10.1111/j.1365-3059.2008.01959.x
Jacquot JP, Rivera-Madrid R, Marinho P et al (1994) Arabidopsis thaliana NAPHP thioredoxin reductase. cDNA characterization and expression of the recombinant protein in Escherichia coli. J Mol Biol 235:1357–1363. https://doi.org/10.1006/jmbi.1994.1091
Katrusiak AE, Paterson PG, Kamencic H et al (2001) Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl–glycine, homocysteine and glutathione in plasma and cell extracts. J Chromatogr B: Biomed Sci Appl 758:207–212. https://doi.org/10.1016/S0378-4347(01)00182-7
Kharbech O, Ben Massoud M, Sakouhi L, Djebali W, Jose Mur LA, Chaoui A (2020) Exogenous application of hydrogen sulfide reduces chromium toxicity in maize seedlings by suppressing NADPH oxidase activities and methylglyoxal accumulation. Plant Physiol Biochem 154:646–656. https://doi.org/10.1016/j.plaphy.2020.06.002
Khraiwesh B, Arif MA, Seumel GI et al (2010) transcriptional control of gene expression by MicroRNAs. Cell 140:111–122. https://doi.org/10.1016/j.cell.2009.12.023
Li P, Zhao C, Zhang Y et al (2016) Calcium alleviates cadmium-induced inhibition on root growth by maintaining auxin homeostasis in Arabidopsis seedlings. Protoplasma 253:185–200. https://doi.org/10.1007/s00709-015-0810-9
Li Z, Mei X, Li T et al (2021) Effects of calcium application on activities of membrane transporters in Panax notoginseng under cadmium stress. Chemosphere 262:127905. https://doi.org/10.1016/j.chemosphere.2020.127905
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
López-Climent MF, Arbona V, Pérez-Clemente RM et al (2014) Effect of cadmium and calcium treatments on phytochelatin and glutathione levels in citrus plants. Plant Biol J 16:79–87. https://doi.org/10.1111/plb.12006
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175 https://doi.org/10.1016/S0021-9258(19)45228-9
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299. https://doi.org/10.1016/S0168-9452(00)00392-7
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nouairi I, Jalali K, Essid S et al (2019) Alleviation of cadmium-induced genotoxicity and cytotoxicity by calcium chloride in faba bean (Vicia faba L. var. minor) roots. Physiol Mol Biol Plant 25:921–931. https://doi.org/10.1007/s12298-019-00681-5
Parejo I, Viladomat F, Bastida J et al (2003) Investigation of Bolivian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci 73:1667–1681. https://doi.org/10.1016/S0024-3205(03)00488-0
Potters G, Horemans N, Bellone S et al (2004) Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol 134:1479–1487. https://doi.org/10.1104/pp.103.033548
Rafiq M, Shahid M, Shamshad S et al (2018) A comparative study to evaluate efficiency of EDTA and calcium in alleviating arsenic toxicity to germinating and young Vicia faba L. seedlings. J Soil Sediment 18:2271–2281. https://doi.org/10.1007/s11368-017-1693-5
Rahoui S, Chaoui A, Ben C et al (2015) Effect of cadmium pollution on mobilization of embryo reserves in seedlings of six contrasted Medicago truncatula lines. Phytochemistry 111:98–106. https://doi.org/10.1016/j.phytochem.2014.12.002
Rahoui S, Martinez Y, Sakouhi L et al (2016) Cadmium-induced changes in antioxidative systems and differentiation in roots of contrasted Medicago truncatula lines. Protoplasma. https://doi.org/10.1007/s00709-016-0968-9
Romero-Puertas MC, Palma JM, Gomez M et al (2002) Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ 25:677–686. https://doi.org/10.1046/j.1365-3040.2002.00850.x
Sakouhi L, Rahoui S, Ben Massoud M et al (2016) Calcium and EGTA alleviate cadmium toxicity in germinating chickpea seeds. J Plant Growth Regul 35:1064–1073. https://doi.org/10.1007/s00344-016-9605-2
Sakouhi L, Rahoui S, Gharsallah C et al (2018) Effects of calcium and EGTA on thiol homeostasis and defense-related enzymes in Cd-exposed chickpea roots. Acta Physiol Plant 40:20. https://doi.org/10.1007/s11738-017-2596-1
Salin ML, Lyon DS (1983) Iron-containing superoxide dismutase in eukaryotes: localization in chloroplasts in water lily, Nuphar luteum. In: Oxy radicals and their scavenger systems, Cohen G, Greenwald RA. Elsevier Science Publishing Co., New York, pp 344–347
Semane B, Dupae J, Cuypers A et al (2010) Leaf proteome responses of Arabidopsis thaliana exposed to mild cadmium stress. J Plant Physiol 167:247–254. https://doi.org/10.1016/j.jplph.2009.09.015
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121–124
Singh S, Tripathi DK, Chauhan DK, Dubey NK (2016) Chapter 11 - Glutathione and phytochelatins mediated redox homeostasis and stress signal transduction in plants: an integrated overview. In: Ahmad P (ed) Plant Metal Interact. Elsevier, pp 285–310. https://doi.org/10.1016/B978-0-12-803158-2.00011-4
Song J, Markewitz D, Wu S et al (2018) Exogenous oxalic acid and citric acid improve lead (Pb) tolerance of Larix olgensis A. Henry seedlings. Forests 9:510. https://doi.org/10.3390/f9090510
Soyingbe OS, Ntanzi C, Makhafola TJ, Kappo AP (2020) Ameliorative effect of exogenously applied oxalic acid on nickel (heavy metal) induced stress in Zea mays. Pak J Bot 52(2):413–418. https://doi.org/10.30848/PJB2020-2(14)
Srivastava RK, Rajpoot R, Pandey P et al (2018) Cadmium alters mitochondrial membrane potential, inhibits electron transport chain activity and induces callose deposition in rice seedlings. J Plant Growth Regul 37:335–344. https://doi.org/10.1007/s00344-017-9726-2
Steinhorst L, Kudla J (2014) Signaling in cells and organisms — calcium holds the line. Curr Opin Plant Biol 22:14–21. https://doi.org/10.1016/j.pbi.2014.08.003
Štolfa I, Pfeiffer TŽ, Špoljarić D et al (2015) Heavy metal-induced oxidative stress in plants: response of the antioxidative system. In: Gupta DK, Palma JM, Corpas FJ (eds) Reactive oxygen species and oxidative damage in plants under stress. Springer International Publishing, Cham, pp 127–163. https://doi.org/10.1007/978-3-319-20421-5_6
Valivand M, Amooaghaie R (2020) Calcium signaling confers nickel tolerance in Cucurbita pepo L. Int J Phytoremediation 0:1–12. https://doi.org/10.1080/15226514.2020.1814992
Wan G, Najeeb U, Jilani G et al (2011) Calcium invigorates the cadmium-stressed Brassica napus L. plants by strengthening their photosynthetic system. Environ Sci Pollut Res 18:1478–1486. https://doi.org/10.1007/s11356-011-0509-1
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179. https://doi.org/10.1016/j.sajb.2009.10.007
Yang T, Poovaiah BW (2003) Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci 8:505–512. https://doi.org/10.1016/j.tplants.2003.09.004
Zaffagnini M, Bedhomme M, Lemaire SD, Trost P (2012) The emerging roles of protein glutathionylation in chloroplasts. Plant Science. 185–186:86–96. 10.1016/j.plantsci.2012.01.005
Zaffagnini M, Fermani S, Marchand CH, Costa A, Sparla F, Rouhier N et al (2019) Redox homeostasis in photosynthetic organisms: novel and established thiol-based molecular mechanisms. Antioxid Redox Signal. 31:155–210. https://doi.org/10.1089/ars.2018.7617
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432. https://doi.org/10.3390/ijms14047405
Zu Y, Mei X, Li B, et al. (2020) Effects of calcium application on the yields of flavonoids and saponins in Panax notoginseng under cadmium stress. Int J Environ Anal Chem 0:1–12. https://doi.org/10.1080/03067319.2020.1781835
Funding
This work was financially supported by the Tunisian Ministry of High Education and Scientific Research (LR18ES38) and the Graduate School of Environmental and Life Science, Okayama University (Grant to Lamia Sakouhi).
Author information
Authors and Affiliations
Contributions
LS performed all laboratory experiments, statistically analyzed the data, and wrote the manuscript; OK and MBM helped in manuscript drafting and revised the language; CG performed PCR analysis; SBH performed HPLC analysis; SM designed the primers and revised the manuscript; YM revised critically the manuscript; AC supervised the experiments and helped in the physiological analyses and revision step. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakouhi, L., Kharbech, O., Massoud, M.B. et al. Calcium and ethylene glycol tetraacetic acid mitigate toxicity and alteration of gene expression associated with cadmium stress in chickpea (Cicer arietinum L.) shoots. Protoplasma 258, 849–861 (2021). https://doi.org/10.1007/s00709-020-01605-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01605-x