Abstract
Purpose
To assess the usefulness of epidural air injection during the RFA treatment of spinal osteoid osteoma.
Methods
A retrospective review of 17 patients who underwent RFA for spinal osteoid osteoma between September 2006 and May 2017 was performed. All the procedures were performed by a single radiologist. We reviewed the perioperative CT studies to assess the distribution of air relative to the osteoid osteoma. The clinical outcome of each patient group was evaluated during routine follow-up.
Results
Seventeen patients were treated for spinal OO (male:female 13:4; mean age was 16, ranging from 4 to 42). The nidus size ranged from 5.8 to 17.2 mm (mean 11.2). In nine cases epidural air injection was performed. In three cases the neuroprotective air was deemed satisfactory with a clear layer of air between the osteoid osteoma and the dural sac being visualised. In six patients adherence between the cortical bone immediately adjacent to the osteoid osteoma and the dural sac in contact was observed.
In 15 patients the procedure was successful in terms of pain relief. No neural damage or other complication was reported in either group.
Conclusion
RFA is a safe treatment for spinal osteoid osteoma. Neuroprotective air injection does not appear to be necessary when performing the treatment in the spine.
Key Points
• Seventeen patients with spinal OO were treated with RFA, nine with air injection and eight without. Clinically successful treatment was achieved in 15 patients, with 2 subsequently undergoing surgery
• In 6/9 cases the injected air failed to achieve separation between the osteoid osteoma and the thecal sac because of inflammatory adhesion
• No complications were observed, regardless of whether neuroprotective air was instilled. Neuroprotective air injection appears unnecessary when treating spinal OO
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiofrequency ablation (RFA) is considered a safe and effective procedure for the treatment of osteoid osteoma (OO) in the appendicular skeleton [1,2,3,4,5,6]. There is an increasing body of evidence that its use for spinal OO is also justified given the minimally invasive nature of the treatment relative to standard spinal surgical techniques [7, 8].
RFA of spinal lesions is technically challenging because of the complex vertebral anatomy and potential for spinal cord injury. The radiofrequency cannula produces an ellipsoid ablation volume with the radius depending on the length of the exposed tip (5 or 10 mm). Hence, a distance of between 5 and 10 mm between the tip of the cannula and the neural elements is generally considered safe. Lesions located closer than 5 mm pose a significant challenge for the radiologist in view of potential for thermal shock to the adjacent cord and nerve roots.
Interventional MSK radiologists throughout the years approached this challenge in different ways. The first approach consists in treating only lesions presenting sufficient clearance from the spinal cord, usually 1 cm [1]. Some authors reported that lesions with a nidus as close as 2 mm are suitable for RFA, but only if the procedure is performed with moderate sedation under local anaesthesia and the potential for neural damage can be assessed in real time [9, 10]. Therefore, OOs less than 2 mm from the neural elements represent an indication for surgical excision.
Another approach was attempted to offer RFA to patients presenting with an OO in contact with the neural structures. This consists of the epidural injection of a thermal insulator to prevent the cord from potential injury due to the high intra-procedural temperatures (90°C) encountered during RFA [8]. Rybak et al proposed the injection of air to obtain a separation between the dura and the theca to safely perform the procedure [11]. Klass and co-workers proposed epidural irrigation with a bolus (10 ml) of room temperature sterile water [12]. Gangi et al. describe the use of a slow epidural infusion (70ml/h) of saline water at room temperature to protect the neural structures located nearby [6].
All these techniques are performed with a separate epidural injection using needles of thicknesses ranging from 26 G [12] to 22 G [11]. These cause an increase of the risks associated to epidural injections in general: Haemorrhage if a blood vessel is inadvertently damaged [13], infection [14] and injury to the neural structures [15].
The purpose of our study was to review our experience of the usefulness of neuroprotective air injection during RFA of spinal OO and compare it against the current practice. Institutional review board approval was obtained for the retrospective data collection used in this study.
Materials and methods
We performed a retrospective review of 17 patients (male:female 13:4; mean age 16, ranging from 4 to 42) treated with RFA in our hospital with a diagnosis of OO involving the spine from September 2006 to May 2017. The RFA was performed in all cases by a fellowship-trained MSK radiologist using an electrode with an active tip of 5 or 10 mm according to the size of the nidus (20-gauge 15-cm radiofrequency cannula, NeuroTherm). One cycle at 90°C for 6 min was performed in all cases [8].
The first nine patients in this case series underwent epidural air injection. Air was injected in small aliquots of 3 ml (20-ml syringe and 21-gauge needle). An intraoperative CT scan was performed to establish the distribution of air relative to the OO location following air injection. The decision to not inject neuroprotective air in the last eight patients in this series was made by the senior author based on experience in the first nine cases. The amount of air injected was considered satisfactory when air was visualised on CT between the dura and the lamina/pedicle wall. We considered the neuroprotection successful when a separation between the dural sac and the periosteum was observed on CT and defined by a low attenuation area of air (at least 1mm in thickness) interposed between the OO and the dural sac on the consecutive axial slices included between one slice cranial to the nidus to at least one slice below it. Unsuccessful neuroprotective injection was described as persistent contact on one or more axial slices between the dural sac and the bone adjacent to the nidus.
Access to the lesion was obtained through a 14G Bonopty penetration set (AprioMed).
All patients were followed up in a spinal outpatient clinic. A consultant spinal surgeon evaluated the presence of post-procedural neural damage and the clinical effectiveness of the procedure in terms of pain relief.
Results
Seventeen patients underwent RFA for spinal OO in this case series. The size of the nidus varied from 5.8 to 17.2 mm (mean 11.2). In two cases the lesion was localised in the cervical spine, in seven cases it involved the thoracic spine, and in eight cases the lumbar region was affected.
The vertebral structure involved was the body (n = 3), the lateral mass (n = 2), the pedicle (n = 3) and posterior elements (including the transverse process, spinous process and laminae, n = 9). The anatomic location of the OO did not affect the decision to perform neuroprotective air injection.
Antalgic scoliosis was diagnosed in nine patients on pre-procedural scans (radiographs).
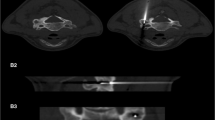
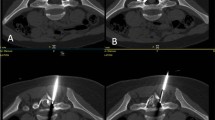
Nine patients were treated with neuroprotective injection of air. The amount of air between the dura and the lesion was graded as satisfactory in 3 cases (Figs. 1 and 2). In six patients we observed an adherence between the cortical bone at the level of the osteoid osteoma and the dural sac in contact (Figs. 3 and 4). The air did not create a plane between the dura and the involved portion of the spine. Air tended to pass towards the opposite side of the dura and in some instances caused a minor displacement of the thecal sac towards the area being treated. This happened because the air went to the contralateral side in all cases and in four cases led to displacement of the thecal sac towards the OO rather than away from it.
Axial CT: the inflammatory adherence phenomenon in a patient with lumbar spine osteoid osteoma: (a) the position of the needle for neuroprotective air injection; (b) the post-injection scan shows a satisfactory amount of air injected determined by the presence of air around the nerve roots (dashed arrows). However, there is no separation between the cortical bone and the thecal sac (arrow). The RFA was performed and no short- or long-term complications were reported (c)
Considering all 17 patients, satisfactory post-procedural pain relief was obtained in 15 patients. Two patients presented persistent symptoms after RFA and were treated with subsequent surgical excision (one underwent RFA with neuroprotective injection, one without). A second RFA was not attempted in any case in the series. Subsequent review of the imaging in the two patients where treatment failed was performed. The first treatment failure occurred when RFA of an OO in the lamina in the cervical spine was performed. The needle was positioned eccentrically within the nidus on imaging review. The second instance of failure was in the largest lesion treated in this series (17.2 mm). The needle was positioned centrally in the nidus using a 10-mm exposed needle tip and it is probable that the OO was incompletely treated because of the size of the lesion.
Discussion
Our study showed no differences in terms of outcome or complications between patients treated with and without the injection of epidural air as a neuroprotective agent. Furthermore, we found that technical success of the injection of a thermal insulator was limited (3/9), potentially because of the presence of inflammatory adherence between the thecal sac and adjacent bone.
RFA is increasingly being utilised in the treatment for spinal OO. Compared with surgery, there are fewer intra- and postoperative (long- and short-term) complications and there are similar results in terms of pain relief [8]. Surgical excision still plays a role in lesions that are not suitable for RFA and recurrent cases.
The potential proximity of the OO to the spinal cord has naturally raised concerns about the safety of RFA [1, 2, 10, 11, 16, 17]. A temperature of 90° C is usually applied for 6 min to achieve a satisfactory ablation of the nidus, above the limit for irreversible denaturation of proteins (80°) [18]. Therefore, adverse effects on the surrounding vital tissues have to be taken into consideration, in particular those secondary to nerve cell or fibre degeneration or necrosis. The most serious adverse effects include focal myelopathy at the ablated level, paresis and paralysis [17]. A number of authors have suggested the use of epidural injection to separate the dura and the affected bone to minimise the risk of neural injury [6, 11, 12]. The potential harm related to RFA was hypothesised by Nour et al. in 2002 [17] and Bitsch et al. in 2006 [16]. The first author reported histological evidence of neural damage in the nerve roots [17]. However, the procedure was performed under MRI guidance with lower resolution compared to CT, not allowing the accurate localisation of needle position. The study by Bitsch is biased on the use of ex vivo non-viable tissue without a periosteal blood supply or CSF circulation [16]. Therefore, the temperatures detected in the perilesional soft tissues are not reliable and again cannot be extrapolated accurately for in vivo RFA.
These concerns, however, were considered by Rybak et al. in 2007 [11] and Klass et al. in 2009 [12], who performed neuroprotective injection of air and sterile water in the epidural space. Both the authors reported the complete absence of permanent or transient neural consequences. Rybak observed an increase in the distance between the lesion and the neural elements, without giving a specific success rate [11].
Klass injected sterile water in seven patients. He was unable to assess the actual degree of separation between the bone and dura as water has the same density as CSF on CT [12].
Other authors [19] have demonstrated in vivo and in vitro that during RFA in the therapeutic range (90° for 6 min) the temperatures reaching the neural elements when the cortical bone is intact are around 40°. Apoptosis and cellular necrosis are obtained only if thermal doses of respectively 44° or 45° are maintained for 120 min [20].
In our series, we performed air injection in nine cases. Only in three cases was complete separation between the dura and the affected cortical bone observed. In the other patients (n = 6) we detected an adherence between the bone and the dura. We interpreted this finding as a response of the dural sac to the inflammatory reaction associated with the OO. This phenomenon is well known in appendicular lesions where associated joint effusions and synovitis may be observed in periarticular cases of OO.
In some instances air even tended to displace the thecal sac and the cord towards the lesion, which was clearly the adverse effect that we desired. In fact, we observed passage of air to the other side of the thecal sac, which represented the track of least resistance. This phenomenon resulted in a displacement of the neural structures towards the OO with an increased proximity of the two structures and possibly higher temperatures reaching the cord and the roots.
In light of this, the neuroprotective injection of air was not performed in the last eight patients of our series. These were treated using the same RFA protocol, and no evidence of neural injury or complication was reported.
No differences in terms of clinical outcome were observed between the two groups.
In conclusion, RFA of spinal OO is a safe procedure, even when the nidus is located less than 10 mm from the neural structures. No neural damage or other complication related to RFA is reported in our series. The use of neuroprotective air in our experience does not appear to be required and no longer forms part of our routine clinical practice in the treatment of spinal OO.
We believe the CSF circulation, the rich blood supply to the periosteum and the integrity of the cortical bone provide an effective thermal insulation when RFA is performed in the therapeutic range (90°C applied for 6 min).
Abbreviations
- OO:
-
Osteoid osteoma
- MSK:
-
Musculoskeletal
- RFA:
-
Radiofrequency ablation
References
Rosenthal DI, Springfield DS, Gebhardt MC, Rosemberg AE, Mankin HJ (1995) Osteoid osteoma: percutaneous radio-frequency ablation. Radiology 197:451–454
Motamedi D, Learch TJ, Ishimitsu DN et al (2009) Thermal ablation of osteoid osteoma: overview and step-by-step guide. Radiographics 29:2127–2141
Bourgault C, Vervoort T, Szymanski C, Chastanet P, Maynou C (2014) Percutaneous CT-guided radiofrequency thermocoagulation in the treatment of osteoid osteoma: A 87 patient series. Orthop Traumatol Surg Res 100:323–327
Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ (2003) Osteoid osteoma: Percutaneous treatment with radiofrequency energy. Radiology 229:171–175
Hoffmann RT, Jakobs TF, Kubisch CH et al (2010) Radiofrequency ablation in the treatment of osteoid osteoma-5-year experience. Eur J Radiol 73:374–379
Gangi AA, Houman Wong L, Buy X, Dietemann J-L, Roy C (2007) Osteoid osteoma: Percutaneous laser ablation and follow-up in 114 patients. Radiology 242:293–301
Albisinni U, Facchini G, Spinnato P, Gasbarrini A, Bazzocchi A (2017) Spinal osteoid osteoma: efficacy and safety of radiofrequency ablation. Skeletal Radiol 46:1087–1094
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ (1998) Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. JBJS 80:815–821
Vanderschueren GM, Obermann WR, Dijkstra SPD, Taminiau AHM, Bloem JL, van Erkel AR (2009) Radiofrequency ablation of spinal osteoid osteoma: Clinical outcome. Spine (Phila Pa 1976) 34:901–903
Wang B, Han SB, Jiang L et al (2017) Percutaneous radiofrequency ablation for spinal osteoid osteoma and osteoblastoma. Eur Spine J 26:1884–1892
Rybak LD, Gangi A, Buy X, Vieira RLR, Wittig J (2010) Thermal ablation of spinal osteoid osteomas close to neural elements: Technical considerations. AJR Am J Roentgenol 195:293–298
Klass D, Marshall T, Toms A (2009) CT-guided radiofrequency ablation of spinal osteoid osteomas with concomitant perineural and epidural irrigation for neuroprotection. Eur Radiol 19:2238–2243
Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Fellows B (2012) A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain Physician 15:131–140
Noh SH, Heo DH (2015) Whole cerebrospinal axis infection after lumbar epidural injection: a case report. Eur Spine J 24:525–528
Manchikanti L, Kaye A, Hirsch J (2014) The risks of epidural and transforaminal steroid injections in the spine: Commentary and a comprehensive review of the literature. Surg Neurol Int 5:38
Bitsch RG, Rupp R, Bernd L, Ludwig K (2006) Osteoid osteoma in an ex vivo animal model: temperature changes in surrounding soft tissue during CT-guided radiofrequency ablation. Radiology 238:107–112
Nour SG, Aschoff AJ, Mitchell ICS, Emancipator SN, Duerk JL, Lewin JS (2002) Radiology MR imaging-guided radio-frequency thermal ablation of the lumbar vertebrae in porcine models. Radiology 224:452–462
Matsuura Y, Takehira M, Joti Y et al (2015) Thermodynamics of protein denaturation at temperatures over 100 °C: CutA1 mutant proteins substituted with hydrophobic and charged residues. Sci Rep 5:15545
Dupuy DE, Hong R, Oliver B, Goldberg SN (2000) Radiofrequency ablation of spinal tumors. Am J Roentgenol 175:1263–1266
Song AS, Najjar AM, Diller KR (2014) Thermally induced apoptosis, necrosis, and heat shock protein expression in 3D culture. J Biomech Eng 136:71006
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr Steven James.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Vidoni, A., Grainger, M. & James, S. Experience of neuroprotective air injection during radiofrequency ablation (RFA) of spinal osteoid osteoma. Eur Radiol 28, 4146–4150 (2018). https://doi.org/10.1007/s00330-018-5406-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5406-2