Abstract
Here we report our experience of a neuroprotective adaptation of the technique of CT-guided radiofrequency (RF) ablation of spinal osteoid osteomas. Over 9 years seven patients underwent eight CT-guided RF treatments for osteoid osteoma. CT-guided RF ablation was performed with general anaesthesia. The lesion was heated to 90°C for 2 min for two cycles by using a Cosman SMK TC-10 RF electrode. This was preceded by a bolus of room temperature sterile water (10 ml) injected through a 26G curved spinal needle into the exit foramen and adjacent epidural space for neuroprotection. The age of the patient, sex, lesion location, biopsy results and complications were recorded. All the biopsies (n = 7) demonstrated histological features of osteoid osteoma. All the procedures were technically successful. Clinical success was assessed up to 3 years post procedure. There was an 85% clinical success rate (6 of the 7 patients), with recurrence of a lesion at 6 months, necessitating a repeat procedure (successful). CT-guided percutaneous RF ablation of spinal osteoid osteoma preceded by bolus of sterile water, injected through a spinal needle into the exit foramen and adjacent epidural space for neuroprotection, is a safe and effective procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoid osteoma (OO) is a benign yet painful bone tumour occurring in young adults and children, particularly in the lower limbs [1, 2]. OO are common making up about 10% of benign primary bone tumours [3, 4]. The diagnosis is only made following imaging and conventional radiography remains the initial investigation of choice. CT or MRI is used to confirm the diagnosis [4]. Skeletal scintigraphy is still used [5] although the frequency is decreasing as the popularity of cross-sectional imaging increases.
There are three approaches to treatment: surgery (open and minimal access) [1, 4–9], conservative (medical) and image-guided minimal access techniques [9–12]. The evolution of image-guided percutaneous treatments has allowed excision or ablation by using small calibre needles [9–12]. Initial results of percutaneous procedures have been favourable when compared with surgical treatment [11, 13, 14]. Percutaneous ablation of tumours in the spine has, in small numbers of patients, produced comparable results to open surgery [5, 11, 13, 15, 16]. However experience of percutaneous treatment of spinal OO is still relatively limited and the fear of thermal injury to adjacent neurological structures remains [2, 4, 11, 16, 17]. Probably because of this surgery still remains a popular treatment for spinal OO.
The purpose of this study is to report our experience of using a modification of the standard CT-guided ablation technique that is designed to protect neurological structures from thermal damage during RF ablation.
Materials and methods
Patients
Selection criteria for RF ablation required patients with both clinical symptoms and radiological signs of an osteoid osteoma in the spine, who had chosen radiological treatment, after alternative options had been considered. Seven OOs were identified in three men and four women (mean age 17, range 13–32) who opted for percutaneous RF ablation. The seven OOs were located in the cervical (n = 2), thoracic (n = 1) and lumbar spine (n = 3), and sacrum (n = 1). Between February 1998 and August 2006 the patients underwent a total of eight CT-guided RF treatments. All patients underwent a single treatment except for one patient who underwent two procedures. All patients were informed of the risks of the procedure and alternative treatment options were explained. Four of the patients underwent preprocedure biopsy.
Procedure
All procedures were performed by the same radiologist. General anaesthesia, using a laryngeal mask airway, was used in all cases. The shortest distance from the skin to the lesion was preferred, and thus all procedures were performed prone. The lesions were localised on CT (GE Lightspeed 4, GE Healthcare, UK) before marking the skin. Lidocaine (1%) was used for local anaesthesia, and a small incision was made in the skin.
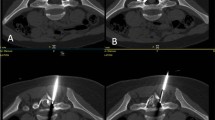
The osteoid osteoma was accessed using a Bonopty bone biopsy needle (Radi Medical Systems, Uppsala, Sweden) to drill through the outer cortex. If a tissue diagnosis had not been made as a result of a previous biopsy, a biopsy was performed at this stage (n = 3). The radiofrequency needle (Cosman SMK TC-10 electrode, Cosman Medical Inc, Burlington, MA, US) positioned through an insulated Neurotherm 22G cannula (Neurotherm Inc, Middleton, MA, US) was then introduced into the nidus, using a coaxial technique, through the Bonopty needle. The ablation volume is ellipsoid with a radius of 5 mm; therefore if the lesion was greater than 1 cm, more than one radiofrequency ablation was performed, with the needle tip in different positions to cover the nidus. This includes the craniocaudal dimension of the lesion (Fig. 1). If the OO was elongated in this dimension, separate ablations were performed along this plane as well. Once the Neurotherm cannula and the Cosman SMK electrode were in place (Fig. 2), a 21G spinal needle was guided down into the adjacent exit foramen. Using a coaxial technique a curved 26G spinal needle was inserted through the 21G needle to negotiate the lateral mass structures and access the exit foramen (Fig. 3). The 26G needle is more flexible and manoeuvrable allowing for more precise and easier placement (Fig. 4).
Contrast medium was injected (2 ml iohexol, GE Healthcare AS, Nydalen, Norway) to confirm the position of the needle tip. Just before the ablation cycle, a bolus of room temperature sterile water (10 ml) was injected through the 26G spinal needle into the exit foramen and adjacent epidural space to reduce the chances of thermal damage to the adjacent neural or vascular structures (Fig. 5). The sterile water was injected over a period of 30 s to allow for adequate distribution around the nerve roots and epidural space.
Two cycles of ablation at 90°C for 2 min for lesions smaller than 1 cm (n = 5) were performed. For lesions greater than 1 cm (n = 2), a further two 2-min cycles in a different position were performed. If a further ablation cycle was needed, a further bolus of sterile room temperature water was injected, through the 26G spinal needle in the same position as for the previous ablation cycle. After the ablation cycles a small volume (1–3 ml) of 0.5% bupivacaine was injected into the exit foramen for symptom relief for a few hours after the procedure.
All patients were examined after the procedure in the recovery room and again before discharge. The wound site was checked for bleeding, swelling and tissue burns. A neurovascular examination was performed and pain levels were assessed using a visual analogue scale (VAS) [18]. Five patients remained in hospital overnight, and two of the patients were discharged on the same day following examination. Patients were advised not to take part in any sporting activity for 2 weeks following the procedure. Patients were reviewed at orthopaedic outpatient clinic (median time 11 months, range 5–17 months) following the procedure. If no record of follow-up was found, patients were contacted by telephone.
Results
The seven OOs (2 cervical, 1 thoracic, 3 lumbar, 1 sacral) were located in the following positions within the vertebra: pedicle (n = 4), lateral mass (n = 2), sacral alar (n = 1). The median diameter of the OO was 6.9 mm (range 4.6–10.3 mm). The median shortest distance to the adjacent thecal sac was 7.1 mm (range 2.3–13.4 mm) and to the nearest neural structure 4.8 mm (range 3.7–6.8 mm). All eight procedures were technically successful as judged by the needle position in the centre of the nidus and the immediate post procedural examination [19]. If the lesion edge was more than 5 mm from the edge of the tumour to the needle tip (n = 2) a further ablation was carried out in a different position.
All patients (n = 7) had satisfactory immediate post procedural (n = 8) assessments. There were no wound complications. The results of all neurovascular examinations were normal with no focal loss of power or sensory abnormalities. The majority of the patients requested analgesia (n = 5), with a combination of paracetamol (15 mg/kg) and ibuprofen (5 mg/kg) being administered orally. One patient did not request any post procedural analgesia. No opiate analgesia was administered.
At repeat assessment, before discharge, the post procedural course (n = 8) was uncomplicated. Neurovascular examinations were unchanged. None of the patients requested analgesia at discharge, but all were discharged with paracetamol and ibuprofen. The median patient hospital stay was 22 h from admission (range 10–27 h). All patients were discharged within 24 h of the procedure, and 85% (n = 6) of the patients reported no ongoing symptoms of pain and were satisfied with the procedure at outpatient follow-up. Analysis of data of patients followed up for 12 months clinically showed only a single patient (15%) needing a second procedure.
The single repeat procedure was successful and carried out a year following the first.
All the patients experienced some degree of pain post procedure (n = 7); visual analogue scores (VAS) ranged from 1 to 6 (mean 2.3). Pain was relieved by paracetamol and ibuprofen.
Follow-up clinic assessments ranged from 5–17 months post procedure. Six patients attended the follow-up clinic appointments. The patient lost to clinical follow-up was a regional referral and did not attend locally for follow-up. No documentation of VAS for pain was found in the clinic notes. Five patients had no documented pain at the time of the clinic visit, and one patient had mild, intermittent pain, which did not affect sporting activities, and required occasional paracetamol, but no NSAIDs.
A telephone questionnaire regarding pain and current physical activity was carried out by one of the authors (DK) at the time of the retrospective study. The telephone assessment ranged from 20 months to 4 years after the procedure. Only four patients could be contacted. The remaining three patients’ personal details had changed, and they could not be contacted. All four patients contacted had a VAS of 0 for pain at rest, and had returned to their normal activities, some of which included active, noncompetitive sports.
Discussion
Treatment of OOs is the first musculoskeletal application of radiofrequency ablation, and it has modified their treatment significantly, providing a safe alternative to surgery [10, 14, 19, 20]. Surgery is not without risk, as these tumours can often be difficult to access, be close to major vessels, or nerves, or require en bloc resection. Tumours of the spine carry the added risks associated with spinal surgery, and are resected less often than peripheral tumours.
Percutaneous options include RF ablation [2, 3, 10, 11, 13–15], percutaneous excision [8], with low calibre needles and drills [21], MR-guided therapy [22], CT-guided drilling of the nidus [20] and laser photocoagulation [5, 23–27]. The use of bipolar ablation has been described in a small group of patients with promising results [28]. The largest published series of percutaneous treatment of spinal OOs is 12 [5] treated with laser photocoagulation. Although relatively small, our series of seven patients is the largest series treated with RF ablation. As with laser photocoagulation there were no periprocedural complications associated with RF ablation, using a neuroprotective sterile water infusion, in our experience [5].
In our series one out of seven (15%) OOs recurred within 6 months which is comparable to other published data [10, 12, 14, 29]. Although it is possible that the OO may recur in some of these patients after the 2-year period of follow-up this is thought to be uncommon and a 2-year follow-up is standard in the orthopaedic literature [10, 15]. It is recognised that recurrence of OO after percutaneous treatment is often related to size, but in our series the size of the single recurring lesion was not statistically significant [20].
At our institution we use the Bonopty needle to gain access to the nidus. Our anecdotal experience is that the Bonopty needle is manoeuvrable in small places. The central drill bit trocar is very helpful for careful drilling through the outer cortex because it does not need much pressure to cut through cortical bone and therefore positional control is maintained. This limits the risk of damage to the adjacent structures and the needle is of an optimal size for passing the radiofrequency needle through it if need be. Our modification to the radiofrequency technique in order to decrease the incidence of neurological injury involves guiding a 21G spinal needle through the soft tissues, to the lateral mass structures. Using a coaxial technique, a 26G curved needle is passed through the 21G needle. The 21G needle acts a buttress for the 26G through the soft tissues. The 26G needle is then used to negotiate the lateral mass structures and access the exit foramen around the anterior aspect of the facet joint. The technique is the same as for CT-guided foraminal nerve root injections.
Just before the ablation cycle, a bolus (10 ml) of room temperature sterile water is injected over 30 s through the 26G spinal needle into the exit foramen and adjacent epidural space to reduce the chances of thermal damage to the adjacent neural structures.
Limitations of the study include the relatively small sample size. Although small, this appears to be the largest series of RF ablation of spinal OOs to date using this neuroprotective technique. A similar technique using laser photocoagulation and a similar method of neuroprotection has been described in five patients [5]. There is likely to be some selection bias given this is a retrospective study and patients were not randomised. One of the patients in this series was reviewed by a surgical team at a major orthopaedic hospital, and deemed not suitable for surgery due to the risk of morbidity. This procedure was carried out at our institution and illustrates the possibility that only those patients who were not deemed to be surgically remediable are referred for radiological intervention and may therefore be the more difficult cases.
The use of RF ablation in treating peripheral bone lesions has been well described but concerns about neurological thermal injury remain when treating OOs in the spine [2, 4, 11, 17]. Epidural irrigation with water may be one way of minimising this risk. Despite not being a tertiary referral orthopaedic or paediatric centre our experience suggests that RF ablation, with neuroprotective modifications, can be performed safely and effectively in the spine.
Conclusion
Radiofrequency ablation of spinal osteoid osteomas, using a modified neuroprotective technique of concomitant epidural irrigation with water, appears to be a safe and effective treatment.
References
Zileli M, Cagli S, Basdemir G, Ersahin Y (2003) Osteoid osteomas and osteoblastomas of the spine. Neurosurg Focus 15;15(5):E5
Samaha EI, Ghanem IB, Moussa RF, Kharrat KE, Okais NM, Dagher FM (2005) Percutaneous radiofrequency coagulation of osteoid osteoma of the “neural spinal ring”. Eur Spine J 14:702–705
Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ (1995) Osteoid osteoma: percutaneous radio-frequency ablation. Radiology 197(2):451–454
Hadjipavlou AG, Lander PH, Marchesi D, Katonis PG, Gaitanis IN (2003) Minimally invasive surgery for ablation of osteoid osteoma of the spine. Spine 28(22):E472–E477
Gangi A, Alizadeh H, Wong L, Buy X, Dietemann JL, Roy C (2007) Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology 242(1):293–301
Kirwan EO, Hutton PA, Pozo JL, Ransford AO (1984) Osteoid osteoma and benign osteoblastoma of the spine. Clinical presentation and treatment. J Bone Joint Surg Br 66(1):81–86
Poey C, Clement JL, Baunin C, Assoun C, Puget-Mechinaud C, Giron J et al (1991) Percutaneous extraction of an osteoid osteoma of the lumbar spine under CT guidance. J Comput Assist Tomogr 15(6):1056–1058
Labbe JL, Clement JL, Duparc B, Poey C, Railhac JJ (1995) Percutaneous extraction of vertebral osteoid osteoma under computed tomography guidance. Eur Spine J 4(6):368–371
Sans N, Galy-Fourcade, Assoun J, Jarlaud T, Chiavassa H, Bonnevialle P, Railhac N, Giron J, Morera-Maupome H, Railhac J-J (1999) Osteoid osteoma: CT-guided percutaneous resection and follow-up in 38 patients. Radiology 212(3):687–692
Vanderschueren GM, Taminiau AHM, Obermann WR, Bloem JL (2002) Osteoid osteoma: clinical results with thermocoagulation. Radiology 224(1):82–86
Laus M, Albisinni U, Alfonso C, Zappoli FA (2007) Osteoid osteoma of the cervical spine: surgical treatment or percutaneous radiofrequency coagulation? Eur Spine J 16:2078–2082
Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N (2001) Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol 12(6):717–722
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ (1998) Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am 7(5):422–425
Venbrux AC, Montague BJ, Murphy KP, Bobonis LA, Washington SB, Soltes AP, Frassica FJ (2003) Image-guided percutaneous radiofrequency ablation for osteoid osteomas. J Vasc Interv Radiol 14(3):375–380
Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ (2003) Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology 229(1):171–175
Yamada T, Tateishi A, Cho S (1992) The effects of hyperthermia on the spinal cord. Spine 17:1386–1391
Osti OL, Sebben R (1998) High-frequency radio-wave ablation of osteoid osteoma in the lumbar spine. Eur Spine J 7(5):422–425
Wewers ME, Lowe MK (1990) A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 13(4):227–236
Rosenthal DI (1997) Percutaneous radiofrequency treatment of osteoid osteomas. Semin Musculoskelet Radiol 1(2):265–272
Vanderschueren GM, Taminiau AHM, Obermann WR, van den Berg-Huysmans AA, Bloem JL (2004) Osteoid osteoma: factors for increased risk of unsuccessful thermal coagulation. Radiology 233(3):757–761
Van Royan BJ, Baayen JC, Pijpers R, Noske DP, Schakenraad D, Wuisman PIJM (2005) Osteoid osteoma of the spine: a novel technique using combined computer-assisted and gamma probe-guided high-speed intralesional drill excision. Spine 30(3):369–373
Nour SG, Aschoff AJ, Mitchell ICS, Emancipator SN, Duerk JL, Lewin JS (2002) MR imaging-guided radio-frequency ablation of the lumbar vertebrae in porcine models. Radiology 224(2):252–262
Gangi A, Dietemann JL, Gasser B, Mortazavi R, Brunner P, Mourou MY, Dosch JC, Durckel J, Marescaux J, Roy C (1997) Interstitial laser photocoagulation of osteoid osteomas with use of CT guidance. Radiology 203:843–848
Gangi A, Dietemann J-L, Guth S, Vinclair L, Sibilia J, Mortazavi R, Steib JP, Roy C (1998) Percutaneous laser photocoagulation of spinal osteoid osteomas under CT guidance. Am J Neuroradiol 19:1955–1958
Sequieiros RB, Hyvonen P, Sequeiros AB, Jyrkinen L, Ojala R, Klemola R, Vaara T, Tervonen O (2003) MR imaging guided laser ablation of osteoid osteoma with use of optical instrument guidance at 0.23T. Eur Radiol 13(10):2309–2314
DeFriend DE, Smith SP, Hughes PM (2003) Percutaneous laser photo coagulation of osteoid osteomas under CT guidance. Clin Radiol 58(3):222–226
Moser T, Giacomelli MC, Clavert JM, Buy X, Dietemann JL, Gangi A (2008) Image-guided laser ablation of osteoid osteoma in pediatric patients. J Pediatr Orthop 28(2):265–270
Mahnken AH, Tacke JA, Wildberger JE, Günther RW (2006) Radiofrequency ablation of osteoid osteoma: initial results with a bipolar ablation device. J Vasc Interv Radiol 17(9):1465–1470
Hadjipavlou AG, Tzermiadianos MN, Kakavelakis KN, Lander P (2008) Percutaneous core excision and radiofrequency thermo-coagulation for the ablation of osteoid osteoma of the spine. Eur Spine J. doi:10.1007/s00586-008-0791-x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klass, D., Marshall, T. & Toms, A. CT-guided radiofrequency ablation of spinal osteoid osteomas with concomitant perineural and epidural irrigation for neuroprotection. Eur Radiol 19, 2238–2243 (2009). https://doi.org/10.1007/s00330-009-1404-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1404-8