Abstract
Objective
The aim of this study was to evaluate the efficacy and complications of CT-guided radiofrequency ablation (RFA) of spinal osteoid osteoma (OO).
Materials and methods
Between 2002 and 2012, a total of 61 patients (46 male and 15 female, mean age 26.4 ± 12.7 years) were subjected to RFA for spinal OO. The diagnosis of OO was made after a period of pain and symptoms of 20.6 ± 14.4 months. RFA was performed under conscious sedation and local analgesia. Clinical symptoms were evaluated at 3, 6, and12 months, and at the end of the time of the present investigation. Mean follow-up was 41.5 ± 7.1 months.
Results
The primary efficacy of RFA, complete regression of symptoms, was obtained in 57 out of 61 patients (93.4%). Four out of 61 (6.5%) patients showed a relapse of OO (after 3 months); 2 out of 4 were subjected to a second RFA, the remaining ones were subjected to surgery. There was one complication (case of lower limb paresthesia for 30 days after the ablation) and one possible complication (a disc herniation).
Conclusion
CT-guided RFA is an excellent treatment for spinal OO. Our data suggest that this procedure should be considered for the first stage of therapy for this disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoid osteoma (OO), first described by Jaffe in 1935 [1], is a benign osteoblastic lesion. It represents 10–12% of all benign bone tumors, and 3% of all primary bone tumors. OO usually occurs in people aged 5–25 years, with male predominance (male to female ratio = 3:1). Most lesions are found in the long bones of the lower extremity, particularly the meta-diaphyseal regions of the femur and tibia. Other common sites include the spine, hands, and feet. In 10% of the cases OO occurs in the spine [2]. OO is characterized by a focus of vascularized osteoid tissue and sometimes mineralized immature bone, often surrounded by sclerotic reactive bone. The tumor is less than 2 cm in diameter [3] by definition. Most spinal OOs are located in the posterior element of the vertebra. The lumbar spine is most commonly affected, followed by the cervical, thoracic, and sacral segments. When OO affects the spine, they typically present with back pain and reactive scoliosis. In the case of anterior vertebral involvement, spinal stiffness is common, and has been reported in 89% of patients [4].
The typical clinical presentation with pain escalating at night and relieved by nonsteroidal anti-inflammatory drugs (salicylates, e.g., aspirin) is not always present, and diagnosis may be delayed for many months. Moreover, detection and localization of the tumor on radiographs is very difficult. CT is generally the preferred cross-sectional technique for demonstration and localization. CT typically shows a focally lucent lesion within surrounding sclerotic reactive bone. Although MRI is sensitive, it can be nonspecific as the hyperemia and resultant bone marrow edema pattern may obscure the tumor.
In the past, surgical excision was the standard treatment for OO, especially for spinal OO. Surgical complications for spinal lesions include incomplete removal, postoperative hematoma, and nerve injury [2]. Rosenthal et al. in the 1990s proposed imaging-guided radiofrequency thermal ablation (RFA) as an option to treat OO [5]. RFA gained a vast consensus and quickly became the treatment of choice for nonspinal OO [6]. RFA was also proposed for the treatment of OO in vertebral locations [2, 7,8,9,10,11]. However, the potential complications of neural damage have been a cause for concern, and deserve special attention. An increasing number of reports indicate that RFA seems to be the optimal solution for spinal OO, with the potential to replace surgical and more invasive approaches whenever possible, unless compression of neural elements is present (Table 1). However, study populations are still limited and some technical issues exist.
Our purpose was to retrospectively evaluate the clinical long-term results of CT-guided RFA in a large series of patients with spinal OO.
Materials and methods
In our series (more than 800 OO cases), spinal location was involved in less than 8% of all cases (61). All patients who underwent CT-guided RFA of spinal OO between November 2002 and November 2012 at our institution were included in this analysis.
The diagnosis of spinal OO was suspected on the basis of the clinical history and presentation and was confirmed by imaging studies (radiographs, MRI, and thin-slice CT). CT imaging was performed to confirm imaging diagnosis and for treatment planning. A needle biopsy was attempted at the time of treatment to obtain histological proof of the lesion.
Pain was scored according to a visual analog scale (VAS) score as collected before the intervention, and after 3, 6, and 12 months, and at the time of the present investigation (end of follow-up: 5.8 ± 2.6 years, range 4 months to 10 years).
Unsuccessful treatment was defined as the presence of residual clinical symptoms, persisting at least 3 months after RFA, or recurrence of symptoms resembling the initial symptoms with a positive imaging correlation (MRI with dynamic gadolinium-enhanced imaging).
Anamnesis and clinical data of patients before and after the treatment in addition to all imaging examinations were collected from the archive of our hospital. The following parameters were collected from the medical record for potential influence on the outcome of RFA procedure: sex, age, time from onset of symptoms, location, and vertebral level.
Lesion size was measured in millimeters. Similarly, the shortest distance between the lesion and the closest neural structures was measured on the CT images, including the presence of a cortex between the lesion and the closest nerve. All parameters of the RF procedure were included in the analysis of results.
The study was approved by the local ethics committee and complies with the Declaration of Helsinki.
RFA procedure
The patient was placed in a prone position and a posterior approach was used. The procedure was performed under deep sedation and local anesthesia.
The thermal ablation was carried out under CT guidance (high-speed CT; GE Healthcare, Milwaukee, WI, USA). A preliminary scan allowed evaluation and planning of the adequate approach to the lesion, the target point, and the length of the active tip to be used. To target the OO nidus, a Bonopty (Radi MS, Uppsala, Sweden) set was used—external caliber 14G. Before starting RFA treatment we always tried to obtain a biopsy sample, preserving the OO nidus, to conserve the insulation as far as possible. We approached the target with the electrode, with an active tip of 10, 15 or 20 mm (in one case) according to the size of the nidus and the localization of the lesion (monopolar, nonrefrigerated; SMK; Radionics, Burlington, MA, USA). A pad was placed on the skin of the patient and afterwards, RF was gradually administered with an RF generator (RFG-3C; Radionics). We performed a 5°/30-s protocol to raise the temperature to 90°, with a 2- to 3-min plateau at 60°. It usually takes 8 min to reach the maximum temperature; however, there were cases in which a longer time was needed and accounted for the large range reported in the table. Mean time at 90° is around 15 min. The patient’s neurological status is monitored during the procedure by asking the patient to move their legs and also if they notice any changes in the perception of sensitivity in the anatomical area.
At the end of the procedure, the needle and trocar were retrieved; the wound was cleaned and closed with sterile strips.
Statistical analysis
Results are reported as frequencies or mean and standard deviation. Data were analyzed using Mann–Whitney, Pearson Chi-squared and Fisher’s exact tests. Two-tailed p were considered significant if values were less than 0.05. The SPSS statistical package (version 13.1 for Windows; SPSS, Chicago, IL, USA) was used for the statistical analysis. The following parameters were put under investigation for their potential influence on the outcome of RFA procedure: sex, age, time from early symptoms, location, vertebral level, lesion size, distance between lesion and neural structures, pre-procedure VAS score.
Results
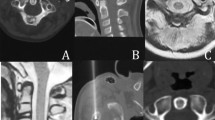
Sixty-one patients underwent RFA to treat spinal OO (46 male and 15 female, mean age 26.4 ± 12.7, range 8–68 years). Seven lesions (11.4%) were found in the cervical spine (Fig. 1), 12 (19.6%) in the thoracic spine (Fig. 2), 28 (45.9%) in the lumbar spine (Fig. 3), and 14 in the sacral spine (22.9%).
Thin-slice CT scan. a A small, ossified core OO is located at L2 (11 mm in diameter). b The final position of the radiofrequency electrode is shown, on the three spatial planes (1, 2, 3), with the active tip inside the nidus. The needle was deliberately positioned at the periphery of the lesion at a greater distance from the spinal canal
Mean time from symptoms onset to diagnosis was 5.7 ± 2.1 months (range 3–12 months). Mean follow-up was 41.5 ± 7.1 months.
The mean single largest lesion diameter was 11.4 mm (range 7–20 mm). RFA treatment parameters are shown in Table 2. In 14 out of 61 cases (23.6%), the OO involved posterior elements of the vertebra (7 out of 61, 11.8% pedicle, 5/61, 8.8% lamina, 2 out of 61, 3% spinous process). The mean distance between the lesion and the closest neural element was 7.4 mm (range 1–35 mm). At histological examination, a formal diagnosis of OO could be established in only 25 out of 61 cases (40.9%). In one patient, the post-procedure pathological examination resulted in a diagnosis of osteoblastoma—the lesion was 20 mm in diameter.
The procedures lasted an average time of 1 h (range 50–70 min). The patients usually reported that the typical OO pain disappeared 12–24 h after the procedure. Mild pain persisted as a consequence of the procedure for 2 to 7 days in 4 patients (4 out of 61, 6.5%). In 5 patients, a corset was indicated (5 out of 61, 8.1%) and in 1 patient a neck brace was necessary (1 out of 61, 1.6%), following the recommendations of the orthopedic surgeons, to reduce the load on the treated area when RFA was performed in or close to a facet joint.
The primary efficacy (pain relief, VAS = 0) of the procedure was proved for 57 out of 61 patients (93.4%; p 0.001). Mean VAS at baseline was 8, whereas after the procedure complete pain relief (VAS = 0) was reported in all patients at each clinical follow-up (3, 6, and 12 months), apart from in 4 patients. In these 4 patients, there was a short-term relief (few weeks) and a recurrence of symptoms occurred (6.6% spine level C3, T10, T12, and S3). C3 was on the articular surface of the left articular process, T10 was on the left pedicle, T12 was in the right part of the soma, S3 was in the soma. The recurrence presented after a mean follow-up of 11 months, range 4–17 months. Two lesions (3.2%) received a second treatment with the same modality, and 2 underwent surgery (3.2%; C3 and T10). Secondary efficacy of RFA was 100%.
The patient affected by osteoblastoma (C4 spine level, pedicle, diameter 20 mm, active tip 20 mm) was effectively treated, with no evidence of recurrence at 32 months’ follow-up.
Radiofrequency ablation was safely performed in all patients, with no reported intra-procedure complications. Two patients with thoracic localization had some discomfort during the procedure, but it was not necessary to discontinue treatment.
We did not find a significant statistical difference in the final results with regard to sex, age, time from early symptoms, location, vertebral level, lesion size, distance between the lesion and neural structures, and pre-procedure VAS score. One minor postprocedure complication (1 out of 61, 1.6%) occurred: lower limb neuropathy for 30 days after the ablation. An unrelated event, a disc herniation, 3 months after the procedure, in 1 patient (OO in the left pedicle), was treated medically, with no need for intervention.
We did not perform any radiological follow-up, unless there was clinical suspicion of a relapse (or persistence) of symptoms. We performed contrast-enhanced MRI in the 4 patients with recurrence. In the literature, MR perfusion has a high diagnostic performance for OO recurrence. The identification of an early and steep enhancement with a short time to peak and a short delay between the arterial and nidus peaks on MR perfusion in the postoperative setting is highly indicative of an OO recurrence.
Discussion
The efficacy of RFA for nonspinal OO was reported to be in the range of 76-100% by different authors, and the rate of complications was reported to be around 1% [19, 20]. A study comparing the results of surgery with those of percutaneous RFA showed that the recurrence rate was similar, but the hospitalization time was longer for surgery than for RFA [21]. Our results for RFA treatment of spinal OO showed that 93.4% had primary success and 96.3% had overall success (2 patients were ultimately subjected to surgery), comparable with those of previous reports [19, 20].
For years, lesions of the spine were considered untreatable with RFA because of the inherent risk posed by the proximity to the neural elements or the position of the OO in the back part of the vertebral body [15, 22]. With this study, we want to confirm that CT-guided RFA can be a safe technique for the treatment of OO of the spine, with results comparable with those seen in extremity lesions.
The work of Rybak et al. [9] addressed many of these issues, but they had a small series of patients and preferred to use a spinal neuro-protector to avoid medullary damage. Our experience demonstrates that neuro-protection does not seem to be necessary. To date, the recommendation is to perform thermal ablation of OO only if there is a 1-cm margin from vital (or neurological) structures, in view of the risk of unwanted thermal injury [5]. Dupuy et al. [23] performed in vivo and ex vivo thermal ablation experiments in animals and found that preserved cortical bone between the lesion and the osseous spine provided a thermal insulation effect and thus a margin of safety.
In our data collection, there was a mean distance of 7 mm (range 1–35 mm) between the lesion and the neural structures; moreover, in some cases there was no preserved bone surrounding part of the lesion.
Some authors believe that the size of the OO (patients with a lesion of 10 mm or larger) does predict the risk of recurrence [10]. In our series, we treated different sized OOs (range 7–20 mm), but we did not encounter any difference in terms of final results (our 4 treatment failure cases had a mean nidus size of 10 mm (range 8–12 mm).
For lesions that are very close to neural elements we prefer deep sedation (rather than general anesthesia) to be able to evaluate neurological status during the procedure. In our series, we did not have cases where treatment had to be terminated because of pain. Neurological examination was necessary to highlight the early onset of neurological complications.
We suspect that when the OO is close to the theca, epidural veins probably shield the dura from the effects of heat. The more difficult situations are those when the OO is near nerve roots or those tumors that push the dura closer to the cord. Even in these cases, where the OO was adjacent to an exiting nerve root, we performed the procedure under deep sedation (Figs. 4, 5). Only in 2 cases (thoracic localization), the patients had some discomfort during the procedure, but this never had to be abandoned.
Sagittal CT reconstruction. a A small OO (dotted arrow) of the back part of the soma of the thoracic vertebra (T10). b The final position of the radiofrequency electrode is shown with the active tip inside the nidus; the electrode reaches the OO, passing through the inferior vertebra and the intervertebral disc
Complications of RFA, such as skin burns, necrosis, fracture, and infection, have been reported in the literature; however, most authors have reported either no or only minor complications that did not require further treatment. In our series, no such complications were found.
In only one case was transient neuropathy observed, possibly because of the absence of an intact cortex between the lesion and the closest nerve. It is therefore our opinion that the presence of intact cortical bone represents an important factor to consider to avoid injuries during the procedure.
Histological confirmation of the diagnosis was attempted for all patients and achieved a final diagnosis in 40,9% of patients, a comparable result with other several authors [11]. The occurrence of a substantial number of nondiagnostic biopsy findings is not surprising given the small size of the biopsy sample. Rosenthal et al. [21] reported that most patients with nondiagnostic biopsy findings have an OO.
This study confirms that RFA is a safe and effective method for treating spinal OO and should be regarded as a first-line therapy. The major limitation of this study is the retrospective design, and the lack of comparison with other available techniques.
We conclude that CT-guided RFA of spinal OO is an effective treatment for spinal OO, and can be safely performed close to the spinal elements or exiting nerve root. Surgery should be reserved for lesions located in specific parts of the vertebral body and when sedation is not possible.
References
Jaffe H. Osteoid osteoma: a benign osteoblastic tumor composed of osteoid and atypical bone. Arch Surg. 1935;31(5):709–728
Gasbarrini A, Cappuccio M, Bandiera S, Amendola L, van Urk P, Boriani S. Osteoid osteoma of the mobile spine: surgical outcomes in 81 patients. Spine (Phila Pa 1976). 2011;36(24):2089–93. doi:10.1097/BRS.0b013e3181ffeb5e.
Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone (IARC WHO Classification of Tumours). 4th ed. Lyon: IARC; 2013. p. 427
Rodallec MH, Feydy A, Larousserie F, et al. Diagnostic imaging of solitary tumors of the spine: what to do and say. Radiographics. 2008;28(4):1019–41. doi:10.1148/rg.284075156.
Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ. Osteoid osteoma: percutaneous radio-frequency ablation. Radiology. 1995;197(2):451–4.
Albisinni U, Bazzocchi A, Bettelli G, et al. Treatment of osteoid osteoma of the elbow by radiofrequency thermal ablation. J Shoulder Elbow Surg. 2014;23(1):e1–7. doi:10.1016/j.jse.2013.08.011.
Osti OL, Sebben R. High-frequency radio-wave ablation of osteoid osteoma in the lumbar spine. Eur Spine J. 1998;7(5):422–5.
Rehnitz C, Sprengel SD, Lehner B, et al. CT-guided radiofrequency ablation of osteoid osteoma and osteoblastoma: clinical success and long-term follow up in 77 patients. Eur J Radiol. 2012;81(11):3426–34. doi:10.1016/j.ejrad.2012.04.037.
Rybak LD, Gangi A, Buy X, La Rocca VR, Wittig J. Thermal ablation of spinal osteoid osteomas close to neural elements: technical considerations. AJR Am J Roentgenol. 2010;195(4):W293–8. doi:10.2214/AJR.10.4192.
Vanderschueren GM, Taminiau AH, Obermann WR, van den Berg-Huysmans AA, Bloem JL. Osteoid osteoma: factors for increased risk of unsuccessful thermal coagulation. Radiology. 2004;233(3):757–62.
Hoffmann RT, Jakobs TF, Kubisch CH, Trumm CG, Weber C, Duerr HR, et al. Radiofrequency ablation in the treatment of osteoid osteoma—5-year experience. Eur J Radiol. 2009;73:374–9.
Cové JA, Taminiau AH, Obermann WR, Vanderschueren GM. Osteoid osteoma of the spine treated with percutaneous computed tomography-guided thermocoagulation. Spine (Phila Pa 1976). 2000;25(10):1283–6.
Hadjipavlou AG, Lander PH, Marchesi D, Katonis PG, Gaitanis IN. Minimally invasive surgery for ablation of osteoid osteoma of the spine. Spine (Phila Pa 1976). 2003;28(22):E472–7.
Laus M, Albisinni U, Alfonso C, Zappoli FA. Osteoid osteoma of the cervical spine: surgical treatment or percutaneous radiofrequency coagulation? Eur Spine J. 2007;16(12):2078–82.
Vanderschueren GM, Obermann WR, Dijkstra SP, Taminiau AH, Bloem JL, van Erkel AR. Radiofrequency ablation of spinal osteoid osteoma: clinical outcome. Spine (Phila Pa 1976). 2009;34(9):901–4. doi:10.1097/BRS.0b013e3181995d39.
Martel J, Bueno A, Nieto-Morales ML, Ortiz EJ. Osteoid osteoma of the spine: CT-guided monopolar radiofrequency ablation. Eur J Radiol. 2009;71(3):564–9. doi:10.1016/j.ejrad.2008.04.020.
Klass D, Marshall T, Toms A. CT-guided radiofrequency ablation of spinal osteoid osteomas with concomitant perineural and epidural irrigation for neuroprotection. Eur Radiol. 2009;19(9):2238–43. doi:10.1007/s00330-009-1404-8.
Hadjipavlou AG, Tzermiadianos MN, Kakavelakis KN, Lander P. Percutaneous core excision and radiofrequency thermo-coagulation for the ablation of osteoid osteoma of the spine. Eur Spine J. 2009;18(3):345–51. doi:10.1007/s00586-008-0791-x.
Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol. 2001;12:717–22.
Sung KS, Seo JG, Shim JS, Lee YS. Computed-tomography-guided percutaneous radiofrequency thermoablation for the treatment of osteoid osteoma: 2 to 5 years follow-up. Int Orthop. 2009;33:215–8.
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80:815–21.
Erlemann R. Imaging and differential diagnosis of primary bone tumors and tumor-like lesions of the spine. Eur J Radiol. 2006;58(1):48–67.
Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175:1263–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Albisinni, U., Facchini, G., Spinnato, P. et al. Spinal osteoid osteoma: efficacy and safety of radiofrequency ablation. Skeletal Radiol 46, 1087–1094 (2017). https://doi.org/10.1007/s00256-017-2662-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2662-1