Abstract
Purpose
Osteoid osteomas in the spine constitute a challenging group for both surgical and percutaneous approaches. Purpose of the present study is to report a case report of a spinal osteoid osteoma in a challenging spinal location and review literature for safety and efficacy of the technique.
Methods
We report a case of spinal osteoid osteoma extending in the epidural space and abutting the dura in a pediatric patient treated by percutaneous computed tomography-guided radiofrequency ablation. This is not a systematic review of the literature. A number of separate literature searches were performed. Non-English studies and case reports were excluded from the study. All references of the obtained articles were also evaluated for any additional information.
Results
Although all prophylactic measures were taken (hydrodissection, thermocouples and neurophysiologic monitoring) and the procedure was uneventful, patient within three hours, was unable to raise or bend the unilateral lower extremity below the knee. Pain reduction was significant from the first morning post-ablation and during the follow-up period of 18 months. MR scan was within normal limits. Dexamethasone was iv injected for 24 h and prescribed per os for 7 days. At follow-up 1 week later mobility of the lower extremity had returned to normal.
Conclusion
As far as spine ablation is concerned, all prophylactic measures should be taken; neurophysiologic monitoring seems to be more sensitive than temperature measurement. Intravenous and per os corticosteroids are extremely useful in case of nerve damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinal osteoid osteomas account for 7–20% of all cases and are more commonly located on the posterior elements of the lumbar spine [1]. Percutaneous thermal ablation techniques (Radiofrequency and laser ablation) are considered standard therapies for osteoid osteoma with surgery being reserved for exceptions, more commonly associated to proximity of the lesion to neural structures. Magnetic Resonance-guided High Intensity Focused Ultrasound (MR-guided HIFU) is a totally non-invasive ablative technique that can generate heat at the targeted tissue; MR-guidance provides high-resolution anatomical imaging allowing accurate targeting and treatment planning, as well as real-time 3D temperature monitoring [2]. Osteoid osteomas in the spine constitute a challenging group for both surgical and percutaneous approaches, both of which seem to be associated to higher peri- and postoperative morbidity, longer hospitalization and rehabilitation periods, lower efficacy and higher complication rates [1,2,3,4,5].
Here we present a case of spinal osteoid osteoma extending in the epidural space and abutting the dura in a pediatric patient treated by percutaneous computed tomography-guided radiofrequency ablation. This case presentation shows the necessity for prophylactic measures during spine ablation and the effectiveness of intravenous dexamethasone administration as first-line therapy in the early stage of post-ablation neural deficit. Additionally a literature review was performed. This is not a systematic review of the literature. A number of separate literature searches were performed. Non-English studies and case reports were excluded from the study. All references of the obtained articles were also evaluated for any additional information.
Case report
We report a case of a pediatric patient with a spinal osteoid osteoma located in the right lamina of S1 vertebral body, extending inside the epidural space. The lesion was treated with percutaneous radiofrequency ablation under computed tomography guidance. Institutional review board approval was obtained for preparation of this report.
A 12-year-old girl presented with low back pain and right S1 neuralgia due to an exophytic cortical osteoid osteoma, located in the right lamina of S1 vertebral body and extending in the epidural space. In agreement with the orthopedics and neurosurgeons, percutaneous ablation was proposed. The ablation session was performed with the patient under general anesthesia and neurophysiologic monitoring of the sensitive-evoked and motor-evoked potentials due to lesion’s close proximity to the dural sac. Computed tomography guidance with sequential scanning (120 kV peak, 240 mAs wavelength and 0.9 mm slice thickness) was used for planning, targeting and intra-procedural modification during the ablation session. Post the initial CT scan, skin entry point was selected (Fig. 1a). Under extended local sterility a 22 Gauge spinal needle was inserted intradurally and myelography was performed in order to be able to distinguish the dural sac throughout the ablation session. The needle was withdrawn, repositioned epidurally and hydrodissection right next to the osteoid osteoma nidus was performed by means of Dextrose 5% mixed with contrast medium (60 ml of Dextrose solution mixed with 3 ml iodinated contrast medium). A thermosensor was placed for temperature monitoring right next to the nerve root (Fig. 1b). Following, a bone trocar (OBM, Arrow OnControl, Teleflex, Shavano Park, TX, USA) was inserted in the lesion of interest and its approach was evaluated with sequential CT scans. Once in the correct location, coaxially the bone biopsy needle was inserted for sampling (Fig. 1c). A 15-cm long, 17-gauge Radiofrequency electrode with 1 cm single active tip (RF AMICA probe, Hospital Service S.P.A. Rome/Italy) was then introduced into the osteoid osteomas nidus through the coaxial system; the trocar was withdrawn till being outside the expected ablation zone and monopolar radiofrequency ablation was performed at 85–90 °C for 6 min (Fig. 1d). The position of the thermocouple and RF electrode was monitored by intermittent imaging during the procedure. Continuous thermal monitoring, during the ablation, via the thermocouple, showed no temperature increase above 39 °C. Intermittent neurophysiologic control of the sensitive-evoked and motor-evoked potentials was performed during the ablation with no pathologic registration. Although there was a small interference to the registration from the RF generator, no passive wave deformity was observed in comparison with the contralateral side. Active monitoring with stimulation was performed every two minutes, during which the RF energy deposition was paused. Systematic examination of both sides (afflicted and normal) showed no pathologic registration. Computed tomography assessed any potential immediate complications at the end of the ablation treatment. The procedure and recovery from general anesthesia was uneventful. On the ward, within three hours, patient was unable to raise or bend the unilateral lower extremity below the knee. Immediately the patient was clinically evaluated by a neurosurgeon who diagnosed L4 paresis and ordered a new MR examination (Fig. 2). The scan was without pathologic findings. Patient remained in the hospital overnight and dexamethasone (4 mg of dexamethasone phosphate/ml) was injected intravenously. Approximately four hours later extremity’s mobility gradually started to return. Patient was discharged the next day pain free with minor mobility loss of the lower extremity which had returned to normal on the follow-up visit 1 week later. During this period patient was advised to avoid excessive stressful weight bearing and prolonged strenuous activity while oral dexamethasone phosphate per os was prescribed for 7 days (0.3 mg/kg/day in three or four divided doses). Pain reduction was significant from the first morning post-ablation; patient reported no pain from the 1st week clinical visit during the follow-up period of 18 months. Osteoid osteoma diagnosis was verified by the histologic findings, the report of which mentioned presence of nidus in the biopsy sample.
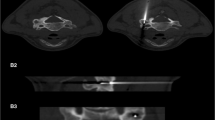
a Computed tomography axial scan illustrating the nidus of the osteoid osteoma (white arrow) in the posterior medial wall of S1 vertebral body extending inside the epidural space right next to the dural sac. b Computed tomography axial scan illustrating the 22 Gauge thermosensor (white arrow) right next to the sac and nidus. c Computed tomography axial scan illustrating the bone biopsy needle coaxially inserted through the trocar in the nidus. d Computed tomography axial scan illustrating the radiofrequency electrode coaxially inserted through the trocar in the nidus; trocar is withdrawn outside the expected ablation zone (minimum 1 cm away)
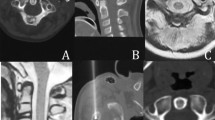
a Immediate post-ablation Magnetic Resonance Imaging—sagittal T1 weighted sequence with fat signal suppression prior to iv gadolinium injection depicting the osteoid osteoma nidus (white arrow). b Immediate post-ablation Magnetic Resonance Imaging—sagittal T1 weighted sequence with fat signal suppression post iv gadolinium injection depicting the osteoid osteoma nidus (white arrow). There is absence of enhancement inside the nidus accompanied by peripheral post-ablative inflammatory enhancement. c Immediate post-ablation Magnetic Resonance Imaging—axial STIR sequence prior iv gadolinium injection depicting minor edema (white arrow) in proximity to the osteoid osteoma nidus and at the surrounding soft tissues (white dashed arrow).d Immediate post-ablation Magnetic Resonance Imaging—sagittal STIR sequence prior to iv gadolinium injection depicting the osteoid osteoma nidus as well as lack of any pathologic signal intensity in the visible part of spinal cord and cauda equina
Authors were unable to identify the cause of the paresis, which seemed to respond very well to the corticosteroid treatment, but it could have equally been just spontaneous regression. One of the theories one can evoke is that the incidence is unrelated to the ablation technique, but rather related to the ancillary manoeuvres such as the epidural injection of Dextrose 5%, as a protective medium, which could have created an osmotic/volume effect on the L4 root, which was above the treated level.
Discussion
Since the first ever case report of osteoid osteoma treated with percutaneous radiofrequency ablation, things have fallen into place and nowadays the technique is considered gold-standard therapy [6]. Apart from radiofrequency alternative ablative techniques include laser interstitial therapy (LITT), microwave (MWA), cryoablation and high intensity focused ultrasound under MR guidance (MR-guided HIFU) [7,8,9,10,11,12]. Although MR-guided HIFU cannot systematically treat non-invasively all osteoid osteomas, the number of eligible cases may increase when HIFU is combined to minimally-invasive thermo-protective techniques [2]. At the moment cumulative literature data support the evidence for long-term efficacy and safety of percutaneous radiofrequency ablation [7, 13,14,15,16,17,18,19,20]. When working in pediatric cases, instrumentation suitable for the patient’s smaller size, general anesthesia with intubation and exposure to ionizing radiation are areas of additional concern [21].
Spine due to the proximity with sensitive neural structures is considered a challenging location for percutaneous treatment of osteoid osteoma (Table 1). Beyer et al. [22] in a European multi-centered study enrolled 77 spinal osteoid osteomas treated with CT-guided RFA reporting significant and persistent pain reduction effect and concluding that the technique a safe and efficient method to treat spinal OO and should be regarded as first-line therapy after interdisciplinary case discussion. Apart from the standard RF electrodes, bipolar systems specifically designed for the spine have been described for the treatment of spinal osteoid osteoma [23].
Application of neuroprotective air injection during spine osteoid osteoma RFA has been described adding to increased safety [24, 25]. Real-time monitoring of the temperature close to a sensitive neural structure (must be kept below 42 °C), neurophysiologic monitoring and dissection for displacement of the sac constitute protective techniques for ablation in challenging locations; neurophysiologic monitoring seems to be more sensitive that temperature monitoring [5, 26, 27]. In case of nerve injury advantages of local and/or systematic corticosteroid administration include membrane stabilizing and analgesic effect that changes pain behaviors, silence of neural firing, reduction of inflammatory mediator synthesis as well as inhibition of neurogenic extravasation and edema formation [28]. Since the reported case as a standard protocol in the department, authors perform an epidural injection of steroids after each spine ablation session.
When compared to open surgery, percutaneous CT-guided ablation is less invasive, easily repeatable, governed by lower morbidity and lower cost with high rates of technical and clinical success rates; on the other hand, open surgical resection is more complicated and associated to increased tissue injury, blood loss and hospitalization [29, 30]. Overall post-surgical complications range from 5.5 to 30% including among others pneumothorax, hematoma, infection, hook dislodgement, incidental durotomy, wound dehiscence and post-operative neuropathic pain [3, 31,32,33]. Yu et al. [29] in a retrospective cohort study comparing open surgical resection and radiofrequency ablation for spinal osteoid osteomas evaluated 28 consecutive patients; authors concluded that the presence of cerebrospinal fluid (at least 1 mm) between the spinal OO lesion and spinal cord/nerve root adds to the efficacy of radiofrequency ablation while in cases with spinal cord/nerve root compression open surgical resection should be the treatment of choice. Similarly in a retrospective study by Wang et al. [34] authors consider cerebrospinal fluid around the lesion is an appropriate indication for percutaneous RFA. Pipola et al. [35] performed a retrospective comparison analysis of data prospectively collected from 2 cohorts of consecutive patients diagnosed with OO of the spine reporting no statistically significant difference in local recurrence rate stratified for level and site of lesion. However, authors reported a statistically significant difference in the disease-free survival at longest follow-up favoring surgery concluding that spinal osteoid osteoma treatment should be tailored according to the relationship of lesions with neural structures and to advantages and disadvantages of each technique [35]. In conclusion, percutaneous radiofrequency ablation in the spine combined with the necessary protective techniques (hydro- or gas dissection, temperature monitoring and most importantly neurophysiologic monitoring) can be considered a safe and efficacious treatment for spinal osteoid osteoma. Cortisone therapy both locally and systematically administered can provide effective management as an add-on in the treatment of nerve damage.
References
Tsoumakidou G, Thénint MA, Garnon J, Buy X, Steib JP, Gangi A (2016) Percutaneous image-guided laser photocoagulation of spinal osteoid osteoma: a single-institution series. Radiology 278:936–943
Bing F, Vappou J, de Mathelin M, Gangi A (2018) Targetability of osteoid osteomas and bone metastases by MR-guided high intensity focused ultrasound (MRgHIFU). Int J Hyperthermia 35(1):471–479
Gasbarrini A, Cappuccio M, Bandiera S, Amendola L, van Urk P, Boriani S (2011) Osteoid osteoma of the mobile spine: surgical outcomes in 81 patients. Spine 36:2089–2093
Vanderschueren GM, Obermann WR, Dijkstra SP, Taminiau AH, Bloem JL, van Erkel AR (2009) Radiofrequency ablation of spinal osteoid osteoma: clinical outcome. Spine 34:901–904
Kurup AN, Schmit GD, Morris JM, Atwell TD, Schmitz JJ, Weisbrod AJ et al (2017) Avoiding complications in bone and soft tissue ablation. Cardiovasc Intervent Radiol 40:166–176
Rosenthal DI, Alexander A, Rosenberg AE, Springfield D (1992) Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology 183(1):29–33
Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW et al (2009) Thermal ablation of osteoid osteoma: overview and step-by-step guide. Radiographics 29(7):2127–2141
Gangi A, Alizadeh H, Wong L, Buy X, Dietemann JL, Roy C (2007) Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology 242(1):293–301
Mahnken AH, Tacke JA, Wildberger JE, Günther RW (2006) Radiofrequency ablation of osteoid osteoma: initial results with a bipolar ablation device. J Vasc Interv Radiol 17(9):1465–1470
Dasenbrock HH, Gandhi D, Kathuria S (2012) Percutaneous plasma mediated radiofrequency ablation of spinal osteoid osteomas. J Neurointerv Surg 4(3):226–228
Kostrzewa M, Diezler P, Michaely H, Rathmann N, Attenberger UI, Schoenberg SO, Diehl SJ (2013) Microwave ablation of osteoid osteomas using dynamic mr imaging for early treatment assessment: preliminary experience. J Vasc Interv Radiol 25(1):106–111
Basile A, Failla G, Reforgiato A, Scavone G, Mundo E, Messina M, Caltabiano G, Arena F, Ricceri V, Scavone A, Masala S (2014) The use of microwaves ablation in the treatment of epiphyseal osteoid osteomas. Cardiovasc Intervent Radiol 37(3):737–742
Filippiadis DK, Tutton S, Kelekis A (2014) Percutaneous bone lesion ablation. Radiol Med 119(7):462–469
Rosenthal D, Callstrom MR (2012) Critical review and state of the art in interventional oncology: benign and metastatic disease involving bone. Radiology 262(3):765–780
Filippiadis DK, Tutton S, Mazioti A, Kelekis A (2014) Percutaneous image-guided ablation of bone and soft tissue tumours: a review of available techniques and protective measures. Insights Imag 5(3):339–346
Hage AN, Chick JFB, Gemmete JJ, Grove JJ, Srinivasa RN (2018) Percutaneous radiofrequency ablation for the treatment of osteoid osteoma in children and adults: a comparative analysis in 92 patients. Cardiovasc Intervent Radiol 41(9):1384–1390
Filippiadis DK, Velonakis G, Kostantos C, Kouloulias V, Brountzos E, Kelekis N et al (2017) Computed tomography-guided radiofrequency ablation of intra-articular osteoid osteoma: a single centre’s experience. Int J Hyperthermia 33(6):670–674
Albisinni U, Facchini G, Spinnato P, Gasbarrini A, Bazzocchi A (2017) Spinal osteoid osteoma: efficacy and safety of radiofrequency ablation. Skeletal Radiol 46(8):1087–1094
Vanderschueren GM, Obermann WR, Dijkstra SP, Taminiau AH, Bloem JL, van Erkel AR (2009) Radiofrequency ablation of spinal osteoid osteoma: clinical outcome. Spine (Phila Pa 1976) 34(9):901–4
Faddoul J, Faddoul Y, Kobaiter-Maarrawi S, Moussa R, Rizk T, Nohra G, Okais N, Samaha E, Maarrawi J (2017) Radiofrequency ablation of spinal osteoid osteoma: a prospective study. J Neurosurg Spine 26(3):313–318
Burrill J, Manraj S, Heran KS (2012) Nonvascular pediatric interventional radiology. Can Assoc Radiol J 63(3):S49–S58
Beyer T, van Rijswijk CSP, Villagrán JM, Rehnitz C, Muto M, von Falck C, Gielen J, Thierfelder KM, Weber MA (2019) European multicentre study on technical success and long-term clinical outcome of radiofrequency ablation for the treatment of spinal osteoid osteomas and osteoblastomas. Neuroradiology 61(8):943
Tomasian A, Jennings JW (2018) Spinal osteoid osteoma: percutaneous radiofrequency ablation using a navigational bipolar electrode system. AJR Am J Roentgenol 211(4):856–860
Vidoni A, Grainger M, James S (2018) Experience of neuroprotective air injection during radiofrequency ablation (RFA) of spinal osteoid osteoma. Eur Radiol 28(10):4146–4150
Esteban Cuesta H, Martel Villagran J, Bueno Horcajadas A, Kassarjian A, Rodriguez CG (2018) Percutaneous radiofrequency ablation in osteoid osteoma: tips and tricks in special scenarios. Eur J Radiol 102:169–175
Yoon JT, Nesbitt J, Raynor BL, Roth M, Zertan CC, Jennings JW (2020) Utility of motor and somatosensory evoked potentials for neural thermoprotection in ablation of musculoskeletal tumors. J Vasc Interv Radiol 31(6):903–911
Nöel MA, Segura MJ, Sierre S, Francheri Wilson IA, Tello CA, Galaretto E, Remondino RG, Talarico ME, Bersusky ES, Piantoni L (2017) Neurophysiological monitoring in radiofrequency ablation of spinal osteoid osteoma with a progressive time and temperature protocol in children. Spine Deform 5(5):351–359
Eker HE, Cok OY, Aribogan A, Arslan G (2012) Management of neuropathic pain with methylprednisolone at the site of nerve injury. Pain Med 13(3):443–451
Yu X, Wang B, Yang S, Han S, Jiang L, Liu X, Wei F, Wu F, Dang L, Liu Z (2019) Percutaneous radiofrequency ablation versus open surgical resection for spinal osteoid osteoma. Spine J 19(3):509–515
Wu H, Lu C, Chen M (2017) Evaluation of minimally invasive laser ablation in children with osteoid osteoma. Oncol Lett 13(1):155–158
Quraishi NA, Boriani S, Sabou S, Varga PP, Luzzati A, Gokaslan ZL et al (2017) A multicenter cohort study of spinal osteoid osteomas: results of surgical treatment and analysis of local recurrence. Spine J 17(3):401–408
Kadhim M, Binitie O, O’Toole P, Grigoriou E, De Mattos CB, Dormans JP (2017) Surgical resection of osteoid osteoma and osteoblastoma of the spine. J Pediatr Orthop B 26(4):362–369
Etemadifar MR, Hadi A (2015) Clinical findings and results of surgical resection in 19 cases of spinal osteoid osteoma. Asian Spine J 9(3):386–393
Wang B, Han SB, Jiang L, Yuan HS, Liu C, Zhu B, Liu ZJ, Liu XG (2017) Percutaneous radiofrequency ablation for spinal osteoid osteoma and osteoblastoma. Eur Spine J 26(7):1884–1892
Pipola V, Tedesco G, Spinnato P, Facchini G, Gala RB, Bandiera S, Bròdano GB, Terzi S, Ghermandi R, Evangelisti G, Ricci A, Griffoni C, Pezzi A, Gasbarrini A (2021) Surgery versus radiofrequency ablation in the management of spinal osteoid osteomas: a spine oncology referral center comparison analysis of 138 cases. World Neurosurg 145:e298–e304
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent for publication was obtained for every individual person’s data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Filippiadis, D., Mavrogenis, A., Spiliopoulos, S. et al. Percutaneous computed tomography-guided radiofrequency ablation of a spinal osteoid osteoma abutting the dura: a case report and review of the literature. Eur J Orthop Surg Traumatol 31, 1625–1630 (2021). https://doi.org/10.1007/s00590-021-02922-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-021-02922-4