Abstract
The biodegradable biopolymer chitosan had its linear structure chemically modified in a two-step reaction, first by exploring the amino reactivity with glycidylmethacrylate, whose intermediate product, containing an aldehyde group, was then reacted with 1,2-ethanedithiol. Chitosan and the synthesized biopolymers were characterized by elemental analyses, infrared spectroscopy, nuclear magnetic resonance of the carbon nucleus in the solid state, X-ray diffractometry, thermogravimetry and scanning electron microscopy, to give a degree of immobilization of 3.00 mmol g−1. The available basic nitrogen, sulfur and oxygen Lewis centers attached to the enlarged pendant chains enriched the ability of the biopolymer for copper, lead and cadmium sorption from aqueous solution, to give maximum capacities of 2.05 ± 0.01; 2.53 ± 0.02 and 1.88 ± 0.01 mmol g−1, when compared to chitosan with 1.54 ± 0.33; 1.22 ± 0.04 and 1.12 ± 0.08 mmol g−1, respectively, using the Langmuir sorption isotherms. Based on the present results, the highest amount of these cation sorptions, especially with lead that is associated with sulfur–cation soft interactions, is dependent directly on not only the presence of long pendant chain attached, but also the availability of favorable Lewis base centers. The experimental data adjusted to the Langmuir, the Freundlich and the Temkin sorption isotherms using linear and non-linear regression methods and are in agreement with the best fit for Langmuir model type I.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, social pressure on governmental authorities with respect to water resources has been increased mainly related to toxic pollutants in reservoirs, rivers and ground water that may contaminate potable waters [1, 2]. Among these pollutant agents an intense concern is on the presence of toxic transition and heavy metals that can be introduced into aquatic systems through effluent discharge from various daily industrial operations [3–6].
Heavy metal pollution may be traceable to industrial activities such as smelting of ores or refining, which operations introduce metals into air, soil and water. Widespread concern over cumulative toxicity and environmental impact has led to extensive research into developing alternative technologies for the removal these damaging metals from wastewaters, as they are unwanted, mainly due to their non-biodegradable nature and to their environmental, health and economic impacts and on the ecosystems [7, 8].
Taking into account the toxicity of these metals, some procedures have been used for their removal from wastewater such as ion exchange, precipitation, membrane processes and sorption methods. This last methodology is very useful not only from a practical standpoint as it obtains high efficiency and is a low-cost process for pollutant removal from wastewater. The choice of low-cost sorbents is a task that requires special care to obtain with satisfactory success that is advantageous in comparison to other possible methods [9–11].
To meet the demand for effective potential sorbents, polysaccharides are presented as the most widespread compounds to be used as biomaterials for pollutant removal, especially chitosan [1, 12, 13]. The most reactive amino group, located at carbon 2 in the original structure of chitosan, has the ability to immobilize organic chains to attach basic centers. After blocking the active amino center of the precursor structure, the hydroxyl group on carbon 6 is free for the desired reaction and in the next stage, neutralizing the proton leaves the free amino group for a new reaction [14]. Another possibility to reach the same condition is, in principle, to block the amino group with a special reagent and remove it after having reacted carbon 6, to again yield chemically modified chitosan [15], in which the attached basic center on pendant chains are enriched by the free amino group to chelate cations from aqueous solutions. Thus, chitosan has the ability to chelate metal ions from aqueous solutions, but when chemically modified with the incorporation of pendant chains containing basic centers, the ease in removal of metals is expected to be increased [16–19].
As set forth, the research field related to the chemical modification of chitosan is rich in syntheses of new biopolymers that depend on the purpose of applications. In the present case, the main objective is to explore the synthesis of new biopolymers containing basic Lewis centers. To meet this goal chitosan reacts in two steps, first with glycidylmethacrylate, followed by 1,2-ethanedithiol to enlarge the pendant chains. The characterized product was applied for copper, cadmium and lead sorptions from aqueous solution. The capacity of the final biopolymer was evaluated through the Langmuir sorption isotherms with batch methodology. The equilibrium data were fit to the Langmuir, the Freundlich and the Temkin isotherms using linear and non-linear regression method to obtain data related to these isotherms.
Experimental
Materials
Powdered chitosan, with 78 % degree of deacetylation, determined from infrared spectroscopy, was extracted from crab shells and supplied by Primex Ingredients (Norway), 1,2-ethanedithiol (Aldrich), glycidylmethacrylate (Aldrich), ethanol (Synth), lead, cadmium and copper nitrates (Vetec) were all of analytical grade.
Characterization
The carbon, nitrogen and sulfur contents of the original chitosan and its derivative were determined through Perkin Elmer, model 2400, elemental analyzer. Infrared spectra of the samples as KBr pellets were obtained by accumulating 32 scans on a Bomem MB-series spectrophotometer, in the 4,000–400 cm−1 range, with 4 cm−1 of resolution. Nuclear magnetic resonance spectra of carbon nucleus in solid state were obtained on a Bruker Avance II plus 400 MHz spectrometer at room temperature, using the cross-polymerization and magic angle spinning (CP/MAS) technique, with pulse repetitions of 5 s and contact times of 1 ms, at 100.62 MHz and with magic angle spinning of 10 kHz. X-ray diffraction patterns were obtained on a Shimadzu XD-3A diffractometer (35 kV, 25 mA), in the 2θ = 1.5–50° range, with nickel-filtered CuKα radiation, having a wavelength of 0.154 nm. Thermogravimetric curves were obtained using a Shimadzu TGA 50 apparatus, under an argon atmosphere at a flow rate of 1.67 cm3 s−1, with a heating rate of 0.167 K s−1. Scanning electron microscopy (SEM) data were obtained from detection of the secondary electron images on a JEOL JSM 6360LV scanning electron microscope, operating at 20 kV, with samples supported on a copper ribbon. After preparation, the samples were subjected to gold plating and then were analyzed. The amount of cation sorbed was determined using a Perkin Elmer 3000 DV through inductively coupled plasma optical emission spectrometry (ICP-OES) apparatus.
Synthesis
Chitosan modification is based on a two-step reaction, first with glycidylmethacrylate [19], followed by reaction with 1,2-ethanedithiol. In the first step, 4.0 g of chitosan was suspended in 200 cm3 of water in a 250 cm3 three-necked flask and stirred for 15 min at 353 K. To this suspension 2.65 cm3 of glycidylmethacrylate was slowly added and the reaction was kept under mechanical stirring for 2 h. The product (CHg) obtained was filtered, washed with water, ethanol and dried under vacuum at 318 K for 6 h. Then, 4.0 g of CHg was added to 150 cm3 of ethanol in a 250 cm3 three-necked flask while stirring at 333 K, followed by slowly addition of 1.67 cm3 of 1,2-ethanedithiol in the presence of 1.0 cm3 of triethylamine as a catalyst and this mixture was maintained under reflux with mechanical stirring for 72 h. The new material (CHgt) was filtered, washed with ethanol and deionized water and dried under vacuum at 318 K for 6 h.
Sorption
Sorption experiments were carried out by adding nearly 20 mg of CHgt into a series of polyethylene flasks containing 25.0 cm3 of cation solutions, having concentrations ranging from 7.0 × 10−4 to 2.0 × 10−2 mol dm−3. The experiments were performed in duplicate in a batch-wise process in an orbital bath at 298 ± 1 K. The time needed to obtain the maximum saturation was determined and kinetics experiments were also performed using similar cation solutions. Sorption equilibrium was obtained at 4 h, however, the samples was kept for 6 h to ensure the best equilibrium conditions. The supernatant solutions were separated from the solid through decantation and aliquots of supernatant were taken to determine the remaining amounts of cation by ICP-OES. The amount of the cation sorbed onto chemically modified biopolymer is calculated by Eq. 1,
where N f is the number of moles sorbed (mmol g−1), n i and n s are the number of moles in the initial solution and the supernatant after equilibrium and m is the mass of the sorbent [16, 17, 19, 20].
Isotherm models
Different sorption isotherms were used to describe sorbates interaction with sorbents and to optimize this process. Three most commonly used, models Langmuir, Freundlich and Temkin, are applied to determine the sorption capacity of chemically modified chitosan. The relationship between the amount of cations sorbed and the equilibrium concentration of cations in the solution is described by Langmuir sorption isotherm [17, 19].
Langmuir model
The modified form of Langmuir models LangX (X = 1 to 4) is described as expressed in Eq. 2:
where N f (mmol g−1) and C s (mmol dm−3) are the amounts of cations sorbed by chemically modified chitosan and equilibrium concentration of cations in solution, respectively, Ns (mmol g−1) is the maximum sorption capacity and b (dm3 mmol−1) is the sorption equilibrium constant, which quantitatively reflects the affinity between the sorbent and sorbate [21]. The Langmuir isotherm can be transformed to four linear forms [22].
Freundlich model
The non-linear and linear Freundlich isotherms are given in Eqs. 3 and 4.
where N f (mmol g−1) and C s (mmol dm−3), K F (mmol dm−3) and n are Freundlich constant and intensity factors, respectively. K F and n values are calculated from the slope and intercept of plots of log N f versus log C s [23].
The Freundlich sorption isotherm enlightens about the surface heterogeneity, the exponential distribution of active sites and their energies. This isotherm does not predict any saturation of the sorbent by the sorbate, thus infinite surface coverage is predicted mathematically, indicating a multilayer sorption on the surface [24].
Temkin model
The Temkin isotherms in non-linear and linear forms are shown in Eqs. 5 and 6:
A ln C s versus N f plot enables determination of K T, n T and b values. The constant b is related to the heat of sorption, which can be calculated using the following Eq. 7:
The Temkin isotherm assumes that the heat of sorption of the molecules in a layer decreases linearly with coverage due to sorbent/sorbate interactions. The sorption is characterized by a uniform distribution of the binding energies [25].
Error function was used to evaluate the fit of the isothermal equation to the experimental equilibrium. Three parameters, correlation coefficient (R), chi square (χ 2) and the standard error (SE) were used to know how an isotherm fit to experimental data. The mathematical equation that represents the standard error (SE) and chi square (χ 2) are shown in Eqs. 8 and 9:
where N f exp is the number of moles sorbed, N f calc is the number of moles calculated from each model, m is the number of points present in each isotherm and P is the number of parameters in each equation [25].
Results and discussion
Elemental analysis
The sequence of reactions of the precursor biopolymer chitosan (CH) based on a two-step reaction, starting from glycidylmethacrylate to form the intermediate (CHg) that subsequently reacted with 1,2-ethanedithiol yielded the chemically modified biopolymer (CHgt), as shown in Fig. 1, which biopolymers had the elemental analyses determined. The amount (L 0) of each element [19] and C/N molar ratios were calculated based on data obtained from elemental analyses data, using Eq. 10, as shown in Table 1.
The amount of nitrogen content in the intermediate for CHg, 4.20 mmol g−1, decreased when compared to the precursor chitosan with value of 5.27 mmol g−1, reflecting the chemical incorporation of the organic moiety glycidylmethacrylate in the structure. In the next step, the intermediate product CHg was further chemically modified with 1,2-ethanedithiol, whose molecule contains sulfur and carbon atoms, causing a proportional decrease of the nitrogen content of 2.64 mmol g−1 in the final chemically modified chitosan (CHgt). For this biopolymer derivative the calculated C/N molar ratio gave high value, as compared 14.99 with the experimental 12.43 value. However, the calculated percentage of sulfur atom gave 16.12 % that corresponds to 5.03 mmol g−1. Thus, S/N molar ratio is independent on any unavailable carbon content in the final product, which could be present without reacting in the course of the proposed reaction. The obtained 1.90 molar ratio for such value clearly corroborated with the success of the sequence of immobilizations. Again, based on sulfur content, the degree of immobilization of 3.00 mmol g−1 was calculated for the later pendant chain incorporated.
Infrared spectroscopy
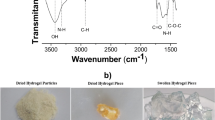
The spectrum of chemically modified intermediate biopolymer CHg was compared with precursor chitosan, as shown in Fig. 2. Typical C–H stretching vibrations bands at 2,916 and 2,877 cm−1 were present in pristine chitosan. A broad band at 3,405 cm−1 attributed to OH stretching vibrations, which can also overlap with the possible amino stretching frequency in the same region. Original chitosan also showed important characteristic bands at 1,655 cm−1 associated to amide I and at 1,592 cm−1 due to N–H bond. The band at 1,426 cm−1 related to primary OH alcohol bond. Sharp bands at 1,379 and 1,419 cm−1 related to the CH3 symmetrical deformation mode. Broad band at 1,076 cm−1 shows the presence of C–O stretching vibration [19, 20].
In the final chemically modified chitosan CHgt a band in the 2,550–2,600 cm−1 range should be present that corresponds to the S–H bond from the incorporated organic moiety as shown in Fig. 1. However, the C–S and S–H bonds are highly polarized, and thus give stronger spectral activity in the Raman spectrum than that of infrared region. The S–H bond probably corresponds to one absorption band in the infrared which can be regarded as useful tool for general characterization of sulfur-containing compounds [26], however, the new band at 674 cm−1 corresponds to C–S stretching [27]. Furthermore, the disappearance of the band at 1,635 cm−1 is related to double bond of glycidylmetacrylate at 1,635 cm−1, which confirms the chemical modification, as shown in Fig. 2.
Nuclear magnetic resonance
For chitosan a sequence of five distinct peaks are assigned to C1, C2, C4, C6 and C3/C5 at 105, 58, 84, 62 and 75 ppm, respectively [16], as given by the numbered carbon atoms in the main and pendant chain structures in Fig. 1. Two other peaks at 25 and 175 ppm are attributed to methyl and carbonyl carbons attached to monomeric form remaining from chitin, due to its incomplete deacetylation, as shown in Fig. 3. As expected, the intermediate product CHg presented peaks at 137 and 128 ppm that correspond to vinyl carbons of glycidylmethacrylate. The peaks at 19 and 169 ppm are attributed to methyl and carbonyl carbon C10 from ester functionality of glycidylmethacrylate. The peaks at 54 and 62 ppm for C2 and C6 of chitosan are well separated due to the increased number of carbon–nitrogen bonds [28], as observed for the intermediate product, which separation is less pronounced for the final biopolymer. For CHgt biopolymer addition peaks that correspond to carbon–sulfur bond appeared at 26 and 35 ppm for C13 and C14, respectively. The peak at 22 ppm related to methylene was assigned to the carbon bonded to sulfur hydrogen C15.
Thermogravimetry
Thermal degradation profiles of the original and chemically modified chitosans present slight difference, as evidenced by thermogravimetric curves in Fig. 4. The degradation profile shows the decomposition process of two thermal events as clearly illustrated from derivative curves with two distinct peaks, being the first event more pronounced by chitosan [14]. This biopolymer presented the mass loss comprising the temperatures ranging from 326 to 350 K that is attributed to the release of water linked by hydrogen bonds or physically sorbed on the chitosan surface, to give the maximum peak at 333 K, corresponding to 9 %, as shown in Fig. 4a. From 551 to 601 K range a maximum peak at 570 K, with a mass loss of 57 % is related to the biopolymer thermal degradation. The mass loss content has not chemically identified, however, pyrolysis of polysaccharides usually starts with a random division of the glycosidic bonds, followed by a decomposition to form acetic and butyric acids, and a series of lower fatty acids, predominantly at position C2, C3 and C6 of the biopolymer chitosan [29].
Chemically modified chitosan presents high degree of immobilization of sulfur based on elemental analysis, presented lower thermal stability than pristine chitosan, as shown in Fig. 4b. The first mass loss of 3 % is also related to water released that occurs at 326 K and the second stage with maximum mass loss of 71 % at 546 K, also presented lower temperature in comparison to pure chitosan. This last mass loss due to the biopolymer decomposition corroborated with the presence of organic moiety bonded to the linear biopolymeric structure. This chemically process gave a less thermally stable biopolymer than the precursor, which pendant chains can be easily decomposed on heating, as expected, to cause decrease in its stability [19]. On the other hand, the insertion of anchored molecule into the chitosan structure leads to the disruption of intermolecular hydrogen bond, to also contribute the decreasing in its thermal stability [14]. The present results correlate with the elemental analysis, infrared and nuclear magnetic resonance spectroscopy, which confirm chemical modification of the precursor chitosan.
Scanning electron microscopy
The morphologies of the chitosan were visualized through an electronic microscope as presented in Fig. 5a, b. The original chitosan morphologically present, as shown in Fig. 5a, an almost smooth surface consisting on the well-aligned superposition of ribbons, attached by either hydrogen bonds or cross-linked covalent interactions, as previously observed [30]. After chemical modification, the new material chitosan CHgt, as shown in Fig. 5b, presented a very distinguished morphology, which consists on part similar to the unmodified chitosan and other part with separated plate-like particles with size between 0.4 and 2.0 μm at larger dimensions and thickness ranging from tens up to few hundreds of nanometers. Most of these particles agglomerate in groups of bunch, resembling the morphology commonly observed for some clays and other inorganic layered materials [31]. These changes result from the chemical modification at smaller ribbon pieces, which became separated from the larger particles.
Sorption study
Precursor and chemically modified chitosan CHgt were applied to study the sorption process of divalent cations from deionized aqueous solutions. The quantity of cations sorbed N f, the sorption equilibrium constant Ns, the interaction energy b and the correlation coefficient (R) obtained from the linearized form of the Langmuir model are summarized in Table 2 for chemically modified chitosan. It is observed that the immobilization of molecules containing Lewis basic centers in the chitosan caused an increase in sorption capacity [14, 19]. The maximum sorption capacity Ns values for this chemically modified chitosan 2.05 ± 0.01; 2.53 ± 0.02 and 1.88 ± 0.01 mmol g−1 for copper, lead and cadmium listed in Table 2 are higher when compared to the same determination for original chitosan that gave 1.54 ± 0.03; 1.22 ± 0.04 and 1.12 ± 0.08 mol g−1 for this sequence of cations. The higher sorption capacity for this biomaterial can be interpreted not only due to the amount of basic centers attached to the pendant chains, but also on the characteristic of the specific basic centers [16, 19]. For the synthesized biopolymer, the softer Lewis sulfur base center favors the interaction with soft lead. This behavior in sorption process, the Langmuir sorption isotherm values for cations were linearized, is shown in Fig. 6.
The three most common isotherms to describe sorption systems in equilibrium at solid/liquid interface are the Langmuir, the Freundlich and the Temkin models, applied for various systems [10, 16, 17, 19, 20, 32–35]. It is observed that the predicted values of constant and correlation coefficients for four different linear forms of the Langmuir isotherms gave excellent results and very close for each model, as shown in Table 2. The correlation coefficient values close to unity and minimum values for error functions, SE, and chi square (χ 2) were obtained from the Langmuir isotherm; however, type I model suggests that it fits better to the experimental data in comparison with the other linear forms of the Langmuir isotherms, which best visualization is shown as an example for copper in Fig. 7. The excellent fit of this isotherm type 1 to the experimental data confirms that the sorption occurs in monolayer, each molecule has the same activation energy and sorbate/sorbate interaction is negligible. The error functions for the Freundlich isotherms showed higher values than the Langmuir isotherm of type I, as can be observed in the data listed in Table 3 for chemically modified chitosan. However, the Temkin model gave lower values of error compared to the other forms of linear Langmuir isotherms.
When comparing the error value functions of the other three linear forms of the Langmuir isotherm with the Freundlich, this is less appropriate to describe the sorption system than the other three models of the Langmuir. The values of Langmuir isotherm constant, determined by non-linear method, are very close to the results of the Langmuir model type I. However, the values of different error functions corresponding to the non-linear Langmuir equation are smaller than those of the Freundlich and the Temkin models as shown in Table 4. Typically, the non-linear regression analysis corresponds to the best way to select the isotherm that fits the experimental data. A clear illustration of the well-adjusted experimental copper data to the Langmuir model is shown in Fig. 8. This method involves an attempt to minimize the distribution of errors between experimental data and the isotherm considered [14]. Both linear and non-linear regression analyses produce different models as the best fitting isotherm for the given set of data, thus indicating a significant difference between the analytical methods.
In attempting to compare the maximum amounts of divalent copper, lead and cadmium sorbed in chitosan and chemically modified derivatives [9, 28, 36–42], significant examples are listed in Table 5. The biopolymer/cation interactive effect depends not only on the properties of the sorbent and sorbate, but also on various environmental aspects such as pH, ionic strength, temperature, sorbent concentration and contact time. However, in case of chitosan specific characteristics related to origin and degree of deacetylation are of enormous importance, in which availability of free amino group governs the interactions with cations. The precursor biopolymer chitosan with the most investigated copper cation [28, 36–38] presented values varying from 2.62 to 0.26 mmol g−1, while cadmium the less studied metal [37] gave lowest sorption value of 0.05 mmol g−1, reflecting the hard amino group with the hardest copper cation among the considered metals, in bond formation [16]. As observed, the obtained values for these cations in the present investigation are higher for lead and cadmium, as shown in Table 5.
The ability of chemically modified chitosan in sorbing cations depends basically on the availability of the basic centers attached to the pendant chains and also the acidic cation properties. For copper the values varied from 0.49 to 0.91 mmol g−1; also it includes chitosan pendant chains containing polyphosphate derivative [39], crown ethers [40, 41], cross-linked with epichlorohydrin [9] and pyridylmethyl derivative [42], which higher amount sorbed expressed its effectiveness in bond formation. For glycidylmethacrylate pendant chain moiety expanded with triamine (CHglyc) [16] also gave higher value of 3.59 mmol g−1 for this cation sorption. This biopolymer gave higher values for lead and cadmium, with a pronounced sorption for this last cation that data illustrated the activity of the basic center available on long pendant chain, which is favored by soft sulfur Lewis base. Based on the previous [16] and present results, it is remarkable that the synthesis of long pendant chains containing favorable basic centers attached is of fundamental importance to increase the sorption process.
Conclusions
Chemically modified chitosan was characterized using different techniques and applied for sorption of copper, lead and cadmium cations from aqueous solution, which showed higher sorption capacity than pure chitosan.
Both linear and non-linear regression analyses were used for the three models, the Langmuir, the Freundlich and the Temkin. In relation to linear and non-linear regression, the Langmuir model type I provided the lowest value of the error than the Freundlich and the Temkin models and fits better to experimental data compared with the latter two models. The four different linear forms of the Langmuir model give different values of error and parameters, which is due to transforming of non-linear into a linear equation, which affects the distribution of errors. A different arrangement of the axes also alters the distribution of error by influencing the accuracy, resulting in the violation of theory behind the isotherm. In case of non-linear method, no problem was associated with the transformation of non-linear to the linear equation form.
The long pendant chain covalently bonded to chitosan polymeric structure containing attached basic centers strongly favors the sorption process, which synthesized new biopolymer sorbed highest cation values, suggesting its use as a useful agent to cation removal from an ecosystem.
References
Gerente C, Lee VKC, Le Cloirec P, McKay G (2007) Application of chitosan for the removal of metals from wastewaters by adsorption—mechanisms and models review. Crit Rev Environ Technol 37:41–127
Rivas BL, Peric IM, Villegas (2010) Synthesis and metal ion uptake properties of water-insoluble functional copolymers: removal of metal ions with environmental impact. Polym Bull 65:917–928
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Rai HS, Bhattacharyya MS, Singh J, Bansal TK, Vats P, Banerjee UC (2005) Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Crit Rev Environ Sci Techonol 35:219–238
Kasgoz H (2006) New sorbent hydrogels for removal of acidic dyes and metal ions from aqueous solutions. Polym Bull 56:517–528
Singha B, Das SK (2013) Adsorptive removal of Cu(II)from aqueous solution and industrial effluent using natural/agricultural wastes. Colloids Surf B 107:97–106
Wang L, Xing R, Liu S, Qin Y, Li K, Yu H, Li R, Li P (2010) Studies on adsorption behavior of Pb(II) onto a thiourea-modified chitosan resin with Pb(II) as template. Carbohydr Polym 81:305–310
Wang LK, Chen JP, Hung YT, Shamas NK (2009) Heavy metals in the environment. CRC Press, Boca Raton
Chen AH, Liu SC, Chen CY, Chen CY (2008) Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J Hazard Mater 154:184–191
Saha TK, Mahmud MF, Karmaker S (2013) Adsorption behavior of [meso-tetrakis(4-carboxylatophenyl)porphyrinato]oxovanadium(IV) tetrasodium in aqueous solution onto chitosan. Polym Bull 70:2047–2063
Oliveira FJVE, Melo MA Jr, Airoldi C (2013) Inorganic–organic hybrids presenting high basic center content: SBA-15 incorporation, toxic metals sorption and energetic behavior. Mater Res Bull 48:1045–1056
Mourya VK, Inamdar NN (2008) Chitosan-modifications and applications: opportunities galore. React Funct Polym 68:1013–1118
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70
Machado MO, Lopes ECN, Sousa KS, Airoldi C (2009) The effectiveness of the protected amino group on crosslinked chitosans for copper removal and the thermodynamics of interaction at the solid/liquid interface. Carbohydr Polym 77:760–766
Oshita K, Seo K, Sabarudin A, Oshima M, Takayanagi T, Motomizu S (2008) Synthesis of chitosan resin possessing a phenylarsonic acid moiety for collection/concentration of uranium and its determination by ICP-AES. Anal Bioanal Chem 390:1927–1932
Khan A, Badshah S, Airoldi C (2011) Biosorption of some toxic metal ions by chitosan modified with glycidylmethacrylate and diethylenetriamine. Chem Eng J 171:159–166
Silva Filho EC, Monteiro PDR, Sousa KS, Airoldi C (2011) Ethylenesulfide as a useful agent for incorporation on the biopolymer chitosan in a solvent-free reaction for use in lead and cadmium removal. J Therm Anal Calorim 106:369–373
Kocak N, Sahin M, Kucukkolbasi S, Erdogan ZO (2012) Synthesis and characterization of novel nano-chitosan Schiff base and use of lead(II) sensor. Int J Biol Macromol 51:1159–1166
Khan A, Badshah S, Airoldi C (2011) Dithiocarbamated chitosan as a potent biopolymer for toxic cation remediation. Colloids Surf B 87:88–95
Badshah S, Airoldi C (2013) Layered inorganic-organic hybrid with talc-like structure for cation removal at the solid/liquid interface. Thermochim Acta 552:28–36
Chowdhury S, Misra R, Kushwaha P, Das P (2011) Optimum sorption isotherm by linear and nonlinear methods for safranin onto alkali-treated rice husk. Bioremediat J 15:77–89
Parimal S, Prasad M, Bhaskar U (2010) Prediction of equilibrium sorption isotherm: comparison of linear and nonlinear methods. Ind Eng Chem Res 49:2882–2888
Kannamba B, Reddy KL, Apparao BV (2010) Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J Hazard Mater 175:939–948
Donat R, Akdogan A, Erdem E, Cetisli A (2005) Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. J Colloid Interface Sci 286:43–52
Sousa S, Silva Filho EC, Airoldi C (2009) Ethylenesulfide as a useful agent for incorporation into the biopolymer chitosan in a solvent-free reaction for use in cation removal. Carbohydr Res 344:1716–1723
Silvertein RM, Bassler GC, Morrel TC (1991) Spectrometric identification of organic compounds. Wiley, London
Coates J (2000) Interpretation of infrared spectra: a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry: applications, theory and instrumentations. Wiley, Chichester
Lopes ECN, Sousa KS, Airoldi C (2009) Chitosan–cyanuric chloride intermediary as a source to incorporate molecules—thermodynamic data of copper/biopolymer interactions. Thermochim Acta 483:21–28
Silva GS, Oliveira PC, Giordani DS, Castro HF (2011) Chitosan/siloxane hybrid polymer: synthesis, characterization and performance as a support for immobilizing enzyme. J Braz Chem Soc 22:1407–1410
Sousa KS, Silva Filho EC, Airoldi C (2011) Ethylenesulfide as a useful agent for incorporation into the biopolymer chitosan in a solvent-free reaction for use in cation removal. Carbohydr Res 344:1716–1723
Pires CTGVMT, Oliveira NG Jr, Airoldi C (2012) Structural incorporation of titanium and/or aluminum in layered silicate magadiite through direct syntheses. Mater Chem Phys 135:870–879
Kumar PS, Gayathri R (2009) Adsorption of Pb2+ ions from aqueous solutions onto bael tree leaf powder: isotherms, kinetics and thermodynamic study. J Eng Sci Technol 4:381–399
Royer B, Cardoso NF, Lima EC, Macedo TR, Airoldi C (2010) Sodic and acidic crystalline lamelar magadiite adsorbents for the removal of methylene blue from aqueous solutions: kinetics and equilibrium studies. Sep Sci Technol 45:129–141
Anirudhan TS, Senan P (2011) Adsorption characteristics of cytochrome C onto cationic Langmuir monolayers of sulfonated poly(glycidylmethacrylate)-grafted cellulose: mass transfer analysis, isotherm modeling and thermodynamics. Chem Eng J 168:678–690
Abesi CY, Abia AA, Igwe JC (2011) Adsorption of iron (III), lead (II) and cadmium (II) ions by unmodified raphia palm (Raphia hookeri) fruit endocarp. Environ Res J 5:104–113
Huang C, Chuung YC, Liou MR (1996) Adsorption of Cu(I1) and Ni(I1) by pelletized biopolymer. J Hazard Mater 45:265–277
Jha I, Iyengar L, Rao A (1988) Removal of cadmium using chitosan. J Environ Eng ASCE 114:962–974
Benavente M, Moreno L, Martinez J (2011) Sorption of heavy metals from gold mining wastewater using chitosan. J Taiwan Inst Chem Eng 42:976–988
Ngah WS, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manage 91:958–969
Varma AJ, Despande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohydr Polym 55:77–93
Yang Z, Wang Y, Tang Y (2000) Synthesis and adsorption properties for metal ions of mesocyclic diamine-grated chitosan-crown ether. J Appl Polym Sci 75:1255–1260
Justi KC, Favere VT, Laranjeira MCM, Neves A, Peralta RA (2005) Kinetics and equilibrium adsorption of Cu(II), Cd(II), and Ni(II) ions by chitosan functionalized with 2[-bis-(pyridylmethyl)aminomethyl]-4-methyl-6-formylphenol. J Colloid Interface Sci 291:369–374
Acknowledgments
The authors thank to TWAS-CNPq for providing fellowships to AK and SB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, A., Badshah, S. & Airoldi, C. Environmentally benign modified biodegradable chitosan for cation removal. Polym. Bull. 72, 353–370 (2015). https://doi.org/10.1007/s00289-014-1278-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1278-z