Abstract
In this work Chitosan (Ch) was chemically modified with ethylenesulfide (Es) under solvent-free conditions to give (ChEs), displaying a high content of thiol groups due to opening of the three member cyclic reagent. ChEs was used in studies of lead and cadmium adsorption from aqueous solution, using the batchwise method and calorimetric studies were accomplished to those interactions, through the calorimetric titration technique. The obtained results show that the modified Ch, ChEs is a material, that besides presenting the advantages of being a biopolymer, it showed a good adsorption capacity of the lead and cadmium cation metallic, that are extremely poisonous and harmful to the environment. The results of the calorimetric titration showed that the related thermodynamic parameters to those adsorptions shown favorable thermodynamic data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitosan (Ch) is a linear copolymer constituted by β(1 → 4) linking 2-acetamido-2-deoxy-β-d-glucopyranose and 2-amino-2-deoxy-β-d-glucopyranose units obtained through N-deacetylation of chitin under strongly basic conditions. From the structural viewpoint, available amino (NH2) and hydroxyl (OH) groups present single-pairs of electrons, even though the N-deacetylation is almost never complete [1, 2]. It is usually obtained trough alkaline deacetylation from chitin that is a natural occurrence polymer made up of acetylglucosamine units, which are widely found in the exoskeleton of shellfish and crustaceans, such as crab, lobsters, and shrimps [3], being the second more abundant polysaccharide of the planet [4] and defeated only by cellulose. Cellulose, chitin, and Ch have related structures and occur in nature, being among the most abundant materials in the world [5, 6]. Chitosan is extensively exploited due to its innumerous advantages, since besides its chemical behavior Ch exhibits also physical and biological properties as non toxicity, biocompatibility, and biodegradability. When the biopolymeric Ch is submitted to chemical modification, this is a promising route to yield new biomaterials. Through the inclusion of desirable pendant molecules covalently bonded to the main chain, some characteristic properties are changed and, consequently, many other interesting areas such as agriculture, medicine, food, industry, pharmaceutical technology and so on become possible technological applications [7]. A point toward these modifications is the Ch sorption capacities studies, detaching its behavior with divalent cations [8].
This investigation deals with the incorporation of ethylenesulfide (Es) molecule in the polysaccharide chain through the reaction of the available amino group. An environmentally friendly solvent-free reaction was explored and the activity in sorbing divalent lead and cadmium from aqueous solution was studied through the proposed model. The cation/basic center interactions at the solid/liquid interface were also followed calorimetrically, and the set of thermodynamic data involved in these interactive processes was determined.
Experimental
Chemicals
Chitosan powder (Primex) with a degree of deacetylation of 82 %, determined through infrared spectroscopy was utilized [9]. Ethylenesulfide (Aldrich) reagent was incorporated on Ch in a solvent-free reaction. Lead and cadmium nitrate solutions were used in the sorption process in deionized water.
Chemical reaction
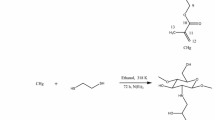
To a sample of Ch (2.0 g) in an appropriate flask protected from humidity, Es in excess (5.6 cm3) was added in the absence of solvent, at 328 K, under magnetic stirring. After 3 h, the solid was separated by filtration and extensively washed with deionized water. The isolated solid, hereafter named ChEs, was dried at 328 K in an oven [8].
Characterizations
The contents of carbon, sulfur, and nitrogen were determined through elemental analysis on a Perkin Elmer, model 2400, elemental analyzer. FTIR spectra of the samples as KBr pellets were obtained by accumulating 32 scans on a Bomem Spectrophotometer, MB-series, in the 4000 to 400 cm−1 range, with 4 cm−1 of resolution. Solid-state 13C NMR spectra of the samples were obtained on a Bruker AC 300/P spectrometer, using the CP/MAS technique, with pulse repetitions of 5 s and contact times of 1 ms; the measurements were at 75.47 MHz, with magic angle spinning of 4 kHz. Thermogravimetric curves were obtained using a Shimadzu TGA 50 apparatus, under an argon atmosphere at a flow rate of 1.67 cm3 s−1, with a heating rate of 0.167 K s−1. The amount of cation sorbed was determined using a Perkin Elmer 3000 DV ICP-OES apparatus.
Sorption experiments
An amount of 20 mg of the ChEs were introduced into a series of polyethylene flasks containing 10.0 cm3 of metallic cation solution having concentrations ranging from 7.0 × 10−4 to 7.0 × 10−3 mol dm−3, for 4 h at 298 + 1 K [10, 11]. The essays were accomplished in pH 6.1, since in acidic pH, lower than 4.0, raw Ch is soluble, and the same occurs to the modified material. Besides in such conditions the proton ions compete with the cations in binding to basic centers of the modified Ch. The amount of the cation adsorbed in the experiment (mmol g−1) was calculated by Eq. 1, where N f is the number of moles adsorbed on modified Chs, n i and n s are the number of moles in the initial solution and the supernatant after equilibrium, and m is the mass of the adsorbent used in each adsorption process [12, 13].
To determine the maximum adsorption capacity, N s, the experimental data related to the number of moles in the supernatant at each point of the titration, C s, and the N f obtained were fitted to a modified Langmuir equation (Eq. 2), b being a constant related to the chemical equilibrium at the solid/liquid interface.
Calorimetric titration
The interactive effect between the cations in solution and the basic centers attached on the modified Ch, ChEs, were measured through calorimetric titrations on a LKB 2277 instrument [14, 15], where three independent titrations must be carried out to complete the thermodynamic cycle: (i) the thermal effect due to ChEs interaction with cations (Q tit), (ii) hydration of the solid biopolymer (Q s), and (iii) dilution of cation solutions (Q dil). For Q tit experimental titration, the metallic solution is added to a suspension of nearly 20 mg of each biopolymer sample in 2.0 cm3 of water, under stirring at 298.15 ± 0.20 K. Increments of 10.0 × 10−3 cm3 of divalent cation solution were added to the biopolymer to obtain the thermal effect of interaction (Q tit). The thermal effect of hydration of the suspended solid samples in water was null [14–16]. Thus, the resulting thermal effect is given by the following equation:
After adjusting the adsorption data to a modified Langmuir equation it is possible to determine the enthalpy associated with the cation/biopolymer interaction and the enthalpy of the formation of a monolayer per unit of mass of adsorbent, ∆mono H, can be determined:
where ∑X is the sum of the mole fractions of the cation solution after adsorption. X is obtained for each point of titrant addition; ΔH (J/mol), the enthalpy of adsorption per gram of adsorbent, is obtained by dividing the thermal effect resulting from adsorption by the number of moles of adsorbate and K is the proportionality constant, which also includes the equilibrium constant. From the angular and linear values of the \( {{\sum X } \mathord{\left/ {\vphantom {{\sum X } {\sum {\Updelta H} }}} \right. \kern-\nulldelimiterspace} {\sum {\Updelta H} }} \) versus ∑X plot it is possible to calculate the Δmono H value.
Results and discussion
Elemental analysis
The nitrogen content in the chemically modified biopolymer, 3.28 mmol g−1, decreased when compared to the precursor biopolymer, 5.49 mmol g−1, and this fact is in agreement with the incorporation of one Es molecule in the available amino groups bonded to the polymeric structure, followed by another molecule of Es bonding to the first to give a S/N molar ratio of 1.94 and the sulfur content was 6.37 mmol g−1. Based in this ratio, the reaction comprised in the described processes is schematically represented in Fig. 1. In addition, there is the possibility of the Es molecule in reacting with the hydroxyl groups on the biopolymer in a similar manner to its reaction with an inorganic silica polymer. However, the greater probability is reacting with the amino groups [17], in agreement with its greater reactivity. This new modified material has as active groups for sorption process besides the remaining amino groups, the introduced sulfur groups, whose last have a great affinity for cations such as lead and cadmium.
Infrared spectroscopy
The pristine Ch possesses characteristic bands that are C–H stretching around 2900 cm−1 and a broad intense band near 3400 cm−1 due to O–H and N–H stretching. The band at 1320 cm−1 is related to aliphatic CH bending vibrations and those between 2920 and 2850 cm−1 correspond to the stretching vibrations of the same groups. Bands corresponding to the acetamide group, remaining from chitin, due its incomplete deacetylation [17], appear at around 1655 and 1380 cm−1, and are attributed to C=O stretching and CH deformation bands. The band at 1320 cm−1 is related to aliphatic CH bending vibrations and those between 2920 and 2850 cm−1 correspond to the stretching vibrations of the same groups. The bands in the 1200–800 cm−1 region are associated with the pyranosidic ring, reflecting C–O–C and β glycosidic linkages as well as the C–O bond related to primary and secondary alcohols. The spectra of the chemically modified Ch showed small changes when compared to the original matrix, which may be explained by considering the difficulty in seeing the SH band in the infrared spectrum. Nevertheless this band can be seen, although with difficulty, in the region around 2550 cm−1. It is also possible to observe a change in bands that belong to the methylenic groups in the 2700 and 3000 cm−1 region. A clear illustration for the effectiveness of the chemical modification process came from the CH:CH2 ratio of 5:1 for the precursor Ch, which changes to 5.5 in the final biopolymer [18, 19].
NMR spectroscopy
The 13C NMR spectra for Ch and the chemically modified biopolymer are shown in Fig. 2. Raw Ch presented characteristic chemical shifts at 105, 55, 85, and 60 ppm, related to the C1, C2, C4, and C6 carbons, respectively, as well as the signal at 75 ppm attributed to C3 and C5 carbon atoms. The signals at 22 and 175 ppm are attributed to methyl and carbonyl groups from the original chitin, due to the expected incomplete deacetylation process, as clearly indicated in the biopolymer structure in Fig. 2a. The spectrum of chemically modified Ch is shown in Fig. 2b, in which peaks in the 30–50 ppm region suggest different chemical environments for CH2 groups. Such different environments are due to the contribution of the Es molecules in the new polymeric structure. A significant modification was found in the peaks related to C6 and C2 carbon atoms, which were completely separated in the ChEs structure, as shown in Fig. 4b. The displacement occurs for one of the carbons present in the 50 and 70 ppm region, proving that the reaction occurred in the amino group located on C2 carbon atom, due to the fact that this group is more reactive than the hydroxyl group on the C6 carbon atom [20].
Adsorption isotherms
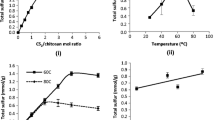
The isotherm of the chemically modified Ch, ChEs, with divalent cations, lead and cadmium, is illustrated in Fig. 3. This data from the adsorption isotherms gave maximum values of 1.94 mmol g−1 ± 0.02 and 1.81 mmol g−1 ± 0.03 for divalent cadmium and lead, respectively. These values are higher when compared to the other sorption cation values showed in the previous study [8], demonstrating higher effectiveness and affinity of Es in such cation removal. The mechanism of sorption can involve interactions that can be interpreted due to the transference of cation from solution to the basic centers of the modified Ch molecule (ChEs), by complexation of the cations through the available Es groups. The affinity between the sulfur atom and the cation will determine the adsorption capacity. The chemically modified Ch has less free amino content in comparison to the precursor biopolymer, to be able to chelate the metallic cations; however, it has additional sulfur centers that can be used as pendant chains, increasing the available basic centers for cation complexation. Based on these structural features it is suggested that the use of Es as modifier agent, in a solvent-free reaction, allows achieving some advantages, by increasing the adsorption capacity of the resulting polymeric biopolymer. Thus, it was found that the chemically modified Ch presented the following order of adsorption for divalent cations: Cd > Pb, due to favorable complexation equilibrium constant, as it is predicted by Irving–Williams series. During such interaction the cations are bonded to the basic center attached on pendant chains to form cation/biopolymer complex at the solid/liquid interface [10].
The parameters obtained from the linearized Langmuir model for divalent metal sorption on ChEs are listed in Table 1. From these data it is observed that cadmium is the cation having the highest adsorption in comparison to the lead cation, although both cations possess an excellent linear coefficient for the Langmuir model.
Calorimetric titration
The thermal effects obtained when cation nitrates interacted with ChEs were determined in separate calorimetric experiments as shown in Fig. 4. The enthalpic data are exothermic for both studied cations interactions and the magnitude of the values presented follows the order Cd > Pb as reflected by the sorption values as shown in Table 2. The negative free energy values indicate that a spontaneous process of complexation of both cations by ChEs has occurred, as previously observed [8, 19, 20]. All systems presented negative entropic values, which are also consistent with previously reported results. These values suggest that order in solvent behavior causes a decrease in entropy as the complexation process is in progress. These negative values obtained for entropy also suggest that the presence of cations bonding to available sulfur groups may order the random disposition of the Ch chains [20].
Conclusions
The synthesis of a new chemically modified Ch possessing a SH bonding in its chain using Es in the absence of solvent was efficiently performed, according to such characterization methods as elemental analysis and carbon nuclear resonance spectroscopies. The chemically modified biopolymer adsorbed metallic cations giving the values 1.94 mmol g−1 ± 0.02 and 1.81 mmol g−1 ± 0.03 for Cd and Pb, respectively. The adsorption ability for each metal is associated with intrinsic parameters of each cation that determine its affinity for the modified surface. These interactions could be calculated by applying the modified Langmuir equation that showed to be a good model to obtain the adsorption parameters as showed by the correlation coefficients.
The interactions between the cation and the basic centers of ChEs at the solid/liquid interface followed by calorimetric titrations, showed negative entropies, but favorable thermodynamic values, suggesting the possibility of application of this modified biopolymer for removal of this series of divalent cations from aqueous solutions.
References
Sorlier P, Denuzière A, Viton C, Domard A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules. 2001;170:765–72.
Methacanon P, Prasitsilp M, Pothsreea T, Pattaraarchachai J. Heterogeneous N-deacetylation of squid chitin in alkaline solution. Carbohydr Polym. 2003;52:119–23.
Cestari AR, Vieira EFS, Oliveira IA, Bruns RE. The removal of Cu(II) and Co(II) from aqueous solutions using cross-linked chitosan-Evaluation by the factorial design methodology. J Hazard Mater. 2007;143:8–16.
Tolaimate A, Desbrières J, Rhazi M, Alagui A, Vincendon M. On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer. 2000;41:2463–9.
Klemm D, Heublein B, Fink HP, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed. 2005;44:3358–93.
Kumar MNVR. A review of chitin and chitosan applications. React Funct Polym. 2000;46:1–27.
Ye X, Yang Q, Wang Y, Li N. Electrochemical behaviour of gold, silver, platinum and palladium on the glassy carbon electrode modified by chitosan and its application. Talanta. 1998;47:1099–106.
Sousa KS, Silva Filho EC, Airoldi C. Ethylenesulfide as a useful agent for incorporation into the biopolymer chitosan in a solvent-free reaction for use in cation removal. Carbohydr Res. 2009;344:1716–23.
Monteiro OAC Jr, Airoldi C. Some thermodynamic data on copper–chitin and copper–chitosan biopolymer interactions. J Colloid Interface Sci. 1999;212:212–9.
Machado MO, Lopes ECN, de Sousa KS, Airoldi C. The effectiveness of the protected amino group on crosslinked chitosans for copper removal and the thermodynamics of interaction at the solid/liquid interface. Carbohydr Polym. 2009;77:760–6.
Da Fonseca MG, Da Silva Filho EC, Machado RSDA Jr, Arakaki LNH, Espínola JGP, Oliveira SF, Airoldi C. Anchored fibrous chrysotile sílica and its ability in using nitrogen basic centers on cátion complexing from aqueous solution. Colloids Surf A. 2004;227:85–91.
Arakaki LNH, Alves APM, SilvaFilho ECd, Fonseca MG, Oliveira SF, Espínola JGP, Airoldi C. Sequestration of Cu(II), Ni(II), and Co(II) by ethyleneimine immobilized on sílica. Thermochim Acta. 2007;453:72–4.
de Melo JCP, da Silva Filho EC, Santana SAA, Airoldi C. Maleic anhydride incorporated onto cellulose and thermodynamics of cátion-exchange process at the solid/liquid interface. Colloids Surf A. 2009;346:138–45.
Oliveira FJVE, da Silva Filho EC, Melo MA Jr, Airoldi C. Modified coupling agents base don thiourea, immobilized onto sílica. Thermodynamics of copper adsorption. Surf Sci. 2009;603:2200–6.
da Silva Filho EC, de Melo JCP, da Fonseca MG, Airoldi C. Cation removal using cellulose chemically modified by a Schiff base procedure applying green principles. J Colloid Interface Sci. 2009;340:8–15.
Arakaki LNH, da Fonseca MG, da Silva Filho EC, Alves APM, de Sousa KS, Silva ALP. Extraction of Pb(II), Cd(II), and Hg(II) from aqueous solution by nitrogen and thiol functionality grafted to silica gel measured by calorimetry. Thermochim Acta. 2006;450:12–5.
Lima IS, Airoldi C. A thermodynamic investigation on chitosan–divalent cation interactions. Thermochim Acta. 2004;421:133–9.
da Silva Filho EC, de Melo JCP, Airoldi C. Preparation of ethylenediamine-anchored cellulose and determination of thermochemical data for the interaction between cations and basic centers at the solid/liquid interface. Carbohydr Res. 2006;341:2842–50.
Santana SAA, Vieira AP, da Silva Filho EC, Melo JCP, Airoldi C. Immobilization of ethylenesulfide on babassu coconut epicarp and mesocarp for divalent cation sorption. J Hazard Mater. 2010;147:714–9.
Lima IS, Lazarin AM, Airoldi C. Cyclic voltammetric investigations on copper α-N, O-succinated chitosan interactions. Carbohydr Polym. 2006;64:385–90.
Acknowledgements
The authors wish to thank Fapesp and CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva Filho, E.C., Monteiro, P.D.R., Sousa, K.S. et al. Ethylenesulfide as a useful agent for incorporation on the biopolymer chitosan in a solvent-free reaction for use in lead and cadmium removal. J Therm Anal Calorim 106, 369–373 (2011). https://doi.org/10.1007/s10973-010-1205-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1205-y