Abstract
Chitosan is a low-cost natural adsorbent. Its derivatives from chemical and physical modification processes possess superior properties for wide applications to meet the growing demands. The chemical modification includes replacement reactions, chain elongation and depolymerization, while the physical modification is to obtain polymeric forms such as powders, nanoparticles and gels. This paper is aimed to highlight the present trends in chitosan preparation and modification, the enhancement in adsorptive properties and the remarks into future directions. The mechanisms involved in adsorption by chitosan derivatives and how the spent adsorbent can be regenerated were also discussed. Meanwhile, for the adsorption of heavy metals from wastewater, chitosan modified with activated carbon showed a better adsorption capacity of 90.90 mg g−1 for Cr(VI) and 50.50 mg g−1 for Cd(II), and for dye adsorption, chitosan modified with activated neem leave showed better adsorption capacity of 90.8 mg g−1 for methylene blue, and for phenol removal, chitosan modified with salicylaldehyde and β-cyclodextrin polymer showed better adsorption capacity of 179.73 mg g−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
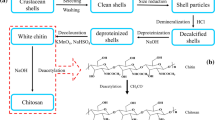
Chitosan is a post-deacetylation chitin derivative and one of the most abundant post-cellulose polysaccharides in nature [1, 2]. It has found wide applications in biotechnology, agriculture, medicine and so on, due to growing demands [3,4,5]. In general, shrimp, crab, crayfish and krill shell can be used to prepare chitosan [6, 7]. However, studies have also shown that alternative sources of chitin (basic raw materials for chitosan preparation) are bees, fungi, coral and crustacean resting eggs [8]. This basic raw material (chitin) for chitosan preparation can be processed through chemical processes involving deproteinization, demineralization and deacetylation [9, 10]. Annually, 2000 tons of chitosan is produced mainly from shrimps and crab shell waste [11, 12].
Crustaceans are found to be more suitable for deacetylation and have different degrees of deacetylation-based solubility and swelling characteristics which are attributable to the parallel arrangement of their main chains due to weak intermolecular hydrogen bonding [13, 14]. This swelling property allows adsorption, floating and chitosan drug diffusion mechanism [15,16,17]. Chitosan is also one of the most available sustainable products in the natural environment, obtained as a result of the chemical or enzymatic deacetylation of chitin in the biosynthesis process [18]. This method leads to partial or complete elimination of acetyl groups from the chitosan group of acetyl-amino. Chitosan’s name is applied to the altered chitin which contains less than 25% of the acetyl groups [19, 20]. Figure 1 presents the chemical structures of chitin and chitosan.
Chemical structure of chitin and chitosan [21]
(1 → 4)-2-Acetamido-2-deoxy-β-d-glucan and (1 → 4)-2-amino-2-deoxy-β-d-glucan are the basic structures of Chitin and chitosan, respectively (Fig. 1). Nevertheless, the difference between them is the degree of deacetylation and their solubility in dilute acid media [22, 23]. When the chitin degree of deacetylation drops to about 50%, it becomes soluble in aqueous acidic media and is called chitosan [24, 25]. Depending on the source, there are three crystalline polymorphic forms of chitin. These include shrimp and crab shell α-chitin, squid pen ß-chitin and cephalopoda stomach cuticles γ-chitin [26].
To improve the sorption potential, resistance to low pH and mechanical strength of chitosan, several physical and chemical modification methods have been applied [27]. Physical modifications include the preparation of chitosan for all applications in various forms such as powder, nanoparticles, gel beads, membranes, sponges, type structure of “honeycomb” and various types of fibre [16]. Chemical modifications include cross-linking with glutaraldehyde, ß-cyclodextrin oxidized, ethylene glycol diglycidyl ether or epichlorohydrin [28]. Figure 2 presents a schematic representation of the cross-linking reaction of chitosan with glutaraldehyde.
Chitosan has found numerous applications in various industries, such as textiles, paper, cosmetics, pharmaceutical, agricultural, medical and environmental engineering [31,32,33]. It is primarily used in environmental engineering as an effective biosorbent [34].
The advantages of chitosan over other adsorbents include its abundance as biopolymer in nature, the presence of characteristics free amino and hydroxyl functional groups which makes it chelate five or more metals than other adsorbents [35] and the presence of positions that can be modified in its chemical structure [36]. In metal sorption, chitosan is selective because it does not take up alkaline and alkaline earth metal ions but transition and post-transition metal ions [17]. These properties of sorption were used for environmental, separation and analytical purposes [16]. Other advantages include its low price, high sensitivity to a variety of contaminants, chemical stability, high reactivity and pollution selectivity [16, 37].
Chitosan modification results in the formation of chitosan derivatives which possess better properties and an increased number of adsorption sites and adsorption capacity [36, 38]. Wang et al. [39] and Kulkarni et al. [40] reported chitosan modification methods to include N-substitution, O-substitution and free radical graft copolymerization. LogithKumar et al. [41] discussed recent research in chitosan modifications by quaternization, carboxyalkylation, hydroxylation, phosphorylation, sulphation and copolymerization for bone tissue engineering. Seedevi et al. [42] showed an increase in the antioxidant property of chitosan from sepia prashadi by chlorosulfonic acid in N,N-dimethylformamide. Campelo et al. [43] recorded an increase in the roughness and hydrophilicity and a decrease in calcium deposit of chitosan by sulphation modification for metallic implants when in contact with blood. Galhoum et al. [44] showed an increase in uranyl removal onto chitosan grafted with diethylenetriamine. Vakili et al. [45] reported an improve adsorption capacity of colorant blue 4 by chitosan modified with hexadecylamine and 3-aminopropyl triethoxysilane. Furthermore, chitosan hydrogels used in wastewater treatment have been greatly improved when modified by protonation, carboxylation and grafting with glutaraldehyde, epichlorohydrin, ethylene glycol, diglycidyl ether and sodium tripolyphosphate [46,47,48]. Stabilization of enzymes like amylase from Aspergillus carbonarius was achieved when chitosan was modified with phthalic anhydride [49] and the stabilization of horseradish peroxide was achieved when chitosan was covalently modified using phthalic anhydride [50].
Several review papers have been published on chitosan extraction [15], chitosan and modified chitosan as low-cost adsorbent [20, 36, 37], chitosan application in the industry, agriculture and medical science [3, 31, 41, 51] and chitosan and modified chitosan regeneration [52]. However, this review attempts to discuss the underlying background of chitosan preparation, its inherent properties for industrial applications and life cycle assessment, highlight the physicochemical modifications leading to the enhanced adsorptive properties of chitosan derivatives for water pollutants removal and nutrient recovery, and summarize the adsorption mechanisms and recovery of spent chitosan derivatives with the view to shedding insight into future directions.
2 Life cycle assessment
The environmental impact of most disposable waste has been evaluated and comparison made using life cycle assessment (LCA). This allowed for the quantification and comparison of the environmental impact among or between stages of production or services within their life cycle [53]. Laceta et al. (2013) reported on the environmental assessment of 1 m2 chitosan-based films after conducting a comparative environmental assessment between polypropylene (PP) commercial food packaging film and developed chitosan-based biodegradable film. Their environmental load in diverse life cycle stages such as material extraction, film manufacture and end of life was studied and a comparison was made. The result revealed that PP films had a higher impact on fossil fuels and carcinogens impact categories. However, chitosan-based films had a higher environmental load in land use, minerals and respiratory inorganic categories. Muñoz et al. (2017) also reported on life cycle assessment of chitosan production from cradle to gate in India and Europe from the viewpoint of the supply chains of their raw materials (snow crab and shrimp waste shells in Canada and India, respectively), their processing in China and India and chitosan manufacture in Europe and India. The result showed a difference in the environmental profiles of both chitosan from crab and shrimp waste shells, which were reflections of the difference in supply chains of raw materials, production location and applications. Beach et al. [54] reported on alternatives for investing microalgae by flocculation using chitosan, ferric sulphate and alum by building a life cycle inventory. The result from the life cycle inventory showed the superiority of chitosan from the viewpoint of the environment as a flocculant for microalgae harvesting.
3 Preparation of chitosan
The process of chitosan preparation involves the deproteinization of autolyzed crustacean shells in 3–5% (w/v) NaOH solution at room temperature for few hours. This is accompanied by demineralization (through processing, extraction of the inorganic minerals) with 3–5% (w/v) HCl aqueous at room temperature to obtain a white to beige coloured chitin. Next, it is treated at 90–120 °C for 4–5 h with 40–60% (w/v) NaOH solution, resulting in chitin deacetylation to form chitosan. The insoluble precipitate is washed with water. The deacetylation conditions determine the molecular weight of the polymer and the degree of deacetylation [13, 55]. Meanwhile, just as chitin undergoes alkaline deacetylation in the presence of sodium hydroxide to give chitosan, it also undergoes hydrolysis in the presence of hydrochloric acid to produce glucosamine as shown in Fig. 3 [56].
Molecular reactions between chitin and chitosan with HCl and NaOH [56]
4 Properties of chitosan for industrial applications
Different chitosan properties determine their end uses. For example, strong hydrophilicity often due to a large number of highly reactive hydroxyl and amine functional groups, specific surface and stability of polymer chain are favourable attributes of chitosan in water pollutants removal [57, 58].
Chitosan is a large number of biopolymers with different degrees of N-deacetylation (40–98%) and molecular weight (50,000–2,000,000 g mol−1). It is characterized by the presence of amine and hydroxyl functional groups which influence its properties. It is a soft base with a d-glucosamine residue and a pKa value of 6.2–7.0 [57, 59]. Chitosan is nontoxic, biodegradable and in the acidic environment, the amine groups of chitosan are protonated to form soluble, positively charged polysaccharides with a high load density [60, 61].
Viscosity is a property of chitosan that increases as its degree of deacetylation and concentration increases, and temperature decreases [16]. It is often in the range of 60 and 780 cPs. Therefore, concentration and temperature are critical factors in determining the viscosity of chitosan solution. The increase in the degree of deacetylation of chitosan that results in the increase in viscosity can be attributed to the difference in conformity in an aqueous solution of high- and low-deacetylated chitosan. Chitosan with strong deacetylation has an extended conformation with a more flexible chain due to the load repulsion in the molecule. Meanwhile, low load density in the polymer chain of chitosan molecule results in the formation of rod-like or coiled shape at a low degree of deacetylation [16].
Chitosan materials possess a density in the range of 0.15 and 0.3 g mL−1 due to their porous nature as in the case of chitosan from marine crab and squilla and commercial chitosan [55, 62].
Fourier transform infrared (FTIR) analysis of chitosan shows a broad absorption band in the range of 3000 to 3500 cm−1 which is attributed to O–H stretching vibrations and the 3263 cm−1 to the vibration of N–H [59, 62]. The stretching vibrations of C-H occur at 2854 cm−1 and the absorption peak at 1558 cm−1 corresponds to the N–H bending vibrations. The amide II band is used as the characteristic band of N-acetylation. The spectra of chitosan show the different vibration that occurs after deacetylation process, which shows that the C = O vibration at 1627 cm−1 region associated with chitin has been reduced in chitosan, as well as the emergence of the absorption band at 894 cm−1 on chitosan which is the vibration for NH2 [62].
Water binding capacity (WBC) of chitosan is within the range of 138 and 805% as reported by [62] for chitosan from Pessu river crab shell, [63] for chitosan synthesized from fish, crab and shrimp, Sarbon et al. (2015) for chitosan from mud crab and [64] for commercial chitosan from shrimp and crab. It is hydroscopic in nature with less than 10% moisture content and often reveals a split amide band and crystalline polymorph due to its parallel structure [55]. Table 1 presents the synthesized chitosan materials from different sources and their physicochemical properties.
Chitosan’s ability to chelate metal ions has made it a potential natural antioxidant for stabilizing and prolonging the shelf life of lipid foods [74]. The presence of organic diacid anhydride in chitosan is useful in the manufacture of cosmetics like shampoos, hair colourants, styling lotions, hair sprays, hair tonics, moisturizer, nail enamel foundation, mouth washer, chewing gum, lipstick and eye shadow [51]. Furthermore, the inherent characters of morphology, size, non-toxicity and density are important in the release of drugs in chitosan base dosage during drug delivery [75]. The biocompatibility and biodegradability properties of chitosan have made it useful in biomedicine and tissue engineering. Low molecular weight chitosan is used as a carrier for solid drug formulations in the drug delivery system. The cationic property of chitosan has found applications in gene therapy system because it provides strong electrostatic interaction with anionic DNA, thus protecting it from nuclease degradation [51, 76]. The antimicrobial property of chitosan has made its film great potential for food preservation [77].
5 Chitosan modification procedures
Physicochemical modification of chitosan has been a growing interest to improve water-acid solubility and adsorption properties, while broadening its applications. The chemical modification involves replacement reactions, chain elongation (cross-linking, copolymerization of graft and polymer networks) and depolymerization, while the physical modification is generally aimed at obtaining conditioned polymeric forms such as powders, nanoparticles and gels (beads, membranes, honeycombs or hollow fibres) [78].
Repo et al. [79] improved the reactivity of chitosan by ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA) functionalization. Chitosan is dissolved in 10% (v/v) acetic acid and diluted with methanol. EDTA anhydride in methanol is added, and the mixture is stirred vigorously at room temperature for 24 h. The precipitate is mixed with ethanol and washed with NaOH solution (pH 11) to remove unreacted EDTA. A similar method was adopted for DTPA-functionalized chitosan. EDTA- and DTPA-modified chitosan exhibit effective adsorption for Co(II) and Ni(II) at 93.0% to 99.5% in 100 mg L−1 aqueous solutions. Figure 4 illustrates the synthesis route of EDTA- and DTPA-modified chitosan.
Synthesis of EDTA- and/or DTPA-modified chitosan [80]
Hariani et al. [81] synthesized bentonite-modified chitosan. Bentonite is added into the chitosan-CH3COOH solution at pH 4. The mixture was stirred for 2 h. The composite is centrifuged and washed with deionized water to a neutral pH. The calcium-rich bentonite/chitosan composite showed an improved capacity of phenol at 12.5 mg g−1 in 125 mg L−1 solution at a pH of 7 for 30 min.
Moosa et al. [82] noted chitosan modification using granulated activated carbon. Activated carbon is immersed in chitosan acetic acid solution at a ratio of 10:50 (w/v) at 25 °C for 24 h. The filtrate is soaked in 0.1 mol L−1 NaOH for 3 h to precipitate the chitosan on activated carbon. Next, it is washed with distilled water prior to use. The material demonstrates a 95.81% removal of methylene blue as against 85.85% by the unmodified one (Table 3).
Saifuddin and Kumaran [83] encapsulated chitosan onto acid-treated oil palm shell charcoal. The process involves the preparation of chitosan gel using 10 wt% oxalic acid at 50 °C. The charcoal is slowly added to the gel solution, and the mixture is agitated for 24 h to form beads. The process is repeated thrice to form a thick chitosan coating. The material shows an increase in chromium ion removal of 154 mg g−1.
Okoya et al. [84] modified chitosan with cocoa husk char. A 100 mL of the chitosan gel in oxalic acid is diluted with water, wherein cocoa husk char is added. The mixture is agitated for 24 h. Then, the composite is soaked in 0.5% (w/v) NaOH solution for 3 h and rinsed with deionized water. The material depicts the Cr6+ and Pb2+ adsorption of 333 mg g−1 and 263.16 mg g−1, respectively, while the unmodified one records the values of 136.98 mg g−1 and 125.0 mg g−1, respectively.
Kyzas and Deliyanni [85] recognized the modification of magnetic chitosan by cross-linking with glutaraldehyde (GLA). GLA-cross-linked chitosan is prepared by adding 15 mL of GLA (approximately 2:1 aldehyde groups (–CHO) of GLA per initial amino group (–NH2) of chitosan) in a 400 mL of acetic acid solution (2% v/v) bearing 2 g of dissolved purified chitosan. The mixture is stirred at 25 °C for 3 h, and the precipitate is washed with ethanol and distilled water and dried in a vacuum oven at 45 °C. Magnetic nanoparticles are prepared by dissolving 3.5 g of FeCl2 · 4H2O and 9.5 g of FeCl3 · 6H2O in 400 mL of distilled water at 60 °C for 1 h under nitrogen flow. Ammonia solution is added dropwise to a pH of 10. The precipitate is decanted and freeze-dried. For magnetic cross-linking, 0.75 g of magnetic nanoparticles is added into chitosan solution and the mixture is sonicated for 30 min. This is followed by the same procedures for GLA cross-linking. The modified chitosan derivatives display enhanced reusability upon regeneration. Similarly, GLA-cross-linked chitosan shows an improved Hg(II) capacity of 145 mg g−1, while that with magnetic nanoparticles is 152 mg g−1. Figure 5 illustrates the schematic preparation of cross-linked chitosan with GLA and magnetic nanoparticles and the possible interactions with Hg(II).
Preparation of magnetic cross-linked chitosan for Hg(II) removal [85]
Vafakish and Wilson [86] reported the grafting of aniline and acetaldehyde onto chitosan. Aniline renders a light-yellow viscous chitosan solution, while acetone forms a white low-viscous solution. The reaction mixtures are stirred at 70 °C for 18 h. A 3 mol L−1 NaOH solution is added gradually under stirring to a pH 7, at which pink precipitate is evolved. The precipitate is separated from the supernatant, washed with water and ethanol, and dried in a vacuum oven at 50 °C for 6 h. The modification enhances the specific surface of chitosan and its capacity towards fluorescein dye to 61.8 mg g−1. Figure 6 presents the synthesis of chitosan by grafting with aniline and acetaldehyde.
One-step synthesis of chitosan by grafting with aniline and acetaldehyde [86]
Al-Ghamdi et al. [87] noted the modification of chitosan by ρ-bromo-β-ketosulfone. The solvent, ρ-bromo-β-ketosulfone is prepared by refluxing a mixture of 2 mmol sodium benzene sulfinate and 2 mmol ethanolic solution of 4-bromo-4-bromoacetophenone for 6 h, and the solid product is washed with water and recrystallized. The solid is dissolved in chitosan solution, and the residual phase is recovered by evaporated and dried. The modified chitosan exhibits a 122.47 mg/g of Hg(II) removal. Significant improvement can be observed from the surface morphology as opposed to the pristine chitosan, but the thermal stability decreased. The synthesis routes are shown in Fig. 7.
Preparation of ρ-bromo-β-ketosulfone for chitosan modification [87]
Hastuti et al. [88] reported the removal of Cr(VI) by epichlorohydrin-modified chitosan. Chitosan gel was prepared by adding NaHCO in chitosan acetic acid solution at ratio of 0.2:2.0 (w/w). The gel is sprayed with 5% NaOH solution to form chitosan beads. The solid is coated in benzaldehyde solution for 2 h. The cross-linking process is carried out by dispersing the beads into dioxane. One mole per litre NaOH solution is slowly added to the stirred solution, followed by epichlorohydrin. The mixture is refluxed for 6 h, and the precipitate is washed with ethanol and water. The modified chitosan demonstrates an improved porosity, increased resistance against acidic medium and enhanced capacity of 89% as compared to 74% by the unmodified one. Figure 8 shows the reaction of chitosan with epichlorohydrin.
Reaction of chitosan with epichlorohydrin [88]
Soni et al. [89] disclosed the modification of chitosan-activated carbon composite with tripolyphosphate. A water in paraffin oil emulsion is added into the homogenous chitosan-charcoal suspension in acetic acid. The mixture is agitated for 2 h to form a stable emulsion. A 0.1 mol L−1 tripolyphosphate is added dropwise to form nanocomposite particles. The solid is washed with toluene and acetone. The adsorbent exhibits an outstanding phenol adsorption capacity of 409 mg g−1. Similarly, Liu et al. [90] recorded the effective removal of phenol and Cu2+ of over 80% by chitosan-activated carbon membrane composite.
Marrakchi et al. [91] revealed a superior removal of methylene blue and reactive orange 16 at 40.99 mg g−1 and 190.97 mg g−1, respectively by epichlorohydrin-cross-linked chitosan-sepiolite composite. Sepiolite powder is dispersed into a chitosan acetic acid solution. The mixture is then added dropwise into a 1 mol L−1 NaOH solution. The solid is washed and to a neutral pH and added into epichlorohydrin solution at 50 °C for 6 h.
Sharififard et al. (2016) used 0.2 mol L−1 oxalic acid to dissolve chitosan at 40 °C to form a viscous gel. A 20 g of acid-treated activated carbon is mixed with chitosan gel and stirred at 40 °C for 12 h to form a chitosan-activated carbon composite. The composite was added in dropwise into a 0.7 mol L−1 NaOH precipitation bath to form beads. The modification has improved its capacity for metal ions, i.e., 90.9 mg g−1 for Cr(VI) and 52.63 mg g−1 for Cd(II). The pristine one recorded the values of 41.94 mg g−1 and 10 mg g−1, respectively. Regunton et al. [92] also attempted similar procedures, where the modified chitosan composite shows a methylene blue removal of 99.77%.
Huang et al. [93] reported the modification of chitosan-activated carbon membrane by cross-linking using epichlorohydrin. Epichlorohydrin-to-membrane ratio is 20 mL g−1, and the cross-linking reaction occurs for 12 h. The cross-linked membrane is treated with concentrated HCl for 90 min for surface protonation and washed with deionized water to a neutral pH. Epichlorohydrin is proven as a suitable cross-linker to boost the phenol and Cr(VI) to 95% at maximum concentrations of 50 mg L−1 and 200 mg L−1, respectively.
López-Cervantes et al. [94] modified chitosan beads by glutaraldehyde cross-linking for direct blue 71 removal. The wet chitosan beads from the NaOH bath are suspended in a 0.025 mol L−1 glutaraldehyde solution (beads-to-solution ratio of 1:10) at room temperature for 16 h and washed with distilled water and ethanol.
Auta and Hameed [95] showed that the preparation of epichlorohydrin-cross-linked chitosan-activated carbon beads. The chitosan acetic acid solution with dispersed waste tea activated carbon is added dropwise into 0.067 mol L−1 NaOH solution to form beads. The beads are cross-linked with epichlorohydrin at 50 °C for 6 h and freeze-dried to preserve the texture. The modified adsorbent is promising for cationic and anionic dyes removal.
Asokogene et al. [62] recognized the synthesis of neem leave-chitosan composite in oxalic acid solution. The activated neem leave is slowly added into the mixture at 50 °C for 2 h. The dried composite is soaked in 0.5% (w/v) NaOH solution for 3 h, rinsed with distilled water and dried. The modified chitosan possesses a better specific surface leading to a higher methylene blue capacity of 90.8 mg g−1 at a dye concentration of 300 mg L−1.
Nevertheless, chitosan synthesis from chitin and some widely used functionalized chitosan derivatives structures is presented in Fig. 9 [96]. Furthermore, in chitosan modification procedures, some important advantages and disadvantages are summarized in Table 2.
Chitosan synthesis from chitin and some widely used functionalized chitosan derivatives structures [96]
6 Chitosan derivatives for water pollutants removal
Among the toxic contaminants in water are heavy metals, phenol and dyes. These solutes are harmful if directly ingested, and their accumulation in the food renders a threat during meat, vegetables and fish consumption. Reactive hydroxyl and other functional groups, strong hydrophilicity and stability of polymer chain endow chitosan with a high adsorption ability towards toxic contaminants in water [57, 97, 98].
Sharififard et al. [99] evaluated the adsorption of Cr (VI) and Cd (II) by chitosan adsorbents. The modified chitosan shows an increase of specific surface at 362.30 m2 g−1 as opposed to the pristine one (16.37 m2 g−1). Similarly, the adsorption capacities for Cr (VI) and Cd (II) improved to 90.90 and 50.50 mg g−1, better than the unmodified one at 41.60 mg g−1 and 10.00 mg g−1, respectively (Table 3). The increase in the surface area offers more interaction probabilities between the solutes and active sites for greater removal. Hastuti et al. [88] studied the adsorption of Cr (VI) from batik industrial wastes using chitosan modified with epichlorohydrin. The modified chitosan shows a solubility resistance to acids and an improved capacity from 7.4 mg g−1 (unmodified chitosan) to 8.9 mg g−1 at 30 min contact time and pH 3. Bahador et al. [100] investigated the effect of chitosan (CS) and iron ore (Fe3O4) magnetic nanoparticles on chromium (Cr) removal behaviour of Moringa leifera activated carbon (AC). The adsorption capacity showed the following: AC (56.78 mg g−1), CS/AC (114.80 mg g−1), Ac/Fe3O4 (121.70 mg g−1) and CS/AC/Fe3O4 (130.80 mg g−1). Modified AC and Fe3O4 nanoparticles by CS increased Cr removal.
It is very difficult to purify sewage-containing dyes because colouring is generally resistant to biological oxidation and chemical oxidants in some cases. Adsorption at low cost and by means of widely available natural adsorbents is an alternative to the traditional and conventional processes. Advanced oxidation methods are effective but relatively expensive in the degradation of colourants and pigments in wastewater. Chitosan is a promising biosorbent to remove dyes from water [103]. Vafakish and Wilson [86] studied the aniline-modified chitosan for the removal of anionic fluorescein dye. The adsorption capacity increased from 1.96 to 61.8 mg g−1. Similarly, Asokogene et al. [62] reported the potential of activated neem leave-chitosan composite for methylene blue removal. The specific surface increased from 226 to 389 m2 g−1, while the capacity rose from 29.93 to 90.8 mg g−1. Carvalho et al. [104] evaluated the adsorption potential of chitosan films from shrimp waste on anthocyanin pigment from red cabbage with the view to providing information about the immobilization of anthocyanin molecules onto chitosan films. The adsorption capacity of the chitosan film was 140 mg g−1. Pinheiro et al. [105] evaluated the adsorption potential of chitosan from shrimp waste and alginate beads on anthocyanins from Pinot Noir grape skin with the view to demonstrating the possibility of concentrating different molecular structures of anthocyanins onto chitosan and alginate beads. The adsorption capacity of chitosan and alginate beads was 216 mg g−1 (65%) at pH 8 and 126.4 mg g−1 (38%) at pH 4, respectively.
Chitosan has been successfully used to extract anions from water in recent years. Chatterjee and Woo [106] used chitosan beads to remove nitrates. The capacity of 92.1 mg g−1 was recorded at pH 3, initial nitrate concentration of 1 g dm−3 and temperature of 30 °C. In a related work, the protonated cross-linked glutaraldehyde chitosan gel beads displayed a pH-independent process of nitrate extraction [107]. The protonated chitosan beads had also been applied as defluoridating medium [108]. The presence of other coexisting anions shows that the adsorption varied with pH, whose maximum capacity lies between 4.72 and 7.32 mg g−1. The hydrogen bonds are indicated as the sorption mechanism between the positively charged amino groups of chitosan and fluoride ions. Affonso et al. [109] evaluated fluoride adsorption from fertilizer industry effluent using carbon nanotubes stabilized in chitosan sponge (CNT-CS) as adsorbent. The removal capacity of fluoride was 975.4 mg g−1, which was an indication of the potential of hybrid material to remove fluoride from the real matrix. Further evaluation showed that after 5 cycles of regeneration, the reuse of adsorbent kept similar adsorption capacities in all cycles.
Chitosan has also been used for the adsorption of nutrients from an aqueous solution. Safie et al. [110] carried out an adsorption performance comparison on ammonium ion (NH4+) of natural zeolite (NZ), activated NZ (ANZ) with high molecular weight chitosan (HMWC) and low molecular weight chitosan (LMWC). The result showed that HMWC, NZ and ANZ attained adsorption equilibrium at 15 h and LMWC at 20 h. However, the removal capacity was 0.769, 0.331, 2.162 and 2.937 mg g−1 for LMWC, HMWC, NZ and ANZ, respectively. Haseena et al. [101] also prepared a novel composite adsorbent of chitosan and bentonite nanoclay in the form of thin films and its adsorption potential for ammonium-nitrogen (NH4+N) from an aqueous solution was investigated and compared with unmodified chitosan. The removal of ammonium-nitrogen from an aqueous solution of initial concentration of 15 ppm, pH of 6 and adsorbent dosage of 0.5 g was 43.19 and 82.11%, respectively for unmodified and modified chitosan (Table 3). The removal efficiency of modified chitosan was almost twice that of the unmodified chitosan. This was an indication that the modified chitosan possessed more surface area for adsorption.
Zhao et al. [111] fabricated a cost-effective, biomass-derived and novel adsorbent by coating polydopamine on lanthanum-chitosan hydrogel (La-CS@PDA) which is rich in amine groups for the adsorption of phosphate in wastewater. Phosphate adsorption was enhanced by the diffusion structure of the channel-network of La-CS@PDA,hence, the adsorption capacity was 195.3 mg g−1 which was superior to other phosphate adsorbent materials in literature. Nevertheless, in the presence of other competitive anions like Cl−, SO42−, HCO3−, NO3−, F− and HCrO4−, La-CS@PDA demonstrated distinct selective adsorption for phosphate due to the presence of selective binding sites of La species in the composite.
Phenols are among the most toxic contaminants in water. They are toxic to aqua species even at low concentrations, in addition to the risk of loss of taste and aroma. New alternative methods to remove phenols effectively from water are continuously sought and investigated. The classical approaches using the biological treatment and activated carbon adsorption reveal low efficiency, while the advanced phenol extraction/oxidation methods are satisfactory but costly [112]. Li et al. [113] examined chitosan derivatives by chemical modification using salicylaldehyde and ß-cyclodextrin polymer for phenol, p-nitrophenol and p-chlorophenol removal. The performance of chitosan for phenols removal is initially poor (1.98–2.58 mg g−1), while the modification strategies boost the removal to as high as 179.73 mg g−1. Table 3 generalizes the effects of chitosan modification on its texture and performance as an adsorbent for solutes removal.
7 Adsorption mechanisms
The adsorptive interactions of chitosan depend on pH, crystallinity, water affinity and deacetylation (amino group content) [114]. Adsorption could also govern by complex formation or electrostatic attraction in acid media, ion exchange with protonated amino groups and chelation at amino groups [115]. Chitosan chelates metals in solution five to six times due to the presence of free amino groups in the chitosan chain. This property is widely used for the uptake, separation or recovery of valuable metals and dyes for environmental purposes. The use of chitosan is somewhat limited in industrial applications due to the cost of materials, the variance of characteristics and the availability of resources, hence the need for its synthesis [16].
The adsorption properties of chitosan are due to its functional groups including improved hydrophilicity by polymer hydroxyl groups, which also helps to increase the diffusion of polymer networks and enables metals and dyes to be adsorbed from the aqueous solution. In several ways, hydroxyl and amino groups can react with aqua solutes. The amino groups are very important for adsorption processes as compared to the hydroxyl groups, for which the degree of deacetylation is an important parameter for assessing the quality of chitosan biopolymer [57]. Figures 10 and 11 illustrate the working mechanisms of methylene blue and chromium, respectively, by chitosan derivatives [116, 117].
Adsorption mechanism of methylene blue on chitosan beads [116]
Mechanisms of chromium ions removal by chitosan-magnetite nanocomposite strip [117]
Yet, certain challenges can occur in the chemical system, including the susceptibility of chitosan solubility in acidic solution, for which it is not desirable to withstand its insoluble texture [16]. Hence, cross-linking reactions may improve the stability of chitosan under acidic and alkaline conditions,however, this process can reduce the adsorption traits.
High nitrogen content in chitosan works as active sites for several chemical reactions in water. The amine groups in chitosan are weak enough to deprotonate water [57],
The pKa value of chitosan (6.3) increases its solution pH when in contact with water [57]. Chitosan adsorption is dependent on solution pH due to the direct effect of an acid–base reaction. The amine groups serve as a binding site for metals via chelation mechanisms in the deprotonated form of chitosan. Chitosan may also bear electrostatic properties for ion-exchange mechanisms to adsorb aqua metals [57].
8 Recovery of spent chitosan
Spent chitosan/chitosan derivatives are in most cases non-biodegradable and toxic after adsorption due to the deposit of adsorbates. The regeneration is carried out by chemical treatment with mineral acids (H2SO4, HCl and HNO3), complexing or chelating, agent (EDTA, EDTA-disodium (Na2EDTA)), alkaline (NaOH, NH4OH), salt (NaCl, KNO3’ Na2CO3, Na2SO4), organic acid (citric acid) solution and distilled water because of the simplicity, convenience, effectiveness and low cost of these desorption agents to restore the properties of chitosan materials [118,119,120,121]. Thermal and biological desorption methods for chitosan are not common because chitosan cannot withstand high temperature [122] and the ease of biodegradability due to accessibility for the microorganism in biological desorption [123].
Mineral acids are mostly used as desorption solvents for cationic pollutants because of the favourable adsorption nature in the basic environment of these cationic pollutants and the mechanism involved in their adsorption on chitosan and chitosan derivatives adsorbents. Therefore, in desorption, these mineral acids supply a high number of cation (H+) which weakens the interaction between the cationic pollutants and their adsorption groups. Meanwhile, the anions from these mineral acids form a complex with the cationic pollutants and are released into the solution [124]. Complexing or chelating agents like EDTA and EDTA-disodium (Na2EDTA) in desorption of cationic pollutants form complexes because of their high-affinity constant which replace the functional groups on the chitosan and its derivatives adsorbent complexed with ions and complexate with ions [125,126,127]. Alkali desorption agents are mostly used to desorb the anions in pollutants from chitosan and its derivative adsorbents due to the higher affinity of these anions to react with Na+ or NH4+ than the adsorption sites, thus weakening the adsorbate and adsorbent bonds in alkali conditions [128, 129]. Salt desorption agents are considered as suitable because of their ability to weaken the interaction between cationic pollutants and the binding sites on the chitosan and its derivatives surface in order to form complexes that are stable [130, 131].
The weak or strong adsorptive forces between the sorbent surface and the sorbate molecules in the regeneration media often determine the efficiency of desorption [95, 132]. Therefore, the adsorption of adsorbate after adsorbent regeneration depends on the efficiency of regeneration of adsorbents after adsorbate desorption. Deposited adsorbates are desorbed using a cheap and eco-friendly agent and the adsorbent regenerated for another cycle. Regeneration is important for keeping the adsorption process cost low [133, 134]. Desorption and regeneration of chitosan and its derivatives are found to increase with increased temperature, contact time and agitation speed [135, 136]. Other factors include the increased concentration of desorption and regeneration agents. However, an excessive increase in concentration could lead to the disintegration of the chitosan structure [135]. Meanwhile, the low mechanical and chemical stability of chitosan as well as its biodegradability are factors that affect chitosan-based adsorbent regeneration [52]. Figure 12 shows the schematic representation of the chitosan adsorbent regeneration procedure.
Schematic representation of adsorption–desorption in chitosan [52]
Desorption efficiencies and other parameters affecting the regeneration of chitosan and its derivatives adsorbents using various agents are summarized in Table 4. However, limited data from the literature on desorption agents and their operating conditions, such as adsorbate concentration, pH, temperature and eluent concentration has made it difficult to compare the performance of the eluents.
9 Conclusion and future prospects
The modification strategies of chitosan have greatly improved the properties of chitosan derivatives for competitive applications especially in water and wastewater treatment and nutrient adsorption/recovery. The existence of amino and hydroxyl groups creates the positions for surface modification either by chemical or physical means. Chitosan derivatives generally hold superior properties of hydrophilicity, mechanical strength and solubility resistance to acids, specific surface, porosity and adsorption. Chitosan also shows superior life cycle assessment from the viewpoint of environmental impact.
A large volume of works may have been published over the years on chitosan and its derivatives for various pollutants removal. Nonetheless, only few of these works clearly presented novel modifications, advantages and limitations, and life cycle assessment of the chitosan structure, while most are still focusing on routine studies by testing the modified adsorbent on different adsorbates. The potential of modifying chitosan is still large and yet to be unlocked and can be significantly developed in the future. Future quest for novel modifiers should ensure that modification strategies are carried out without altering the biodegradability, antimicrobial and antifungal properties and non-toxicity of the pristine chitosan. Secondly, future research areas should make efforts to employ natural resources as chitosan modifiers because of the safety and health concerns associated with end applications. This development will further promote the use of chitosan derivatives on a larger scale because of the impact it will have on bioprocessing, cosmetics and food industries, among others.
References
Pereda M, Amica G, Marcovich NE (2012) Development and characterization of edible chitosan/olive oil emulsion films. Carbohyd Polym 87(2):1318–1325
Ramasamy P, Shangmugam A (2015) Characterization and wound healing property of collagen-chitosan film from sepia kobiensis. Int J Biol Macromol 74:93–102
Hamed I, Özogul F, Regenstein JM (2016) Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Technol 48:40–50
Kaya M, Baran T, Asan-Ozusaglam M, Cakmak Y, Tozak K, Mol A et al (2015) Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol Bioprocess Eng 20(1):168–179
Torii Y, Ikeda H, Shimojoh M, Kurita K (2009) Chemoselective protection of chitosan by dichlorophthaloylation: preparation of a key intermediate for chemical modifications. Polym Bull 62(6):749–759
Marei NH, Abd El-Samie E, Salah T, Saad GR, Elwahy AH (2016) Isolation and characterization of chitosan from different local insects in Egypt. Int J Biol Macromol 82:871–877
Rodríguez-Núñez JR, López-Cervantes J, Sánchez-Machado DI, Ramírez-Wong B, Torres-Chavez P, Cortez-Rocha MO (2012) Antimicrobial activity of chitosan-based films against Salmonella typhimurium and Staphylococcus aureus. Int J Food Sci Technol 47(10):2127–2133
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res J 37:4311–4330
Kumar MNR (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Kurita K, Yoshida Y, Umemura T (2010) Finely selective protections and deprotections of multifunctional chitin and chitosan to synthesize key intermediates for regioselective chemical modifications. Carbohyd Polym 81(2):434–440
Muñoz I, Rodríguez C, Gillet D, Moerschbacher BM (2018) Life cycle assessment of chitosan production in India and Europe. Int J Life Cycle Assess 23:1151–1160
Santos VP, Marques NS, Maia PC, Lima MABD, Franco LDO, Campos-Takaki GMD (2020) Seafood waste as attractive source of chitin and chitosan production and their applications. Int J Mol Sci 21(12):4290
Alves NM, Mano JF (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol 43:401–414
Okolo PO, Akakuru OU, Osuoji OU, Jideonwo A (2013) Studies on the properties of chitosan-starch beads and their application as drug release materials. Bayero J Pure Appl Sci 6(1):118–126
Arbia W, Arbia L, Adour L, Amrane A (2013) Chitin extraction from crustacean shells using biological methods-a review. Food Technol Biotechnol J 51(1):12–25
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38(1):43–74
Muzzarelli RAA (2011) Potential of chitin/chitosan-bearing terials for uranium recovery: an interdisciplinary review. J Carbohyd Polym 84(1):54–63
Patil RS, Ghormade V, Deshpande MV (2000) Chitinolytic enzymes: an exploration. Enzyme Microb Technol J 26(7):473–483
Alaba PA, Oladoja NA, Sani YM, Ayodele OB, Mohammed IY, Olupinla SF, Daud WMW (2018) Insight into wastewater decontamination using polymeric adsorbents. J Environ Chem Eng 6:1651–1672
Crini G, Badot PM (2008) Application of chitosan, a natural amino polysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci J 33(4):399–447
El-hefian EA, Nasef MM, Yahaya AH (2011) Chitosan physical forms: a short review. Aust J Basic Appl Sci 5(5):670–677
Brasselet C, Pierre G, Dubessay P, Dols-Lafargue M, Coulon J, Maupeu J, ...., & Delattre C (2019) Modification of chitosan for the generation of functional derivatives. Appl Sci 9(7):1321
Sorlier P, Denuzière A, Viton C, Domard A (2001) Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromol J 2:765–772
Bhatt LR, Kim BM, Hyun K, Kwak GB, Lee CH et al (2011) Preparation and characterization of chitin benzoic acid esters. Molecules 16:3029–3036
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(7):603–632
Jang M, Kong B, Jeong Y, Hyung Lee C, Nah J (2004) Physicochemical characterization of a-chitin, ß-chitin, and γ-chitin separated from natural resources. J Polym Sci A Polym Chem 42:3423–3432
Madera-Santana TJ, Herrera-Méndez CH, Rodríguez-Núñez JR (2018) An overview of the chemical modifications of chitosan and their advantages. Green Materials 6(4):131–142
Lee ST, Mi FL, Shen YJ, Shyu SS (2001) Equilibrium and kinetic studies of copper (II) ion uptake by chitosan-tripolyphosphate chelating resin. J Polym 42(5):1879–1892
Mohammad HM (2016) Preparation and charactetrization of cross-linked chitosan beads for phosphate adsorption in aqueous solution. Master of Science thesis submitted to the department of chemistry, University of Saskatchewan, Saskatoon, SK (S7N 5C9), Canada
Patrulae V, Anamaria N, Manuela MM, Laura DP, Otilia BS, Vasile O (2013) Optimization of the removal of copper (II) ions from aqueous solution on chitosan and cross-linked chitosan beads. J Bioresour 8(1):1147–1165
de Farias BS, Junior TRSAC, de Almeida Pinto LA (2019) Chitosan-functionalized nanofibers: A comprehensive review on challenges and prospects for food applications. Int J Biol Macromol 123:210–220
Mucha M, Miskiewicz D, Pawlak A (2003) Chitosan and its mixtures Properties and applications. Przem Chem 82(8–9):1138–1142
Struszczyk MH (2002) Chitin and chitosan. Part II. Applications of chitosan. Polimery-Warsaw J 47(6):396–403
da Silva Alves DC, Healy B, Pinto LA, Cadaval TR, Breslin CB (2021) Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 26(3):594
Bailey SE, Olin TJ, Bricka M, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Resour J 33:2469–2479
Kyzas GZ, Dimitrios NB (2015) Recent modifications of chitosan for adsorption applications: a critical and systematic review. J Marine Drugs 13:312–337
Bhatnagar A, Sillanpää M (2009) Applications of chitin-and chitosan-derivatives for the detoxification of water and wastewater-a short review. Adv Colloid Interface Sci J 152(1):26–38
Liu L, Li Y, Li Y, Fang YE (2004) Rapid N-phthaloylation of chitosan by microwave irradiation. Carbohyd Polym 57(1):97–100
Wang J, Wang L, Yu H, Chen Y, Chen Q, Zhou W, ..., & Chen X (2016) Recent progress on synthesis, property and application of modified chitosan: an overview. Int J Biol Macromol 88:333-344
Kulkarni AD, Patel HM, Surana SJ, Vanjari YH, Belgamwar VS, Pardeshi CV (2017) N, N, N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohyd Polym 157:875–902
LogithKumar R, KeshavNarayan A, Dhivya S, Chawla A, Saravanan S, Selvamurugan N (2016) A review of chitosan and its derivatives in bone tissue engineering. Carbohyd Polym 151:172–188
Seedevi P, Moovendhan M, Vairamani S, Shanmugam A (2017) Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. Int J Biol Macromol 99:519–529
Campelo CS, Chevallier P, Vaz JM, Vieira RS, Mantovani D (2017) Sulfonated chitosan and dopamine based coatings for metallic implants in contact with blood. Mater Sci Eng, C 72:682–691
Galhoum AA, Mahfouz MG, Gomaa NM, Vincent T, Guibal E (2017) Chemical modifications of chitosan nano-based magnetic particles for enhanced uranyl sorption. Hydrometallurgy 168:127–134
Vakili M, Rafatullah M, Ibrahim MH, Abdullah AZ, Gholami Z, Salamatinia B (2017) Enhancing reactive blue 4 adsorption through chemical modification of chitosan with hexadecylamine and 3-aminopropyl triethoxysilane. J Water Process Eng 15:49–54
Correa-Murrieta MA, López-Cervantes J, Sánchez-Machado DI, Sánchez-Duarte RG, Rodríguez-Núñez JR, Núñez-Gastélum JA (2012) Fe (II) and Fe (III) adsorption by chitosan-tripolyphosphate beads: kinetic and equilibrium studies. J Water Supply Res Technol Aqua 61(6):331–341
Kousalya GN, Gandhi MR, Meenakshi S (2010) Sorption of chromium (VI) using modified forms of chitosan beads. Int J Biol Macromol 47(2):308–315
Sánchez-Duarte RG, López-Cervantes J, Sánchez-Machado DI, Correa-Murrieta MA, Núñez-Gastélum JA, & Rodríguez-Núñez JR (2016) Chitosan-based adsorbents gels for the removal of tris-azo dye: isotherms and kinetics studies. Environ Eng Manag J 15(11)
Nwagu TN, Okolo B, Aoyagi H, Yoshida S (2017) Chemical modification with phthalic anhydride and chitosan: viable options for the stabilization of raw starch digesting amylase from Aspergillus carbonarius. Int J Biol Macromol 99:641–647
O’Brien AM, Smith AT, Ó’Fágáin C (2003) Effects of phthalic anhydride modification on horseradish peroxidase stability and activity. Biotechnol Bioeng 81(2):233–240
Pokhrel S, Paras NY, Rameshwar A (2015) Applications of chitin and chitosan in industry and medical science: a review. Nepal J Sci Technol 16(1):99–104
Vakili M, Shubo D, Giovanni C, Wei W, Pingping M, Dengchao L, Gang Y (2019) Regeneration of chitosan-based adsorbents used in heavy metal adsorption: a review. J Sep Purif Technol 224:373–387
Zahrim AY, Asis T, Hashim MA, Al-Mizi TMTMA, & Ravindra P (2015) A review on the empty fruit bunch composting: life cycle analysis and the effect of amendment(s). Advances in bioprocess technology (pp. 1–13)
Beach ES, Eckelman MJ, Cui Z, Brentner L, Zimmerman JB (2012) Preferential technological and life cycle environmental performance of chitosan flocculation for harvesting of the green algae Neochloris oleoabundans. Biores Technol 121:445–449
Anand M, Kalaivani R, Maruthupandy M, Kumaraguru AK, Suresh S (2014) Extraction and characterization of chitosan from marine crab and squilla collected from the gulf of Mannar region, South India. J Chitin Chitosan Sci 2:1–8
Rajasree R, Rahate KP (2013) An overview on various modifications of chitosan and its applications. Int J Pharm Sci Res 4(11):4157–4193
Crini G (2005) Recent developments in polysaccharide-base materials used as adsorbents in wastewater treatment. Prog Polym Sci J 30(1):38–70
Rasweefali MK, Sabu S, Sunooj KV, Sasidharan A, Xavier KM (2021) Consequences of chemical deacetylation on physicochemical, structural and functional characteristics of chitosan extracted from deep-sea mud shrimp. Carbohyd Polym Technol Appl 2:100032
Hao G, Hu Y, Shi L, Chen J, Cui A, Weng W, Osako K (2021) Physicochemical characteristics of chitosan from swimming crab (Portunus trituberculatus) shells prepared by subcritical water pretreatment. Sci Rep 11(1):1–9
Ishtiyak Q, Chhipa RC (2017) Synthesis, characterization and evaluation of adsorption properties of activated carbon obtained from neem leaves (azadirachta indica). Orient J Chem 33(4):2095–2102
Pandey VP, Rani J, Jaiswal N, Singh S, Awasthi M, Shasany AK et al (2017) Chitosan immobilized novel peroxidase from Azadirachta indica: characterization and application. Int J Biol Macromol 104:1713–1720
Asokogene OF, Zaini MAA, Idris MM, Abdulsalam S, Usman AEN (2019) Physicochemical properties of oxalic acid-modified chitosan/neem leave composites from Pessu river crab shell. Int J Chem Reactor Eng 17(9):1–12
Suneeta K, Kumar SH, Sahoo A, Pradip Kumar R (2017) Physicochemical properties and characterization of chitosan synthesized from fish, crab and shrimp shells. Int J Biol Macromol 104:1697–1705
Mohanasrinivasan V, Mishra M, Paliwal J, Singh S, Selvarajan E, Suganthi V et al (2014) Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. J Biotechnol 4(2):167–175
Tarafdar A, Biswas G (2013) Extraction of chitosan from prawn shell wastes and examination of its viable commercial applications. Int J Theor Appl Res Mech Eng 2:17–24
Puvvada YS, Vankayalapati S, Sukhavasi S (2012) Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Int Curr Pharm J 1(9):258–263
Sakthivel D, Vijayakumar N, Anandan V (2015) Extraction of chitin and chitosan from Mangrove crab (Sesarmaplicatum) from Thengaithittu estuary Pondicherry Southeast coast of India. Int J Pharm Pharm Res 4(1):12–24
Satpathy AA, Dash S, Das SK, Shyamasuta S, Pradhan S (2021) Functional and bioactive properties of chitosan from Indian major carp scale. Aquacult Int 29(2):417–430
Abdullin VF, Shipovskaya AB, Fomina VI, Artemenko SE, Ovchinnikova GP, Pchelintseva EV (2008) Physicochemical properties of chitosan from different raw material sources. J Fibre Chem 40(1):40–44
Kumaresapillai N, Basha RA, Sathish R (2011) Production and evaluation of chitosan from Aspergillus niger MTCC strains. Iran J Pharm Res 10(3):553–558
Kaur K, Dattajirao V, Shrivastava V, & Bhardwaj U (2012) Isolation and characterization of chitosan-producing bacteria from beaches of Chennai, India. Enzyme Res 1–6
da Silva Lucas AJ, Oreste EQ, Costa HLG, López HM, Saad CDM, Prentice C (2021) Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem 343:128550
Aridi AS, Yusof YA, Chin NL, Ishak NA, Yusof NA, & Manaf YN (2021) Physicochemical properties of chitosan extracted from Leucaena leucocephala pods using deprotenization and decolorization steps. In IOP Conference Series: Earth and Environmental Science (Vol. 709, No. 1, p. 012038). IOP Publishing
Mustafa A, Cadar E, Sirbu R (2015) Pharmaceutical uses of chitosan in the medical field. Eur J Interdiscip Stud 1(3):35–40
Akakuru OU, Louis H, Amos PI, Akakuru OC, Nosike EI (2018) The chemistry of chitin and chitosan justifying their nanomedical utilities. Biochem Pharmacol (Los Angel) 7(241):2167–2501
Ruiz GAM, & Corrales HFZ (2017) Chitosan, chitosan derivatives and their biomedical applications. Biological Activities and Application of Marine Polysaccharides 87
Morin-Crini N, Lichtfouse E, Torri G, & Crini G (2019) Fundamentals and applications of chitosan. In Sustainable Agriculture Reviews 35 (pp. 49–123). Cham: Springer
Harish PKV, Tharanathan RN (2007) Chitin/chitosan: modifications and their unlimited applications potential–an overview. Trends Food Sci Technol 18:117–131
Repo E, Warchol JK, Kurniawan TA, Sillanpää ME (2010) Adsorption of Co (II) and Ni (II) by EDTA-and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chem Eng J 161(1–2):73–82
Nagib S, Inoue K, Yamaguchi T, Tamaru T (1999) Recovery of Ni from a large excess of Al generated from spent hydrodesulfurization catalyst using picolylamine type chelating resin and complexane types of chemically modified chitosan. Hydrometallurgy 51(1):73–85
Hariani PL, Fatma F, Riyanti F, Ratnasari H (2015) Adsorption of phenol pollutants from aqueous solution using Ca-Bentonite/chitosan composite. J Manusia dan Lingkungan 22(2):233–239
Moosa AA, Ridha AM, Kadhim NA (2016) Use of biocomposite adsorbents for the removal of methylene blue dye from aqueous solution. Am J Mater Sci 6(5):135–146
Saifuddin MN, Kumaran P (2005) Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron J Biotechnol 8(1):43–53
Okoya AA, Akinyele AB, Ifeanyi E, Amuda OS, Alayande OS, Makinde OW (2014) Adsorption of heavy metal ions onto chitosan grafted cocoa husk char. Afr J Pure Appl Chem 8(10):147–161
Kyzas GZ, Deliyanni EA (2013) Mercury (II) removal with modified magnetic chitosan adsorbents. Molecules 18(6):6193–6214
Vafakish B, Wilson LD (2019) Surface-modified chitosan: an adsorption study of a “Tweezer-like” biopolymer with fluorescein. Surfaces 2(3):468–484
Al-Ghamdi YO, Alamry KA, Hussein MA, Marwani HM, Asiri AM (2019) Sulfone-modified chitosan as selective adsorbent for the extraction of toxic Hg (II) metal ions. Adsorpt Sci Technol 37(1–2):139–159
Hastuti B, Masykur A, Hadi S (2016) Modification of chitosan by swelling and crosslinking using epichlorohydrin as heavy metal Cr (VI) adsorbent in batik industry wastes. Mater Sci Eng 107(012020):1–10
Soni U, Bajpai J, Singh SK, Bajpai AK (2017) Evaluation of chitosan-carbon based biocomposite for efficient removal of phenols from aqueous solutions. J Water Process Eng 16:56–63
Liu Q, Yang B, Zhang L, Huang R (2014) Simultaneous adsorption of phenol and Cu2+ from aqueous solution by activated carbon/chitosan composite. Korean J Chem Eng 31(9):1608–1615
Marrakchi F, Khanday WA, Asif M, Hameed BH (2016) Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int J Biol Macromol 93:1231–1239
Regunton PCV, Sumalapao DEP, Villarante NR (2018) Biosorption of methylene blue from aqueous solution by coconut (Cocos nucifera) shell-derived activated carbon-chitosan composite. Orient J Chem 34(1):115
Huang R, Yang B, Liu Q, Liu Y (2014) Multifunctional activated carbon/chitosan composite preparation and its simultaneous adsorption of phenol and Cr (VI) from aqueous solutions. Environ Prog Sustain Energy 33(3):814–823
López-Cervantes J, Sánchez-Machado DI, Sánchez-Duarte RG, Correa-Murrieta MA (2018) Study of a fixed-bed column in the adsorption of an azo dye from an aqueous medium using a chitosan–glutaraldehyde biosorbent. Adsorpt Sci Technol 36(1–2):215–232
Auta M, Hameed BH (2013) Coalesced chitosan activated carbon composite for batch and fixed-bed adsorption of cationic and anionic dyes. Colloids Surf B 105:199–206
Jhaveri J, Raichura Z, Khan T, Momin M, Omri A (2021) Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules 26(2):272
Abhinaya M, Parthiban R, Kumar PS, & Vo DVN (2021) A review on cleaner strategies for extraction of chitosan and its application in toxic pollutant removal. Environ Res 110996
Begum S, Yuhana NY, Saleh NM, Kamarudin NN, & Sulong AB (2021) Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohyd Polym 117613
Sharififard H, Nabavinia M, Soleimani M (2017) Evaluation of adsorption efficiency of activated carbon/chitosan composite for removal of Cr (VI) and Cd (II) from single and bi-solute dilute solution. Adv Environ Technol 2(4):215–227
Bahador F, Foroutan R, Esmaeili H, Ramavandi B (2021) Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohyd Polym 251:117085
Haseena PV, Padmavathy KS, Krishnan PR, Madhu G (2016) Adsorption of ammonium nitrogen from aqueous systems using chitosan-bentonite film composite. Procedia Technol 24:733–740
Soares SF, Amorim CO, Amaral JS, Trindade T, Daniel-da-Silva AL (2021) On the efficient removal, regeneration and reuse of quaternary chitosan magnetite nanosorbents for glyphosate herbicide in water. J Environ Chem Eng 9(3):105189
Cheung WH, Szeto YS, McKay G (2009) Enhancing the adsorption capacities of acid dyes by chitosan nano particles. Biores Technol 100(3):1143–1148
Carvalho VV, Gonçalves JO, Silva A, Cadaval TR Jr, Pinto LA, Lopes TJ (2019) Separation of anthocyanins extracted from red cabbage by adsorption onto chitosan films. Int J Biol Macromol 131:905–911
Pinheiro CP, Moreira LM, Alves SS, Cadaval TR Jr, Pinto LA (2021) Anthocyanins concentration by adsorption onto chitosan and alginate beads: Isotherms, kinetics and thermodynamics parameters. Int J Biol Macromol 166:934–939
Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J Hazard Mater 164(2):1012–1018
Jaafari K, Elmaleh S, Coma J, Benkhouja K (2001) Equilibrium and kinetics of nitrate removal by protonated cross-linked chitosan. Water SA J 27(1):9–14
Viswanathan N, Sundaram CS, Meenakshi S (2009) Removal of fluoride from aqueous solution using protonated chitosan beads. J Hazard Mater 161(1):423–430
Affonso LN, Marques Jr JL, Lima VV, Gonçalves JO, Barbosa SC, Primel EG, ..., & Cadaval Jr TR (2020) Removal of fluoride from fertilizer industry effluent using carbon nanotubes stabilized in chitosan sponge. J Hazard Mater 388:122042
Safie NN, Zahrim Yaser A, Hilal N (2020) Ammonium ion removal using activated zeolite and chitosan. Asia-Pac J Chem Eng 15(3):e2448
Zhao Y, Guo L, Shen W, An Q, Xiao Z, Wang H, ..., & Li Z (2020) Function integrated chitosan-based beads with throughout sorption sites and inherent diffusion network for efficient phosphate removal. Carbohyd Polym 230:115639
Jadhav DN, Vanjara AK (2004) Removal of phenol from wastewater using sawdust, polymerized sawdust and sawdust carbon. Indian J Chem Technol 1:35–41
Li JM, Meng XG, Hu CW, Du J (2009) Adsorption of phenol, p-chlorophenol and p-nitrophenol onto functional chitosan. Bioresour Technol J 100(3):1168–1173
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohyd Polym 55(1):77–93
Agarwal A, Vaishali A (2017) Chitosan based adsorbent: a remedy to handle industrial waste water. Int J Eng Sci 6:34–49
Biswas S, Taslim UR, Tonmoy D, Papia H, Mohammed MR (2020) Application of chitosan-clay biocomposite beads for removal of heavy metal and dye from industrial effluent. J Compos Sci 4(16):1–14
Thakre D, Jagtap S, Sakhare N, Labhsetwar N, Meshram S, Rayalu S (2010) Chitosan based mesoporous Ti–Al binary metal oxide supported beads for defluoridation of water. Chem Eng J 158(2):315–324
Gautam RK, & Chattopadhyaya MC (Eds) (2016) Advanced nanomaterials for wastewater remediation. CRC press
Gupta VK, Nayak A, Agarwal S (2015) Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ Eng Res 20(1):1–18
Shah IK, Pre P, Alappat BJ (2013) Steam regeneration of adsorbents: an experimental and technical review. Chem Sci Trans 2(4):1078–1088
Shahadat M, Isamil S (2018) Regeneration performance of clay-based adsorbents for the removal of industrial dyes: a review. RSC Adv 8(43):24571–24587
Diab MA, El-Sonbati AZ, Bader DMD (2011) Thermal stability and degradation of chitosan modified by benzophenone. Spectrochim Acta Part A Mol Biomol Spectrosc 79(5):1057–1062
Stoleru E, Hitruc EG, Vasile C, Oprica L (2017) Biodegradation of poly(lactic acid)/chitosan stratified composites in presence of the phanerochaete chrysosporium fungus. Polym Degrad Stab 143:118–129
Kim KJ, Kim DH, Yoo JC, Baek K (2011) Electrokinetic extraction of heavy metals from dredged marine sediment. Sep Purif Technol 79(2):164–169
Ngah WSW, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manage 91:958–969
Oviedo C, Rodríguez J (2003) EDTA: the chelating agent under environmental scrutiny. Quim Nova 26(6):901–905
Cui H, Chen J, Yang H, Wang W, Liu Y, Zou D, Liu W, Men G (2013) Preparation and application of Aliquat 336 functionalized chitosan adsorbent for the removal of Pb(II). Chem Eng J 232:372–379
Padilla-Rodríguez A, Perales-Pérez O, Román-Velázquez FR (2014) Removal of As(III) and As(V) Oxyanions from aqueous solutions by using chitosan beads with immobilized iron(III). Int J Hazard Mater 2:7–17
Sankararamakrishnan N, Dixit A, Iyengar L, Sanghi R (2006) Removal of hexavalent chromium using a novel cross linked xanthated chitosan. Biores Technol 97(18):2377–2382
Futalan CM, Tsai WC, Lin SS, Hsien KJ, Dalida ML, Wan MW (2012) Copper, nikel and lead adsorption from aqueous solution using chitosan-immobilized on bentonite in ternary system. Sustain Environ Res 22(6):345–355
Heidari A, Younesi H, Mehraban Z, Heikkinen H (2013) Selective adsorption of Pb(II), Cd(II), and Ni(II) ions from aqueous solution using chitosan–MAA nanoparticles. Int J Biol Macromol 61:251–263
Kausar A, Naeem K, Hussain T, Bhatti HN, Jubeen F, Nazir A, Iqbal M (2019) Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: comparison of linear and non-linear regression methods. J Market Res 8(1):1161–1174
Enos WW, Gerald KM, Paul MS, Karanja J, Wa T (2009) Kinetics of copper desorption from regenerated spent bleaching earth. Am-Eur J Sci Res 4:317–323
Wankasi D, Jnr MH, & Spiff AI (2005) Desorption of Pb2+ and Cu2+ from Nipa palm (Nypa fruticans Wurmb) biomass. Afr J Biotechnol 4(9)
Bhuvaneshwari S, Sruthi D, Sivasubramanian V, Kanthimathy K (2012) Regeneration of chitosan after heavy metal sorption. J Sci Ind Res 71:266–269
Sharma P, Shrivastava A (2010) Sorptionand desorption of Eu(III), Sr(II) and Sm(II) on the synthetic analogue of muscovite. Int J Adv Eng Sci Appl Math 1:212–219
Wan MW, Kan CC, Rogel BD, Dalida MLP (2010) Adsorption of copper(II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohyd Polymer 80:891–899
Osifo PO, Neomagus HWJP, Everson RC, Webster A, Gun MA (2009) The adsorption of copper in a packed-bed of chitosan beads: modeling, multiple adsorption and regeneration. J Hazard Mater 167:1242–1245
Ngah WW, Endud C, Mayanar R (2002) Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. Reactive Functional Polymer 50:181–190
Rangel-Mendez JR, Monroy-Zepeda R, Leyva-Ramos E, Diaz-Flores PE, Shirai K (2009) Chitosan selectivity for removing cadmium (II), copper (II), and lead (II) from aqueous phase: pH and organic matter effect. J Hazard Mater 162(1):503–511
Zhao F, Repo E, Yin D, Sillanpää MET (2013) Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: kinetics and isotherms. J Colloid Interface Sci 409:174–182
Boyaci E, Eroglu AE, Shahwan T (2010) Sorption of As(V) from water using chitosan and chitosan-immobilized sodium silicate prior to atomic spectrometric determination. Talanta 80:1452–1460
Zhou YT, Nie HL, Branford-White C, He ZY, Zhu LM (2009) Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with a-ketoglutaric acid. J Colloid Interface Sci 330:29–37
Kahu SS, Shekhawat A, Saravanan D, Jugade RM (2016) Two fold modified chitosan for enhanced adsorption of hexavalent chromium from simulated wastewater and industrial effluents. Carbohyd Polym 146:264–273
Stopa LCB, Yamaura M (2010) Uranium removal by chitosan impregnated with magnetite nanoparticles: adsorption and desorption. Int J Nucl Energy Sci Technol 5(4):283–289
Ahamed M, Marjanovic J, Mbianda X (2016) Statistical optimization, kinetic and isotherm studies on selective adsorption of silver and gold cyanocomplexes using aminoguanidyl-chitosan imprinted polymers. J Adv Chem Eng 6:1–11
Acknowledgements
This work was supported by the Tertiary Education Trust Fund (TETFund) of Nigeria through an Academic Staff Training and Development (AST&D) grant and UTM-Iconic Grant No.09G54.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Francis, A.O., Zaini, M.A.A., Muhammad, I.M. et al. Physicochemical modification of chitosan adsorbent: a perspective. Biomass Conv. Bioref. 13, 5557–5575 (2023). https://doi.org/10.1007/s13399-021-01599-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01599-3