Abstract
Purpose
Benign insulinomas are the most prevalent cause of endogenous hyperinsulinaemic hypoglycaemia (EHH) in adults, and because of their small size are difficult to localise. The purpose of the study was to test the diagnostic accuracy and clinical impact of glucagon-like peptide-1 receptor (GLP-1R) PET/CT using 68Ga-DOTA-exendin-4 in consecutive adult patients referred for localisation of insulinomas. The results were compared with 111In-DOTA-exendin-4 SPECT/CT, study-MRI and previously performed external CT and/or MRI (prior external CT/MRI).
Methods
We prospectively enrolled patients with neuroglycopenic symptoms due to EHH. GLP-1R PET/CT, SPECT/CT and study-MRI were performed in a randomised, crossover order within 3–4 days. The reference standard was surgery with histology and treatment outcome.
Results
From January 2014 until March 2017, 52 patients were recruited. All imaging and invasive procedures before recruitment identified suspicious lesions in 46.2% of patients. GLP-1R PET/CT, SPECT/CT and study-MRI detected suspicious lesions in 78.8%, 63.5% and 63.4% of patients, respectively. In 38 patients, conclusive histology was available for final analysis.
Accuracy (95% confidence interval) for PET/CT, SPECT/CT, study-MRI and prior external CT/MRI was 93.9% (87.8–97.5%), 67.5% (58.1–76.0%), 67.6% (58.0–76.1%) and 40.0% (23.9–57.9%), respectively (all P values < 0.01, except comparison of SPECT/CT and study-MRI with a P value = 1.0). Impact on clinical management was 42.3%, 32.7% and 33.3% for PET/CT, SPECT/CT and study-MRI, respectively. Percentage reading agreement was 89.5%, 75.7%, and 71.1% for PET/CT, SPECT/CT and study-MRI, respectively.
Conclusion
68Ga-DOTA-exendin-4 PET/CT performed significantly better than 111In-DOTA-exendin-4 SPECT/CT and MRI in the localisation of benign insulinomas and should be considered in patients where localisation fails with CT/MRI (ClinicalTrials.gov, NCT02127541).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benign insulinomas are neuroendocrine tumours (NET) usually located in the pancreas. They are the most prevalent cause of endogenous hyperinsulinaemic hypoglycaemia (EHH) in adult patients [1]. At present, surgery remains the only curative treatment. Pancreas-preserving surgery such as limited segmental resection or enucleation is considered the treatment of choice [1,2,3]. Therefore, the exact preoperative localisation of insulinomas is critical in order to plan surgical strategy and improve postoperative outcome.
The small size of insulinomas (usually ≤2 cm) [4] challenges the detectability by conventional imaging techniques such as contrast-enhanced computed tomography (CT), contrast-enhanced magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS). The selective arterial calcium stimulation and venous sampling (ASVS) approach exhibits high sensitivity [5] but detects only the vascular bed of the insulinoma, not the insulinoma itself, and can be associated with relevant risk for complications [5,6,7]. A recent systematic review of 2379 cases reported mean sensitivity of 85%, 76%, 58% and 54% in accurate detection of insulinomas by ASVS, EUS, MRI and CT, respectively [8], indicating that there is still an unmet need for a more sensitive non-invasive tool.
In vitro studies using autoradiography have shown that almost all benign insulinomas express glucagon-like peptide-1 receptors (GLP-1R) at high density [9]. The GLP-1R is a G protein-coupled peptide hormone receptor expressed mainly in the alimentary tract, particularly in the pancreatic islet cells, where it mediates the release of glucagon-like peptide-1 (GLP-1) from the small intestine in response to food intake. An insulinoma consists mainly of islet cells. Previously, we and others have shown that targeting GLP-1R using the specific ligands 111In-DOTA-exendin-4 [10], 111In-DTPA-exendin-4 [11] or 99mTc-HYNIC-exendin-4 [12] as radiotracers for single-photon emission computed tomography (SPECT) is a very sensitive, non-invasive method to localise benign insulinomas. However, all of the aforementioned radiotracers are exclusively SPECT tracers, with limitations in comparison to positron emission tomography (PET). PET possesses higher sensitivity and spatial resolution than SPECT, the radiation exposure is lower than SPECT with 111In-labelled compounds, and accurate quantification of radiotracers is better established with PET than with SPECT [13, 14].
In a proof-of-principle study, GLP-1R PET and SPECT were compared in five patients with EHH after the injection of 68Ga-DOTA-exendin-4 ([Nle14,Lys40(Ahx-DOTA-68Ga)NH2]exendin-4) and 111In-DOTA-exendin-4 ([Nle14,Lys40(Ahx-DOTA-111In)NH2]exendin-4), with excellent image quality for the PET modality [15], consistent with a recent clinical study using 68Ga-NOTA-exendin-4 [16].
At present, GLP-1R PET has not been compared with either GLP-1R SPECT or contrast-enhanced MRI to determine the most sensitive non-invasive morphological imaging modality [8, 17].
We therefore tested the diagnostic accuracy and clinical impact of 68Ga-DOTA-exendin-4 PET/CT in a multi-institutional series of consecutive adult patients referred for localisation of insulinomas. The results are compared with 111In-DOTA-exendin-4 SPECT/CT, study 3-Tesla MRI and previously performed external CT and/or MRI (prior external CT/MRI) according to the Standard for the Reporting of Diagnostic Accuracy (STARD) guidelines (Supplementary Table 1).
Materials and methods
Study design and patients
For this prospective, single-centre, crossover imaging study, 52 consecutive patients (Table 1 and Fig. 1) were recruited from different centres in Europe and the United States between January 2014 and March 2017 and referred to the University Hospital Basel (ClinicalTrials.gov, NCT02127541). Inclusion criteria were biochemically proven EHH with neuroglycopenic symptoms, a positive Whipple triad defined as (1) attacks of fainting, dizziness and sweating on fasting, (2) hypoglycaemia present during attacks, and (3) relief of symptoms after administration of carbohydrates and negative results on sulfonylurea. Exclusion criteria were evidence of a malignant insulinoma on conventional imaging, pregnancy or breastfeeding in women, and renal insufficiency (serum creatinine >140 μmol/L).
Procedures
Imaging and invasive procedures before recruitment included prior external CT/MRI, EUS with or without biopsy, somatostatin receptor imaging (68Ga-DOTATOC PET/CT or OctreoScan®), 18F-DOPA (18F-fluorodopa) PET/CT, ASVS and/or surgery using intraoperative ultrasound as locally available. Prior external CT/MRI was performed by the referring centres not more than 2 months before the beginning of the study.
Patients received one 68Ga-DOTA-exendin-4 PET/CT and two 111In-DOTA-exendin-4 SPECT/CT scans (4 and 72 h scans) in a randomised crossover order within 3–4 days. A standardised study-MRI scan was performed between PET/CT and SPECT/CT scans. The reference standard was successful surgery with histological evaluation and treatment outcome (monitoring glucose levels for at least 4 weeks after surgery) in all patients.
Detailed information about synthesis and labelling of 68Ga-DOTA-exendin-4 and 111In-DOTA-exendin-4, co-administration of glucose infusion, and image acquisition of standardised PET/CT, SPECT/CT and study-MRI, as well as prior external CT/MRI, are summarised in the Supplementary Material. Adverse events were recorded and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 protocol.
Evaluation
In order to localise insulinomas in a standardised manner, the pancreas was categorised into three regions, namely head, body and tail with the portal vein and the superior mesenteric artery serving as anatomic landmarks (Supplementary Fig. 1).
PET/CT, SPECT/CT and study-MRI scans were randomised and independently assessed by three board-certified nuclear medicine physicians (GN, CR, FK) for PET/CT and SPECT/CT scans or three board-certified radiologists (EM, CZ, DB) for MRI each with >10 years of experience in PET/CT and SPECT/CT or MRI reading. All readers were unaware of the patients’ identity, other imaging results or the patient’s clinical history. A non-blinded nuclear medicine physician measured tracer uptake in the tumour and normal pancreas parenchyma (background) as well as the kidneys by drawing volumes of interest and measuring maximal standardised uptake values (SUV) in attenuation- and scatter-corrected PET images or count statistics in 4-h and 72-h attenuation- and scatter-corrected SPECT images. Tumour size was derived by measurements on the T1-weighted MRI images by a non-blinded radiologist and by the surgeons/pathologists.
The impact on clinical management was defined as follows: identifying patients with negative or inconclusive finding on all imaging and invasive procedures before recruitment (prior external CT/MRI, EUS with or without biopsy, somatostatin receptor imaging, 18F-DOPA PET/CT, ASVS and surgery as locally available) and positive findings in the standardised prospective investigations (PET/CT, SPECT/CT and study-MRI) that allowed surgery planning/image-guided surgery.
Histopathologic diagnosis was made at the local referring institution where surgery was performed. The pathologists were blinded to the results of other diagnostic tests but were aware of the patient’s clinical history. In the case of controversial findings, central histological reading was available at the tertiary institution (Institute of Pathology, University Bern).
Statistical analysis
The number of detected lesions (% of total) reflects the average of the three readers’ results. Only tests that showed consistency between imaging, surgery and histological analysis (positive for insulinoma or nesidioblastosis) were considered as true positives. In patients with more than one lesion, the imaging test was regarded as true-positive if at least one insulinoma was correctly localised (per-patient analysis).
As measures of diagnostic accuracy for each method, sensitivity, overall accuracy and positive predictive value (PPV) were estimated with exact binomial 95% confidence intervals. Except for single readings of prior external CT/MRI, estimates were first derived for each reader separately and then averaged for all readers per method, using the R add-on package epiR (Supplemental Material, Supplementary Table 2). Pairwise comparisons of diagnostic measures were made between 68Ga-DOTA-exendin-4 PET/CT and 111In-DOTA-exendin-4 SPECT/CT, and between study-MRI and each of the other methods. For each comparison, a separate unconditional mixed-effects logistic regression model was fitted, including rater as random effect. P values for the corresponding main effect (method) are reported.
Interrater reliability is indicated as the percentage agreement for three blinded readers. This measure is not corrected for chance agreement. Nevertheless, it was chosen since Fleiss’ kappa—which would have been the chance-corrected measure of choice—can reach paradoxically low values when the observed proportions, e.g. sensitivity, are very high (the so-called paradox of kappa statistics) [18]. All analyses were conducted using R version 3.3.3 statistical software, using two-sided statistical tests and a significance level, α, of 0.05. No adjustment for multiple testing was made.
There were no clinical GLP-1R PET/CT data available at the start of this study. In this situation, sample size calculations can only be based on crude estimates. It was therefore decided that the results of 40 operated patients should deliver a meaningful statement for a first larger clinical evaluation of 68Ga-DOTA-exendin-4 PET/CT. The choice of this sample size was based on a feasibility rationale and experience.
Results
Imaging and invasive procedures before recruitment
Baseline characteristics of all 52 participants as well as the results of all imaging and invasive procedures before recruitment are summarised in Table 1 and Supplementary Table 3. Forty-nine of 52 patients underwent prior external CT/MRI, in which 14 patients (29%) had one or more suspicious lesions compatible with an insulinoma.
PET/CT, SPECT/CT and study-MRI
All 52 patients underwent 68Ga-DOTA-exendin-4 PET/CT and 111In-DOTA-exendin-4 SPECT/CT. study-MRI was performed in 51/52 patients (Fig. 1). Patient 43 did not receive study-MRI due to pacemaker implantation. 68Ga-DOTA-exendin-4 PET/CT, 111In-DOTA-exendin-4 SPECT/CT and study-MRI identified suspicious lesions in 78.8%, 63.5% and 63.4% of patients (average reading), respectively.
The highest radiotracer (68Ga-/111In-DOTA-exendin-4) uptake was noted in tumours and kidneys (Figs. 2 and 3). Median (interquartile range) tumour-to-background ratios were 3.6 (2.3–5.8) for 68Ga-DOTA-exendin-4 PET and 2.2 (1.5–3.2) for 111In-DOTA-exendin-4 SPECT. Blood sampling of 68Ga-DOTA-exendin-4 revealed a biexponential blood clearance, with a half-time of 12.2 ± 1.9 min and 41 ± 4.9 min. 111In-DOTA-exendin-4 also revealed a biexponential blood clearance with a half-time of 13.6 ± 2.6 min and 118 ± 25.4 min (data are mean ± SD). Both compounds showed a plasma clearance of about 50% in the α-phase. The clearance occurred exclusively via the kidneys.
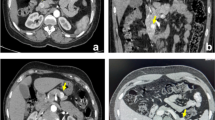
Patient 48 with EHH and negative prior external MRI. Transaxial PET/CT 2.5 h after injection of 68Ga-DOTA-exendin-4 (a), transaxial SPECT/CT 72 h after injection of 111In-DOTA-exendin-4 (b), T1-weighted contrast-enhanced transaxial study-MRI (c) and T2-weighted transaxial study-MRI (d). 68Ga-DOTA-exendin-4 PET/CT (a) shows a clear focal uptake in the transition zone between pancreatic head and body (see arrow). 111In-DOTA-exendin-4 SPECT/CT (b) and study-MRI (c, d) were interpreted as negative scans by all readers. Based on PET/CT findings, surgery was performed, and histological evaluation confirmed the PET/CT reading, which was correctly interpreted by all readers: benign insulinoma of 8 mm
Patient 26 with EHH but without evidence of an insulinoma in the prior external scans (MRI, 18F-DOPA PET/CT). Study transaxial contrast-enhanced T1-weighted MRI (a), transaxial PET/CT 2.5 h after injection of 68Ga-DOTA-exendin-4 (b), transaxial SPECT/CT 4 h (c) and 72 h (d) after injection of 111In-DOTA-exendin-4. study-MRI (a) and 4-h SPECT/CT (c) were interpreted as negative scans by several readers. PET/CT (b) shows a clear focal uptake in the head of the pancreas and the 72-h SPECT/CT (d) shows a more diffuse but more intense uptake at the same location than the 4-h SPECT/CT (c) (see arrows). Based on PET/CT findings, surgery was performed and histological evaluation confirmed a benign insulinoma measuring 9 mm
Nausea and sporadic vomiting are known side effects of exendin-4 radiotracers. Twenty-seven percent (14/52) of patients experienced nausea and 2% (1/52) of patients experienced vomiting after injection of 68Ga-DOTA-exendin-4. Fifty-two percent (27/52) of patients experienced nausea and 44% (23/52) of patients experienced vomiting after injection of 111In-DOTA-exendin-4. In this study, these side-effects were grade 1 according to CTCAE 4.03, confined to the first hour after injection and more pronounced with 111In-DOTA-exendin-4 in comparison to 68Ga-DOTA-exendin-4. No other adverse effects were observed. No severe hypoglycaemic episode occurred after injection of 11.6–23.8 μg 68Ga-DOTA-exendin-4 and 11.0–16.9 μg 111In-DOTA-exendin-4 as all patients received an exogenous glucose (1000 mL, 10%) infusion for 5 h starting just before injection of the radiotracer.
Surgery and histological assessment
Surgical planning was based on all available imaging results. The median (interquartile range) number of days between study imaging and surgery was 46 (24–88). Taking all available preoperative imaging (all imaging and invasive procedures before recruitment as well as study imaging) together, one or more highly suspicious lesions were detected in 43/52 patients (83%). In these patients, surgery was recommended. In four patients (11, 22, 25, 28), none of the imaging modalities or invasive procedures detected a suspicious lesion. In five patients (30, 35, 47, 49, 52), contradicting findings between procedures or readers were found which did not justify surgery. Three patients (4, 12, 19) declined surgery despite unequivocal findings—for example, patient 12 with high suspicion for an ectopic insulinoma (Supplementary Fig. 2).
Altogether 77% of patients (40/52) underwent surgery. In 18/40 patients (45%), the tumour was resected through minimally invasive enucleation. In two patients (patients 23 and 33), symptoms of EHH ceased after surgery, but local and central histological assessment did not confirm the diagnosis of a benign insulinoma or nesidioblastosis. Both patients were excluded from evaluation, as the final diagnosis remained unclear. Consequently, 38 patients had a histological evaluation and hence were included in the main assessment (Fig. 1). Thirty-seven of 38 patients showed a normalisation of blood glucose levels. One patient (patient 31) was operated on according to study-MRI and SPECT/CT findings with enucleation of a lesion in the head of the pancreas, but histology results were negative for an insulinoma, and hypoglycaemia persisted. One or multiple benign insulinomas (median size 12 mm; range 5–23 mm) were confirmed in 36 patients by histology (Figs. 2 and 3), including six patients with a confirmed germline mutation of multiple endocrine neoplasia type 1 (MEN-1). One patient (patient 5) was diagnosed with an adult focal nesidioblastosis. The scan results for this patient, including histopathological confirmation by central assessment, were published previously as a case report [19].
Main outcomes
Table 2 and Supplementary Table 2 summarise averaged accuracy, sensitivity and PPV, as well as percentage reading agreement, for 68Ga-DOTA-exendin-4 PET/CT, 111In-DOTA-exendin-4 SPECT/CT, study-MRI and prior external CT/MRI in all 38 patients with histological confirmation. Prior external CT/MRI was performed in 35/38 patients. In three patients (patients 6, 7 and 8), prior external CT/MRI assessment was not performed because EUS was available. 68Ga-DOTA-exendin-4 PET/CT had the highest accuracy, sensitivity and percentage reading agreement of all tested methods.
Change of clinical management: among the 52 patients, surgery planning/image-guided surgery became possible after initially (before recruitment) negative or inconclusive findings in 42.3%, 32.7 and 33.3% of patients after 68Ga-DOTA-exendin-4 PET/CT, 111In-DOTA-exendin-4 SPECT/CT and study-MRI, respectively (Table 2). All of these patients underwent surgery and showed normalisation of blood glucose (cure) after surgery. The only exceptions were patients 4 and 19 who refused surgery and patient 31 with false-positive imaging. Furthermore, PET/CT localised the insulinoma or nesidioblastosis in 81% of patients with initially (before recruitment) negative invasive procedures (ASVS, EUS with biopsy and surgery with intraoperative ultrasound) (Table 1 and Supplemental Table 3).
Discussion
The main findings in our study can be summarised as follows: (1) GLP-1R PET/CT has significantly higher accuracy and sensitivity, and influences surgical planning/image-guided surgery more than MRI and SPECT/CT. (2) GLP-1R PET/CT showed the highest reader agreement compared to study-MRI or GLP-1R SPECT/CT. (3) Standardisation of MRI improved accuracy, sensitivity and impact on surgery planning. MRI performs equally well as GLP-1R SPECT/CT if MRI is performed meticulously and is read by experienced radiologists.
The superior accuracy and sensitivity of GLP-1R PET/CT in comparison to SPECT/CT can be attributed to the three following factors: higher spatial resolution, higher scanner sensitivity and higher tumour-to-background ratio aiding visual assessment. Notably, in our patient collective, in which the median tumour size was 12 mm, the higher spatial resolution and scanner sensitivity of PET/CT offered a considerable benefit in comparison to SPECT/CT and MRI. The latter is limited in the detection and characterisation of small lesions through motion artefacts, such as respiratory motion, cardiac pulsation, and bowel peristalsis [20]. However, small insulinomas ≤ 10 mm in diameter can be missed even with 68Ga-DOTA-exendin-4 PET/CT, as the single false-negative PET/CT finding was identified in a patient with an insulinoma measuring 5 × 5 × 10 mm (patient 37).
The physiologic high kidney uptake of GLP-1R-specific radiotracers and the inherent high partial volume effect is a limitation of GLP-1R imaging [15]. The better spatial resolution of GLP-1R PET/CT is a substantial advantage over GLP-1R SPECT/CT perceivable in three patients. PET/CT was able to delineate the benign insulinoma in the distal portion of the pancreatic tail in close proximity to the left kidney, whereas SPECT/CT was not able to discriminate the lesions from the kidney uptake (patients 1, 6 and 42).
The superior biexponential blood clearance of 68Ga-DOTA-exendin-4 compared to 111In-DOTA-exendin-4 and the lower partial volume effect of PET compared to SPECT [13] lead to a higher tumour-to-background ratio with PET/CT. This fact may explain the higher reader agreement of PET/CT reading, surpassing that of SPECT/CT and study-MRI, making it a reliable imaging technique. An additional advantage of 68Ga-DOTA-exendin-4 in comparison to 111In-DOTA-exendin-4 is the shorter half-life of 68Ga (68 min vs. 67 h) which results in a lower radiation burden for patients [14]. Furthermore, PET/CT scans are performed 2.5 h after injection of 68Ga-DOTA-exendin-4 which is more convenient for patients than 111In-DOTA-exendin-4 SPECT/CT which should be performed at later time points, e.g. at 24 and 72 h (Fig. 3) [21].
Sensitivity of GLP-1R SPECT/CT and previously performed external CT/MRI were lower in the current study than in our previous published study [11]. This can be attributed to the referral of particularly challenging cases with prior negative or inconclusive imaging procedures. This is reflected in the smaller tumours size in comparison to our previous study (median size; 12 vs. 15 mm) and the fact that 13/52 patients had previous unsuccessful invasive procedures such as ASVS, surgery or biopsy.
The study imaging procedure, in particular PET/CT, significantly influenced the clinical management of patients, defined as successful image-guided surgery in the presence of previous (before recruitment) negative or ambiguous imaging findings or negative invasive procedures. This suggests that the surgical strategy takes into account the correct preoperative localisation, allowing for a laparoscopic or focused pancreatic resection approach [1,2,3], with 45% of all operated patients receiving enucleation (Supplementary Table 3). In addition, GLP-1R PET/CT has a high impact on the surgical management of patients with EHH in the context of MEN-1. MEN-1 patients with EHH often present multiple pancreatic lesions [22] and MRI cannot differentiate insulin-producing from other neuroendocrine tumours. Finally, GLP-1R PET/CT can localise focal nesidioblastosis (patient 5) [19], thereby influencing surgical management in these patients.
Nine of 52 patients did not undergo surgery mainly due to negative or inconclusive imaging results (Supplementary Table 3). This is attributable to the fact that resecting an insulinoma without knowing its exact localisation is associated with extensive pancreatic exploration, including palpation and intraoperative sonography with its associated morbidity [1,2,3]. There may be several reasons for a negative scan in the presence of a documented EHH: (1) The size of the insulinoma is below the detection rate of a given imaging modality. (2) A different aetiology of EHH, in particular nesidioblastosis, may be the cause of a negative scan in the presence of EHH. Previous in vivo and in vitro work suggests that GLP-1Rs are overexpressed in adult nesidioblastosis but to a lesser extent than in benign insulinoma [19]. Indeed, in three patients the GLP-1R imaging showed generally increased uptake, but was considered negative for a focal lesion. One of the patients (patient 11) had an additional 18F-DOPA PET/CT scan that was also compatible with a possible nesidioblastosis. Unfortunately, there was no focal lesion, and the patients thus refused a surgical intervention. All three patients were treated medically (diaxozide or somatostatin analogues). (3) The documentation of EHH (fasting test) was performed by the referral centres, and since it was not standardised, an inadequate procedure cannot be completely excluded.
There is one case that needs particular attention: Patient 31 was the only patient who was operated upon our recommendation and proved to be false-positive. MRI depicted a small lesion of 9 mm in the pancreatic head with a corresponding diffuse uptake in the SPECT/CT scan and a questionable focus in the PET/CT scan spotted by one reader. Surgery was performed and a 3.5-mm lesion was removed in this location which showed possible neuroendocrine tissue on frozen section. However, histological and immunohistochemical evaluation including insulin staining was negative, and the reason for persistence of hypoglycaemia after surgery remained unclear. The same patient had previously undergone a pancreatic tail resection without evidence of an insulinoma or nesidioblastosis. The reason for the false-positive imaging finding remained unclear, but previous studies indicate that the Brunner glands of the duodenum (which homogeneously express GLP-1Rs at high density) might have interfered [11].
Somatostatin receptor PET/CT (e.g. 68Ga-DOTATATE or 68Ga-DOTATOC) and 18F-fluorodopa (18F-DOPA) PET/CT are alternative molecular imaging modalities used for the localisation of insulinomas. Prasad et al. detected insulinomas or nesidioblastosis in 11/13 patients (85%) with 68Ga-DOTATATE or 68Ga-DOTATOC PET/CT [23]. In their study, CT was nearly as good as somatostatin receptor PET/CT, with a detection rate of 77% (10/13 patients), indicating that these were not particularly difficult cases. In our study, 68Ga-DOTATOC PET/CT was performed in nine patients, with histological proof in six patients (patients 1, 5, 21, 24, 34 and 36), resulting in the detection of two insulinomas in six patients (33%). In those six patients, 68Ga-DOTA-exendin-4 PET/CT detected the insulinoma in 94% of patients. Reubi et al. quantified GLP-1R and somatostatin receptor subtype 2 (the main target of 68Ga-DOTATOC and 68Ga-DOTATATE PET/CT) in 26 insulinoma tissue samples using in vitro autoradiography [9]. GLP-1R was expressed in 24/26 samples (92%) at a high density, whereas somatostatin receptor subtype 2 was expressed in 18/26 samples (69%) at a moderate to high density. As a result, GLP-1R PET/CT is likely to perform better than somatostatin receptor PET/CT in the detection of insulinomas.
Kauhanen et al. detected insulinomas or nesidioblastosis in 9/10 patients (90%) with 18F-DOPA PET [24]. Other groups could not repeat the initially excellent results of Kauhanen et al. For example, Nakuz et al. detected the insulinoma in 5/10 patients (50%) with 18F-DOPA PET [25]. Imperiale et al. showed somewhat better results with cabidopa pretreatment that seems to reduce the physiological uptake of 18F-DOPA in the pancreas: insulinoma detection rate of 8/11 patients (73%) [26]. In our patient collective, 18F-DOPA PET/CT without carbidopa pretreatment detected none of five insulinomas correctly. In those five patients, 68Ga-DOTA-exendin-4 PET/CT detected the insulinoma in 93% of patients. As a result, GLP-1R PET/CT is likely to perform better than 18F-DOPA PET/CT in the detection of insulinomas. Taken together, the evidence level for comparative PET/CT studies is scarce and controversial in patients with EHH, and only a direct prospective comparison between the different tracers would allow a firm conclusion.
This study has limitation, as follows: (1) Intentionally, the diagnostic performance of GLP-1R imaging was compared to a study-MRI protocol and not to EUS and/or ASVS, since the latter procedures are not widely available (ASVS) or are more investigator-dependent (EUS, ASVS) than MRI. We, therefore, cannot compare the accuracy and sensitivity of standardised EUS and ASVS with GLP-1R imaging. (2) The inclusion criteria of a pathological fasting test with neuroglycopenic symptoms confirming EHH is, despite possible false-positive results, a rather specific criterion for an insulinoma [27]. Consequently, we cannot evaluate the specificity and the negative predictive value of the tested imaging modalities. (3) Furthermore, 68Ga-DOTA-exendin-4 PET/CT, which was the most sensitive and accurate method (sensitivity of 94.6%), detected at least one suspicious lesion in only 78.8% of all 52 patients. Consequently, diagnostic accuracy and sensitivity is overestimated with the chosen reference standard (histology and clinical outcome). A positive fasting test with neurogylcopenic symptoms is a rather specific criterion for an insulinoma, but it is not suitable as a reference standard, since the purpose of the study was the correct localisation of insulinomas that can best be verified with surgery including histology and treatment outcome. For example, in five patients (9.6% of patients, Fig. 1), imaging findings were inconclusive (different location of lesions), and did not justify surgery. In these patients, the fasting test would not be able to differentiate between true-positive and false-positive findings, which is essential for the evaluation of accuracy and sensitivity.
Conclusion
68Ga-DOTA-exendin-4 PET/CT performs significantly better than 111In-DOTA-exendin-4 SPECT/CT and MRI in the preoperative localisation of insulinoma and nesidioblastosis and changes clinical management. It is also more convenient than GLP-1R SPECT/CT, with a lower radiation burden and a shorter investigation time. It should be recommended in the case of negative or inconclusive results with conventional CT or MRI.
References
Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–98.
Hackert T, Hinz U, Fritz S, Strobel O, Schneider L, Hartwig W, et al. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbeck's Arch Surg. 2011;396:1197–203.
Wenning AS, Kirchner P, Antwi K, Fani M, Wild D, Christ E, et al. Preoperative glucagon-like peptide-1 receptor imaging reduces surgical trauma and pancreatic tissue loss in insulinoma patients: a report of three cases. Patient Saf Surg. 2015;9:23.
Liu H, Peng C, Zhang S, Wu Y, Fang H, Sheng H, et al. Strategy for the surgical management of insulinomas: analysis of 52 cases. Dig Surg. 2007;24:463–70.
Wiesli P, Brändle M, Schmid C, Krähenbühl L, Furrer J, Keller U, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol. 2004;15:1251–6.
Guettier JM, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009;94:1074–80.
Placzkowski KA, Vella A, Thompson GB, Grant CS, Reading CC, Charboneau JW, et al. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab. 2009;94:1069–73.
Mehrabi A, Fischer L, Hafezi M, Dirlewanger A, Grenacher L, Diener MK, et al. A systematic review of localization, surgical treatment options, and outcome of insulinoma. Pancreas. 2014;43:675–86.
Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–93.
Wild D, Mäcke H, Christ E, Gloor B, Reubi JC. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N Engl J Med. 2008;359:766–8.
Christ E, Wild D, Ederer S, Béhé M, Nicolas G, Caplin ME, et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013;1:115–22.
Sowa-Staszczak A, Pach D, Mikołajczak R, Mäcke H, Jabrocka-Hybel A, Stefańska A, et al. Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC- 99mTc/EDDA)NH2]-exendin-4 for the detection of insulinoma. Eur J Nucl Med Mol Imaging. 2013;40:524–31.
Martin WH, Delbeke D, Patton JA, Sandler MP. Detection of malignancies with SPECT versus PET, with 2-[fluorine-18]fluoro-2-deoxy-D-glucose. Radiology. 1996;198:225–31.
Wild D, Wicki A, Mansi R, Béhé M, Keil B, Bernhardt P, et al. Exendin-4-based radiopharmaceuticals for glucagonlike peptide-1 receptor PET/CT and SPECT/CT. J Nucl Med. 2010;51:1059–67.
Antwi K, Fani M, Nicolas G, Rottenburger C, Heye T, Reubi JC, et al. Localization of hidden Insulinomas with 68Ga-DOTA-Exendin-4 PET/CT: a pilot study. J Nucl Med. 2015;56:1075–8.
Luo Y, Pan Q, Yao S, Yu M, Wu W, Xue H, et al. Glucagon-like Peptide-1 receptor PET/CT with 68Ga-NOTA-Exendin-4 for detecting localized Insulinoma: a prospective cohort study. J Nucl Med. 2016;57:715–20.
Zhu L, Xue H, Sun Z, Li P, Qian T, Xing X, et al. Prospective comparison of biphasic contrast-enhanced CT, volume perfusion CT, and 3 Tesla MRI with diffusion-weighted imaging for insulinoma detection. J Magn Reson Imaging. 2017;46:1648–55.
Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990;43:543–9.
Christ E, Wild D, Antwi K, Waser B, Fani M, Schwanda S, et al. Preoperative localization of adult nesidioblastosis using 68Ga-DOTA-exendin-4-PET/CT. Endocrine. 2015;50:821–3.
Ehman RL, McNamara MT, Brasch RC, Felmlee JP, Gray JE, Higgins CB. Influence of physiologic motion on the appearance of tissue in MR images. Radiology. 1986;159:777–82.
Christ E, Wild D, Forrer F, Brändle M, Sahli R, Clerici T, et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94:4398–405.
Triponez F, Cadiot G. Non-functioning tumours of the pancreas in MEN1 patients. J Gastrointestin Liver Dis. 2007;16:295–6.
Prasad V, Sainz-Esteban A, Arsenic R, Plöckinger U, Denecke T, Pape UF, et al. Role of (68)Ga somatostatin receptor PET/CT in the detection of endogenous hyperinsulinaemic focus: an explorative study. Eur J Nucl Med Mol Imaging. 2016;43:1593–600.
Kauhanen S, Seppänen M, Minn H, Gullichsen R, Salonen A, Alanen K, et al. Fluorine-18-L-dihydroxyphenylalanine (18F-DOPA) positron emission tomography as a tool to localize an insulinoma or beta-cell hyperplasia in adult patients. J Clin Endocrinol Metab. 2007;92:1237–44.
Nakuz TS, Berger E, El-Rabadi K, Wadsak W, Haug A, Hacker M, et al. Clinical value of 18F-FDOPA PET/CT with contrast enhancement and without Carbidopa premedication in patients with Insulinoma. Anticancer Res. 2018;38:353–8.
Imperiale A, Sebag F, Vix M, Castinetti F, Kessler L, Moreau F, et al. 18F-FDOPA PET/CT imaging of insulinoma revisited. Eur J Nucl Med Mol Imaging. 2015;42:409–18.
Service FJ, Natt N. The prolonged fast. J Clin Endocrinol Metab. 2000;85:3973–4.
Acknowledgments
We thank all the patients who participated in the trial, the referring physicians and the local investigators who contributed to the trial, and the technicians who did the labelling and the scans. We especially thank Prof. Aurel Perren, Institute of Pathology, University Bern, Switzerland, for pathological review, and Astrid Roesler, Clinical Trial Unit, Department of Clinical Research, University Hospital Basel and University of Basel, Switzerland, for monitoring the study.
Funding
The study was supported by the Swiss National Science Foundation (grant number 320030-152938) and the Desirée and Niels Yde Foundation (grant number 389-12), which had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflict of interest
The authors declare that they have no conflict of interest relevant to this article.
Ethical approval
The study was approved by the regional scientific ethics committee, and all procedures performed in studies involving human participants were in accordance with the ethical standards of the regional scientific ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 748 kb)
Rights and permissions
About this article
Cite this article
Antwi, K., Fani, M., Heye, T. et al. Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imaging 45, 2318–2327 (2018). https://doi.org/10.1007/s00259-018-4101-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4101-5