Abstract
Purpose
To explore the role of 68Ga-DOTATATE/DOTATOC PET/CT (SR PET/CT) in patients with suspicion of or histopathologically proven pancreatogenic hyperinsulinaemic hypoglycaemia.
Methods
We included 13 patients with histopathologically proven or a high clinical suspicion of pancreatogenic hyperinsulinaemia. All the patients underwent a SR PET/CT scan. The results were correlated with histopathological findings. Normalization of blood glucose levels after resection of the pancreatic lesion, as well as a cytological and/or pathological diagnosis of insulinoma, was considered the diagnostic gold standard for insulinoma. The diagnosis of nesidioblastosis was based on exclusion of an insulinoma and conclusive pathological examination of a segment of the pancreas. Malignant insulinoma was defined as the presence of locoregional or distant metastases.
Results
Based on histopathology, 13 patients were found to have pancreatic hyperinsulinaemia: two patients had malignant insulinoma, eight had nonmetastasized insulinoma, and three had nesidioblastosis. SR PET was positive in 11 of the 13 patients (84.6 %) with a final diagnosis of endogenous pancreatic hypoglycaemia. Histopathological staining confirmed 16 foci of hyperinsulinism (insulin positivity). SR PET detected 14 of the 16 lesions, resulting in a sensitivity of 87 %. One intrapancreatic spleen was falsely diagnosed as insulinoma focus on SR PET, resulting in positive predictive value of 93.3 %. Immunohistochemical staining of somatostatin receptor (SSR) subtype 2a was available in ten specimens: two nesidioblastosis, and seven benign and one malignant insulinoma. Eight out of the ten specimens (80 %) stained strongly to moderately positive. Seven of the eight SSR2a-positive lesions were picked up on SR PET. Based on the results of SR PET/CT, nine patients achieved complete remission of the hypoglycaemic events during follow-up.
Conclusion
This explorative study suggests that SR PET in combination with CT may play a significant role in the detection and management of patients with pancreatogenic hyperinsulinaemic hypoglycaemia. A large proportion of insulinomas express SSR2a, and a larger study is needed to fully assess the diagnostic accuracy of SR PET in patients with insulinoma and nesidioblastosis compared with current localizing studies used in clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insulinoma is a rare neuroendocrine tumour (NET) producing an excessive amount of insulin with an incidence of 1 – 3 per million per year [1, 2]. About 97 % of insulinomas are located within the pancreatic parenchyma, approximately 10 % are multiple and 5 – 10 % are associated with multiple endocrine neoplasia type 1 (MEN-1) syndrome. Malignancy can be expected in less than 10 % of patients and more frequently in those with tumours >2 cm [3, 4]. Patients with insulinoma and noninsulinoma pancreatogenic hypoglycaemia syndrome (NIPHS), also referred to as adult nesidioblastosis, suffer from severe hypoglycaemia due to inappropriately increased levels of circulating plasma insulin or proinsulin. The clinical diagnosis can be made by a positive 72-h fasting test demonstrating nonsuppressed plasma insulin, proinsulin, and/or C-peptide levels in the presence of hypoglycaemia. Factitious use of oral blood glucose-lowering drugs should always be excluded [5–7].
The role of imaging is to detect and provide precise anatomical localization and staging of the tumour prior to surgery [8]. Accurate preoperative localization of an insulinoma is desirable because some tumours may not be palpable at the time of surgery. However, due to their small size and similarity to surrounding tissue, localization may be a challenging clinical problem. Conventional imaging with gadolinium-enhanced MRI; three-phase CT and endoscopic ultrasonography (EUS) are the three preferred imaging modalities [8]. Three-phase CT has a sensitivity of 30 – 85 % depending upon the size of the insulinoma [9]. When combined with EUS, the sensitivity may increase to 100 % [10]. MRI has a sensitivity in the range 85 – 95 % in the detection of insulinomas and their metastases [11]. EUS has the highest sensitivity of 94 %. The high spatial resolution allows detection of very small lesions. The sensitivity of the technique, however, is lower if the lesions are located in the tail of the pancreas [12].
The role of somatostatin receptor (SSR) imaging with 111In Octreoscan for the detection of hyperinsulinaemic focus is a matter of controversy, with some reports suggesting that the sensitivity of SSR imaging is about 50 – 60 % and others suggesting a much lower sensitivity of 24 % [13, 14]. This lower sensitivity can primarily be attributed to the limited spatial resolution of scintigraphy techniques, particularly if the lesion is less than 1 cm in diameter. Immunohistochemistry (IHC) as well as reverse transcription polymerase chain reaction (RT-PCR) have, however, shown the presence of SSR subtypes 2a and 5 in abundance on insulinomas. The degree of expression of SSR subtypes is, however, largely dependent on tumour aggressiveness, i.e. the levels of expression of SSR2a and SSR5 are higher in more aggressive tumours [15]. Previous studies in various classes of NET have shown a very good correlation between IHC and RT-PCR and the degree of SSR expression assessed in vivo using 68Ga-labelled SSR PET (SR PET) [16–19]. In general, the combination of three-phase CT and 68Ga-DOTATATE/DOTATOC PET provides significantly better diagnostic accuracy particularly in staging and restaging as it picks up distant metastases in a single imaging session [20].
With this background we performed a retrospective analyses to (a) determine the sensitivity of 68Ga-DOTATATE/DOTATOC PET/CT for localizing the focus of endogenous hyperinsulinaemia in the pancreas, (b) determine the level of SSR2a expression in insulin-producing foci, and (c) explore the impact of SR PET on patient management.
Materials and methods
Data search and patient selection
The inclusion criteria for this retrospective analysis were (a) patients with histopathological, clinical or biochemical confirmation of insulinoma, and (b) patients who had not undergone pancreatectomy prior to the PET/CT examination. All patients sent to the Department of Nuclear Medicine, Campus Virchow-Klinikum, Charité Universitätsmedizin, Berlin, for 68Ga-DOTATOC/DOTATATE PET/CT were screened for the indication. Overall 18 patients had been examined with PET/CT with a high degree of suspicion or histopathologically confirmed endogenous hyperinsulinaemia. Out of these 18 patients, 13 met the inclusion criteria.
Immunohistochemistry
For IHC, 4-μm formalin-fixed, paraffin-embedded tissue sections were immunostained using heat-induced epitope retrieval, the Ventana Benchmark XT platform (Ventana Medical Systems, Tucson, AZ), the Ultraview DAB detection system (Ventana), and synaptophysin (clone 27G12, 1:50; Leica Biosystems), and chromogranin A (CgA, clone LK2H10, prediluted, Ventana Medical Systems). IHC for SSR2a was performed after pretreatment with protease using a rabbit monoclonal antibody directed against SSR2a (clone EP 149, also known as UMB-1; Epitomics, Burlingame, CA). For visualization, the iView DAB detection kit (Ventana) was used. The IHC results were reported as ‘negative’ (no positive cells), or ‘positive’ (at least 20 % of cells positive). The staining intensity was interpreted according to the method of Volante et al. [21]. In addition, all the histopathological specimens were stained for insulin and proinsulin. IHC of CgA and synaptophysin is required to confirm the presence of NET, Ki67 is required for grading, and insulin and proinsulin IHC is required to confirm the presence of insulin-producing cells.
Assessment of endogenous hyperinsulinaemia
Normalization of blood glucose levels after resection of the pancreatic lesion, as well as cytological and/or pathological diagnosis of an insulinoma, was considered the diagnostic gold standard for insulinoma. The diagnosis of nesidioblastosis was based on the presence of endogenous hyperinsulinaemia during hypoglycaemia and the exclusion of an insulinoma and/or conclusive pathological examination of a segment of the pancreas. Malignant insulinoma was defined as the presence of locoregional or distant metastases.
Imaging protocol
68Ga was eluted from a 68Ge/68Ga generator and labelled with either DOTATATE (six patients, from 2010 to June 2013) or DOTATOC (seven patients) according to the respective standard labelling procedure described elsewhere [22]. The selection of either DOTATATE or DOTATOC for imaging was purely based on the availability of the compound due to patent regulations. 68Ga-DOTATATE/DOTATOC PET/CT was performed according to EANM guidelines: after injection of 110 MBq of the radiotracer imaging was performed 60 min after injection [23]. Until June 2010, PET/CT was performed using a Biograph 16 PET/CT system (Siemens AG, Germany) with 120 kV, 230 mAs, five or six bed positions each with an acquisition time of 3 min, collimation of 0.75 mm and slices of 16 × 9 × 0.75 mm. After June 2010 all PET scans were acquired in three-dimensional mode using a Gemini TF 16 PET/CT system (Philips Medical Systems). The standard 3D-LOR algorithm of the system software was used with default parameter settings to reconstruct transaxial slices of 144 × 144 voxels of 4.0 × 4.0 × 4.0 mm. In all patients, a low-dose CT scan acquired immediately before the PET scan was used for attenuation correction (120 kVp, 30 mAs). If contrast-enhanced CT was performed, 70 – 100 ml Ultravist 370 (Bayer Schering Pharma, Berlin, Germany) with a delay of 30 s for the arterial phase, 50 s for the portovenous phase and 70 s for the venous phase was injected intravenously, and images were acquired using bolus tracking methodology.
Images were analysed independently, unblinded, by two nuclear medicine physicians (V.P., T.D.). Any circumscribed focal tracer uptake in the pancreas more than that of the surrounding tissue was considered pathological on PET images. In addition, the maximum standardized uptake value (SUVmax) for each suspicious lesion was also documented. Because of the known influence of size on SUV, particularly for lesions <1 cm in size, no SUV-based cut-off was used for differentiating between normal and pathological uptake.
Results
Demographics
This explorative retrospective analysis included 13 adult patients (mean 53.1 years, range 14–80 years, 4 men, 9 women; Table 1) with histologically proven endogenous hyperinsulinaemic hypoglycaemia. Three patients had MEN-1 syndrome. The results of the 72-h fasting test were retrieved from each patient’s file. The test was positive in eight patients, negative in two and was not performed in the remaining three.
Somatostatin receptor PET/CT
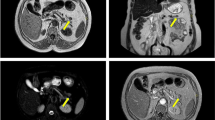
Table 1 summarizes the patient characteristics and fasting test results. Based on pathological results, three patients had nesidioblastosis, two had malignant insulinomas, and the remaining eight had benign insulinoma. SR PET was positive in 11 patients (84.6 %; Table 2). In the remaining two patients, the focus was confirmed on selective intraarterial calcium stimulation test. SR PET showed multiple (seven) SSR-positive lesions in the pancreas in three patients with adult onset nesidioblastosis. In all the patients, the lesions were located near the ductus pancreaticus (size range 8 – 11 mm; Fig. 1).
Example 68Ga-DOTATOC PET/CT imaging in a 61-year-old patient with nesidioblastosis. Three hypervascular lesions are detected in the tail of the pancreas, all near the ductus pancreaticus. The patient underwent distal pancreatectomy. Immunohistochemistry showed Ki67 of 1 %, positive staining for insulin, proinsulin and CgA, as well as synaptophysin. The patient was relieved of hypoglycaemic symptoms after the operation
Immunohistochemistry
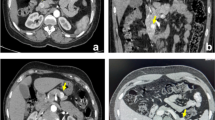
All lesions were positive on IHC for synaptophysin and CgA, suggesting neuroendocrine differentiation. The Ki67 proliferation rate was in the range 1 – 20 %. Histopathological staining of insulin confirmed 15 of 16 foci of hyperinsulinism. The remaining lesion was an intrapancreatic spleen. SR PET correctly detected 14 of 16 lesions resulting in a sensitivity of 87.5 %. One intrapancreatic spleen was falsely diagnosed as an insulinoma focus on SR PET resulting in positive predictive value of 93.3 %. IHC staining of SSR2a could only be performed on ten histopathological specimens (two nesidioblastosis, seven benign and one malignant insulinoma); the other six specimens could not be stained due to lack of sufficient tissue material. Eight out of the ten specimens (80 %) showed SSR2a positivity on IHC whereas two (20 %) were negative. Of the eight specimens positive for SSR2a on IHC, six (75 %) showed 3+ staining, and two (25 %) were moderately positive. SR PET detected seven of eight (88 %) of the SSR2a-positive lesions. In two hyperinsulinaemic foci negative on SR PET, IHC was negative for SSR2a in one and intensely positive (3+) in another patient. Example 68Ga-DOTATATE PET/CT imaging in the detection of insulinoma in a 49-year-old female patient is shown in Fig. 2.
a Example 68Ga-DOTATATE PET/CT imaging in a 49-year-old female patient (patient 9) with insulinoma. A solitary intense SSTR-positive lesion is seen in the pancreas head with a high SUVmax (12.7). The patient underwent enucleation which led to complete remission of hypoglycaemic features. b Immunohistochemistry of the same patient as in a shows positive staining for somatostatin receptor (SSTR) 2a, insulin, CgA and synaptophysin, and is negative for proinsulin. The proliferation rate of the tumour was found to be 1 %
Standardized uptake value and tumour size
The mean SUVmax and standard deviation of the lesions and normal pancreas were 12 ± 10.3 (range 3 – 42.9) and 3.8 ± 1 (1.9 – 5.3). The mean target (lesion) to nontarget (normal pancreas) ratio was 2.9 ± 2.4 (range 1.5 – 10.3) respectively. Three-phase CT and MRI detected 12 of 16 hypervascular lesions; their sizes were in the range 5 – 11 mm. One of the lesions was an intrapancreatic spleen.
Clinical course after imaging
Based upon the results of SR PET/CT, nine patients achieved complete remission of hypoglycaemic events during follow-up (Table 1). In two patients SR PET showed a solitary SSR-positive lesion in the pancreas. Both the patients refused surgery and were treated with somatostatin analogue resulting in successful control of hypoglycaemic symptoms for more than 2 years. In one patient, SR PET showed a low tumour burden in the liver. The patient had multiple comorbidities and was treated with diazoxide. During a follow-up of 7 months the patient was well controlled with morphologically stable metastases in the liver.
Discussion
Insulinoma is a rare NET, usually solitary and almost exclusively localized to the pancreas. The diagnosis can be made by demonstrating nonsuppressed plasma insulin, proinsulin and/or C-peptide concentrations in the presence of hypoglycaemia during a 72-h fasting test. The standard therapy for benign insulinoma is curative surgical resection. After endocrine diagnosis, imaging using various techniques is used to localize the tumour and rule out metastatic disease. As insulinomas are characteristically small, with approximately two thirds <2 cm at presentation, precise anatomical localization may be difficult [1, 2, 7].
Several somatostatin analogues including 68Ga-DOTANOC, 68Ga-DOTATATE and 68Ga-DOTATOC have been developed for PET imaging and are now increasingly used in the diagnostic management of NET [17, 18]. These new compounds offer several advantages compared to somatostatin receptor scintigraphy (SRS). First, PET/CT has a higher spatial resolution than SRS and the combined anatomical and functional information obtained with the hybrid PET/CT technique results in higher sensitivity. Second, many of these radiotracers have a better affinity for SSR2 than 111In Octreoscan. In addition, DOTATOC and DOTANOC cover a broader spectrum of SSRs than 111In Octreoscan.
In our study, SR PET/CT demonstrated a high sensitivity (85 %) in the detection of pancreatic foci responsible for endogenous hypoglycaemia. IHC validation of the presence of SSR2a in 80 % of the insulin-producing foci supports the use of SR PET for detection of pancreatogenic insulin-producing foci. The high detection rate of SR PET in this study in comparison to historical data on SRS may be due to the higher affinity shown by 68Ga-labelled analogues for SSR subtypes 2, 3 and 5. 68Ga-DOTATATE has a very high affinity for SSR2, even surpassing the binding affinity of natural somatostatin. Although the binding affinity for other SSR is low, it is higher than that of 111In-pentetreotide [24]. The SSR density is different in benign and malignant insulinomas and some authors have reported a higher expression of SSR5 in malignant insulinomas [15]. It has also been reported that despite the variability of the SSR subtype expression in insulinomas, SSR4 is the subtype most frequently expressed in both benign and malignant insulinomas [25]. In our analysis, we did not use 68Ga-DOTANOC PET/CT, but because 68Ga-DOTANOC covers a broader spectrum of SSR (subtypes 2, 3 and 5), future studies should be performed to identify the role of 68Ga-DOTANOC PET/CT in both malignant and benign insulinomas.
Interestingly the preliminary results suggest that 68Ga-DOTANOC PET has a limited role in the detection of primary tumour in patients with clinical and biochemical suspicion of insulinoma, with a sensitivity of only 25 % [26]. In contrast, 68Ga-DOTATATE and 68Ga-DOTATOC showed a reasonably good sensitivity compared with 68Ga-DOTANOC that could have been because of better tracer kinetics with a higher target to nontarget ratio [27]. In addition we defined a lesion on PET as positive based on visual assessment of uptake in comparison to that of the local surrounding pancreatic tissue, whereas Sharma et al. [26] used normal liver as the reference. The higher lipophilicity of 68Ga-DOTANOC leads to higher uptake in the liver compared with 68Ga-DOTATOC or 68Ga-DOTATATE [27]. This is the reason we suggest that normal pancreas should be used as the reference for the detection of pancreatic lesions. We found mild to high uptake in the primary lesion as compared to the surrounding tissue with target to nontarget ratios in the range 1.5 – 10.3. The lesions ranged in size from 5 to 11 mm, and morphological imaging missed only few lesions; the latter could be probably because of slight differences in contrast phases of CT acquired on two different scanners.
In comparison with historical data on EUS in insulinoma, in this study SR PET was found to be less sensitive (85 % vs. 94 %). Despite this lower sensitivity, SR PET allows whole-body staging in one session, and when combined with three-phase CT, could potentially have a direct impact on the surgical resection or on choice of medical treatment as shown in our patient cohort in which 9 of 13 patients achieved complete remission of hypoglycaemic events during follow-up.
Whereas the treatment of choice for benign solitary insulinoma is enucleation of the tumour or partial pancreatectomy, for malignant insulinoma the presence of distant metastases changes the treatment strategy. The advantage of PET is in its ability to detect multifocal disease that may occur in up to 10 % of patients, and metastatic disease that occurs in another 10 % of patients. In patients showing distant metastases, high expression of SSR on malignant insulinoma could be used for peptide receptor radionuclide therapy.
On the other end of the spectrum of beta cell diseases of the pancreas is NIPHS. NIPHS is a rare syndrome, characterized by endogenous hyperinsulinaemic hypoglycaemia that is not caused by an insulinoma. Pancreatic specimens from such patients show beta cell hypertrophy, islets with enlarged and hyperchromatic nuclei, and increased periductular islets [28–30]. These histological findings are characteristic features of nesidioblastosis, a term that refers to neoformation of islets of Langerhans from the pancreatic duct epithelium. Localization studies are necessary to distinguish between insulinoma and diffuse islet cell hyperplasia. The disorder was first described by Laidlaw [31]. The diagnosis of nesidioblastosis is considered complex as conventional imaging studies are not helpful and questioning of the patient may reveal a history with a confusing pattern of symptoms. Selective arterial calcium stimulation with hepatic venous sampling has been utilized in some clinical centres to aid the diagnosis [29, 30]. In our study, three patients with NIPHS showed multiple SSR-positive lesions on SR PET. All the patients showed intense SSR2a IHC staining. The presence of SSR in adult nesidioblastosis warrants further exploration of the role of SR PET in the management of patients with NIPHS [32, 33]. It would also be interesting to look into the role of 18F-DOPA PET in the management of patients with adult nesidioblastosis as it has a definite role in the management of children with congenital hyperinsulinism.
Detection of differential SSR expression in insulinoma may sometimes be a problem on SR PET with tracers directed towards SSR2a. Reubi et al. have extensively looked into the SSR on insulinomas using autoradiography techniques, with mixed results. Although conventional imaging including EUS allows detection in the majority of patients there remains an unmet medical need to correctly localize the insulin-producing focus as uncontrolled severe hypoglycaemia is a life-threatening process. Wild et al. reported the first use in humans of 68Ga-labelled or 111In-labelled [Lys(40)(Ahx[6-aminohexanoic acid]-DOTA-(111)In)NH(2)]-exendin-4, a glucagon-like peptide-1 (GLP-1) receptor analogue, to potentially improve the detection of insulinomas [34]. GLP-1 is an intestinal hormone that stimulates insulin secretion through receptors expressed in islet cells. GLP-1 receptors are massively expressed in benign insulinomas. Recent studies have demonstrated that a radiolabelled GLP-1 analogue can detect occult insulinomas, which cannot be localized by other imaging modalities. However, in comparison to SR PET, GLP-1 scintigraphy has been found to have lower sensitivity in the detection of malignant insulinomas. According to Wild et al., this observation is clinically relevant as SSR2 imaging can be recommended before surgery or peptide receptor radionuclide therapy when a malignant insulinoma is suspected [34–37]. It would be interesting to directly compare GLP-1 imaging and the more commonly available SR PET in the detection of pancreatic hyperinsulinaemic foci, particularly in those patients who are negative on SR PET or show multiple SR-positive lesions, e.g. MEN-1 patients.
Limitations of the study and future scope
The main limitations of the study were its retrospective design and small population size. Furthermore, PET/CT was performed on two different scanners with two different peptides, DOTATATE and DOTATOC, which could have independently affected the SUVmax of lesions. In addition, in retrospective studies of three-phase CT, the perfusion pattern may be quite heterogeneous because of the lack of strict timing for bolus tracking. This could have been the reason for CT missing some of the lesions seen on PET alone. It would have been interesting to have all the specimens stained with SSR2a antibodies, but this was technically not possible.
Conclusion
This explorative study suggests that SR PET can play a significant role in the detection and management of patients with pancreatogenic hypoglycaemia. A large proportion of insulinomas express SSR2a. A future study with a larger number of patients is needed to fully assess the accuracy of SR PET in patients with insulinoma and nesidioblastosis in comparison with localizing studies currently used in clinical practice.
References
Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–98.
de Herder WW. Insulinoma. Neuroendocrinology. 2004;80 Suppl 1:20–2.
Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27.
Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25(35):5609–15.
Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144–52.
Service FJ. Insulinoma and other islet-cell tumors. Cancer Treat Res. 1997;89:335–46.
Kenney B, Tormey CA, Qin L, Sosa JA, Jain D, Neto A. Adult nesidioblastosis. Clinicopathologic correlation between pre-operative selective arterial calcium stimulation studies and post-operative pathologic findings. JOP. 2008;9:504–11.
Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98–119.
Noone TC, Hosey J, Firat Z, Semelka RC. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19:195–211.
Gouya H, Vignaux O, Augui J, Dousset B, Palazzo L, Louvel A, et al. CT, endoscopic sonography, and a combined protocol for preoperative evaluation of pancreatic insulinomas. AJR Am J Roentgenol. 2003;181:987–92.
Pamuklar E, Semelka RC. MR imaging of the pancreas. Magn Reson Imaging Clin N Am. 2005;13:313–30.
McLean AM, Fairclough PD. Endoscopic ultrasound in the localisation of pancreatic islet cell tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:177–93.
de Herder WW, Kwekkeboom DJ, Valkema R, Feelders RA, van Aken MO, Lamberts SW, et al. Neuroendocrine tumors and somatostatin: imaging techniques. J Endocrinol Invest. 2005;28(11 Suppl International):132–6.
Vezzosi D, Bennet A, Rochaix P, Courbon F, Selves J, Pradere B, et al. Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur J Endocrinol. 2005;152(5):757–67.
de Sá SV, Corrêa-Giannella ML, Machado MC. Somatostatin receptor subtype 5 (SSTR5) mRNA expression is related to histopathological features of cell proliferation in insulinomas. Endocr Relat Cancer. 2006;13(1):69–78.
Kaemmerer D, Peter L, Lupp A, Schulz S, Sänger J, Prasad V, Kulkarni H, et al. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38(9):1659–68.
Bodei L, Sundin A, Kidd M, Prasad V, Modlin IM. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology. 2015;101:1–17.
Rufini V, Baum RP, Castaldi P, Treglia G, De Gaetano AM, Carreras C, et al. Role of PET/CT in the functional imaging of endocrine pancreatic tumors. Abdom Imaging. 2012;37:1004–20.
Hörsch D, Kulkarni HR, Baum RP. THERANOSTICS – clinical aimshots in surgical warfare against well-differentiated neuroendocrine neoplasms. Ann Transl Med. 2014;2(1):1.
Ruf J, Schiefer J, Furth C, Kosiek O, Kropf S, Heuck F, et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: spotlight on the CT phases of a triple-phase protocol. J Nucl Med. 2011;52(5):697–704.
Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol. 2007;20(11):1172–82.
Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–8.
Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–10.
Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34(7):982–93.
Portela-Gomes GM, Stridsberg M, Grimelius L, Rorstad O, Janson ET. Differential expression of the five somatostatin receptor subtypes in human benign and malignant insulinomas – predominance of receptor subtype 4. Endocr Pathol. 2007;18(2):79–85.
Sharma P, Arora S, Karunanithi S, Khadgawat R, Durgapal P, Sharma R, et al. Somatostatin receptor based PET/CT imaging with 68Ga-DOTA-Nal3-octreotide for localization of clinically and biochemically suspected insulinoma. Q J Nucl Med Mol Imaging. 2016;60:69–76.
Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging. 2010;54(1):61–7.
Service FJ, Natt N, Thompson GB, Grant CS, van Heerden JA, Andrews JC, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab. 1999;84(5):1582–9.
Thompson GB, Service FJ, Andrews JC, Lloyd RV, Natt N, van Heerden JA, et al. Noninsulinoma pancreatogenous hypoglycemia syndrome: an update in 10 surgically treated patients. Surgery. 2000;128(6):937–44.
Anlauf M, Wieben D, Perren A, Sipos B, Komminoth P, Raffel A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. Am J Surg Pathol. 2005;29(4):524–33.
Laidlaw GF. Nesidioblastoma, the islet tumor of the pancreas. Am J Pathol. 1938;14:125–34.
Ferrario C, Stoll D, Boubaker A, Matter M, Yan P, Puder JJ. Diffuse nesidioblastosis with hypoglycemia mimicking an insulinoma: a case report. J Med Case Rep. 2012;6:332. doi:10.1186/1752-1947-6-332.
Francesconi AB, Matos M, Lee JC, Wyld DK, Clouston AD, Macfarlane D. Nesidioblastosis as a cause of focal pancreatic 111In-pentetreotide uptake in a patient with putative VIPoma: another differential diagnosis. Ann Nucl Med. 2009;23(5):497–9.
Wild D, Behe M, Wicki A, Storch D, Waser B, Gotthardt M, et al. [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med. 2006;47:2025–33.
Wild D, Macke H, Christ E, Gloor B, Reubi JC. Glucagonlike peptide-1 receptor scans to localize occult insulinomas. N Engl J Med. 2008;359:766–8.
Christ E, Wild D, Forrer F, Brändle M, Sahli R, Clerici T, et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94:4398–405.
Wild D, Christ E, Caplin ME, Kurzawinski TR, Forrer F, Brändle M, et al. Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J Nucl Med. 2011;52:1073–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest
None.
Ethical approval
This retrospective analysis was performed in accordance with the ethical standards of the institutional research committee and with the principles of the1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Prasad, V., Sainz-Esteban, A., Arsenic, R. et al. Role of 68Ga somatostatin receptor PET/CT in the detection of endogenous hyperinsulinaemic focus: an explorative study. Eur J Nucl Med Mol Imaging 43, 1593–1600 (2016). https://doi.org/10.1007/s00259-016-3331-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3331-7