Abstract
Purpose
18F-FDOPA PET imaging is increasingly used in the work-up of patients with neuroendocrine tumours. It has been shown to be of limited value in localizing pancreatic insulin-secreting tumours in adults with hyperinsulinaemic hypoglycaemia (HH) mainly due to 18F-FDOPA uptake by the whole pancreatic gland. The objective of this study was to review our experience with 18F-FDOPA PET/CT imaging with carbidopa (CD) premedication in patients with HH in comparison with PET/CT studies performed without CD premedication in an independent population.
Methods
A retrospective study including 16 HH patients who were investigated between January 2011 and December 2013 using 18F-FDOPA PET/CT (17 examinations) in two academic endocrine tumour centres was conducted. All PET/CT examinations were performed under CD premedication (200 mg orally, 1 – 2 h prior to tracer injection). The PET/CT acquisition protocol included an early acquisition (5 min after 18F-FDOPA injection) centred over the upper abdomen and a delayed whole-body acquisition starting 20 – 30 min later. An independent series of eight consecutive patients with HH and investigated before 2011 were considered for comparison. All patients had a reference whole-body PET/CT scan performed about 1 h after 18F-FDOPA injection. In all cases, PET/CT was performed without CD premedication.
Results
In the study group, 18F-FDOPA PET/CT with CD premedication was positive in 8 out of 11 patients with histologically proven insulinoma (73 %). All 18F-FDOPA PET/CT-avid insulinomas were detected on early images and 5 of 11 (45 %) on delayed ones. The tumour/normal pancreas uptake ratio was not significantly different between early and delayed acquisitions. Considering all patients with HH, including those without imaging evidence of disease, the detection rate of the primary lesions using CD-assisted 18F-FDOPA PET/CT was 53 %, showing 9 insulinomas in 17 studies performed. In the control group (without CD premedication, eight patients), the final diagnosis was benign insulinoma in four, nesidioblastosis in one, and no definitive diagnosis in the remainder. 18F-FDOPA PET/CT failed to detect any tumour in these patients.
Conclusion
According to our experience, CD administration before 18F-FDOPA injection leads to low residual pancreatic 18F-FDOPA activity preserving tumoral uptake with consequent insulinoma detection in more than half of adult patients with HH and more than 70 % of patients with a final diagnosis of insulinoma. If 18F-FDOPA PET/CT is indicated, we strongly recommend combining CD premedication with early acquisition centred over the pancreas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyperinsulinaemic hypoglycaemia (HH) is the most common disorder of pancreatic islet cell hyperfunction. The diagnosis of HH is based on the typical positive fast test [1]. In adults, HH is usually caused by insulinoma, which is usually sporadic, unique and benign. Approximately 10 % of insulinomas are malignant, and 10 % are multiple, particularly in patients with multiple endocrine neoplasia type 1 (MEN1) syndrome. Localization of insulinoma remains challenging. The diagnostic approach with functional imaging is not mandatory when the tumour is located using conventional imaging diagnostic techniques such as CT, MRI or endoscopic ultrasonography (EUS) examination. However, when conventional anatomical imaging is negative or inconclusive for a single abnormality, functional imaging may be of particular interest for insulinoma detection or characterization. In clinical practice, imaging work-up of patients with HH often requires a combination of anatomical and functional imaging modalities.
Until recently, a highly sensitive functional imaging tool has not been available for localizing insulinomas. The radiopharmaceutical 111In-pentetreotide (Octreoscan), which mainly targets the subtype 2 of somatostatin receptors (sst2), was introduced in the 1990s with excellent results in some cases, performing so-called in vivo histology. Unfortunately, insulinomas express sst2 with an incidence of 60 %. Moreover, the density of sst2 is often variable and sometimes too low for effective somatostatin receptor targeting [2–4]. Furthermore, one-third of insulinomas are under 1 cm in diameter and can therefore be missed by conventional low-resolution gamma cameras [5, 6]. Consequently, the overall sensitivity of scintigraphic examinations using 111In-radiolabelled somatostatin analogues for insulinoma detection remains unsatisfactory [7]. PET imaging using 68Ga-labelled somatostatin analogues has been introduced for imaging neuroendocrine tumours (NET), but to date its sensitivity in imaging insulinomas is largely unknown, with disappointing preliminary results [8].
Although in recent years, a scientific effort focusing on the development of more specific radiopharmaceuticals has been initiated, to date, the gold-standard functional imaging technique remains to be determined. The radiolabelled glucagon-like peptide-1 (GLP-1) analogues for conventional scintigraphic studies or PET/CT imaging have shown promising results related to the overexpression of the GLP-1 receptor (GLP1R) in most insulinomas [9–11]. However, these radiopharmaceuticals are not available at most imaging centres worldwide and should be used in humans in the setting of clinical trials only. 18F-Fluorodihydroxyphenylalanine (18F-FDOPA) has been previously proposed for PET imaging of insulinoma, showing encouraging results in a single series of ten adult patients [12]. However, our initial experience with 18F-FDOPA PET/CT has been somewhat disappointing, a finding that was mainly attributed to the intense and prolonged 18F-FDOPA uptake by the mature exocrine pancreas [13]. Indeed, the pancreas has been shown to have an efficient mechanism for the uptake and decarboxylation of amino acids and their precursors such as l-DOPA. Evidence exists that carbidopa (CD), an efficient inhibitor of the peripheral aromatic amino acid decarboxylase (AADC), may improve both 18F-FDOPA and 11C-5-hydroxytryptophan PET interpretation by significantly increasing tumour uptake while lowering physiological pancreatic uptake [14, 15].
Nevertheless, patient premedication with CD remains inconsistently performed across oncological studies using 18F-DOPA PET imaging [16]. On the other hand, no final consensus has been reached about the usefulness of AADC inhibition by CD premedication before 18F-FDOPA PET in patients with insulinoma or β-cell hyperplasia [17–19]. Furthermore, the CD dosage and the optimal protocol for 18F-FDOPA PET/CT acquisitions are not definitively standardized in clinical practice (200 mg [20], 100 mg [21], 2 mg/kg [22, 23]). The aim of the present study was to assess the performance of an 18F-FDOPA PET/CT protocol combining CD premedication and early PET acquisitions for detecting insulinomas in a cohort of adult patients with HH. This diagnostic approach was also compared with a standard 18F-FDOPA PET/CT protocol without CD administration in an independent patient series.
Materials and methods
Patient population
Among all patients who underwent 18F-FDOPA PET/CT between January 2011 and December 2013 for clinical and biological suspicion of insulinoma in two academic endocrine tumour centres (La Timone University Hospital, Marseille, and Hautepierre University Hospital, Strasbourg), only those who fulfilled the following criteria were retrospectively included: (1) age 18 years or older (i.e., adult patients), (2) HH defined by a positive fasting test [1], (3) absence of personal or familial history of MEN-1 or MEN-1-related syndromic manifestations, (4) patient premedication by CD before 18F-FDOPA injection, and (5) early and delayed PET/CT acquisitions.
In all selected patients, the imaging work-up was performed during the 3 months prior to PET/CT, and included both contrast-enhanced three-phase thoracoabdominal CT and pancreatic MRI. Imaging findings were considered concordant and positive when they showed the same pancreatic abnormality that was subsequently proven histologically to be an insulinoma. Most of the patients were also investigated using EUS. Follow-up data were obtained for at least 12 months following 18F-FDOPA PET/CT. An independent series of nine consecutive patients with HH who were investigated before 2011 with 18F-FDOPA PET/CT without CD premedication were considered for comparison. A subgroup of the cohort has been previously reported [13]. 18F-FDOPA was used in the setting of marketing authorization. In keeping with local institutional guidelines, all patients gave informed consent for the use of anonymized personal data extracted from their medical records for scientific or epidemiological purposes.
18F-FDOPA PET/CT: imaging protocols
PET/CT with carbidopa premedication
A combined PET/CT scanner was used for all scans (Discovery ST, GE Medical Systems). Patients did not fast before radiotracer injection. 18F-FDOPA (4 MBq/kg) was injected intravenously 1 – 2 h after CD premedication (200 mg orally). Pharmaceutical grade CD was purchased from Inresa (Bartenheim, France), and 200-mg capsules were delivered as pharmaceutical compounding (according to the European Pharmacopoeia; Ph Eur monograph 0755) by the hospital central pharmacy to the nuclear medicine unit in both institutions. The 18F-FDOPA PET/CT acquisition protocol included an early acquisition (5 min after injection) centred over the upper abdomen (one 10-min step) and a delayed whole-body acquisition (starting between 20 and 30 min after injection) from the top of the skull to the upper thigh (3 – 5 min/step).
PET/CT without carbidopa premedication
Patients did not receive any antihypoglycaemic medication or glucose infusion and had fasted for 6 h before radiotracer injection. 18F-FDOPA was injected at 4 MBq/kg body mass. All patients had a whole-body reference acquisition about 1 h after radiotracer injection from the skull to the upper thigh. In all patients, 18F-FDOPA PET/CT was performed without CD premedication.
18F-FDOPA PET/CT: image interpretation
PET image datasets from both patient groups were reconstructed iteratively (OSEM algorithm) using CT data for attenuation correction. Coregistered images were displayed on a workstation (Xeleris; GE Healthcare) and independently interpreted by two experienced nuclear medicine physicians who were blinded to the results of other imaging investigations. A positive pancreatic abnormality was defined as a focal area of increased 18F-FDOPA uptake compared to surrounding tissue. 18F-FDOPA PET/CT studies were qualitatively interpreted as positive or negative for pancreas primary tumour location (i.e. insulinoma). In the event of results conflicting between the two reviewers, a third physician was required to reach a consensus. Extrapancreatic uptake foci in nonphysiological areas were considered as metastases.
In positive PET/CT studies, both the tumour maximum standardized uptake value (SUVmax) and the normal pancreatic mean standardized uptake value (SUVmean) were measured for quantitative assessment of 18F-FDOPA uptake. Tumour SUVmax was defined within a spherical volume of interest centred on the tumour and including it completely. The SUVmean was measured in the normal pancreas within a 1-cm3 spherical volume of interest taking care to avoid eventual biliary stasis. Hence, the tumour/normal pancreas ratio was calculated as: tumour SUVmax/normal pancreas SUVmean.
Gold standard
The normalization of blood glucose levels after resection of the pancreatic lesion, as well as the cytological and/or pathological diagnosis of insulinoma, were considered the diagnostic gold standard for solitary insulinoma. The diagnosis of nesidioblastosis was based on exclusion of an insulinoma and conclusive pathological examination of a segment of the pancreas. Malignant insulinoma was defined as the presence of nodal or visceral metastases.
Statistical analysis
Fisher’s exact test was used to compare the diagnostic performance of the two imaging protocols (with and without CD premedication) carried out in two independent populations. The results of early and delayed 18F-FDOPA PET/CT in the same population were compared using the McNemar test and the Sign test. Statistical significance was defined as p < 0.05. Statistical analysis was performed using the Statistica (Statsoft) version 7 software package.
Results
18F-FDOPA PET/CT findings in patients evaluated after carbidopa premedication
During the study period (2011 – 2013), 16 consecutive patients (10 women, 5 men; age range 20 – 81 years) with HH were evaluated using CD-assisted 18F-FDOPA PET/CT before any pancreatic surgery (Table 1). One patient (patient 3) underwent a second PET/CT examination for recurrent HH after surgical excision of an insulinoma. Therefore, 17 18F-FDOPA PET/CT studies were finally performed.
Multiphasic CT and MRI were positive concordant or positive discordant for a pancreatic lesion with typical feature of NET in five and five patients, respectively. Conventional imaging failed to identify any pancreatic abnormality in the seven remaining patients. EUS performed in 13 patients and showed focal pancreatic abnormalities suggestive of solitary insulinoma in 8 of them. In three patients, EUS was positive with normal CT or MRI results. One patient had metastatic disease (nodal and liver involvement) with unresectable primary pancreatic tumour and was subsequently treated with diazoxide to control severe hypoglycaemia. Malignancy was defined by histologically proven liver metastases.
The final diagnosis was benign insulinoma in 11 patients (65 %) and malignant insulinoma in one patient. In the remaining five patients, the tumour remained occult in all imaging studies. Normal pancreatic parenchyma was only faintly visible in all the patients, suggesting the effective inhibitory influence of CD on normal acinar 18F-FDOPA uptake.
PET/CT was positive in 8 of 11 patients (73 %) with histologically proven solitary insulinoma (Fig. 1) and in one patient with malignant insulinoma. PET/CT failed to detect three solitary insulinomas measuring 12, 25 and 26 mm. The median size of the 18F-FDOPA PET/CT-positive benign tumours was 16 mm (range 4 – 40 mm). Among the eight insulinomas accurately detected on early images, three (13, 15 and 16 mm) were missed on delayed images (detection rate 45 %; Fig. 2). However, there was no statistically significant difference in tumour detection rate between early and delayed PET/CT studies.
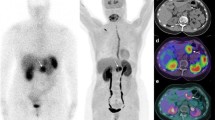
Patient 10 (42-year-old woman; Table 1). a Attenuation-corrected axial carbidopa-assisted 18F-FDOPA PET image (early acquisition) shows focal uptake; c attenuation-corrected axial 18F-FDOPA PET image (delayed acquisition) shows focal uptake; e axial 18F-FDOPA PET/CT fusion image; b, d CT images; f MRI image. Final diagnosis: insulinoma located in the uncinate process
Patient 5 (81-year-old woman; Table 1). a Attenuation-corrected axial carbidopa-assisted 18F-FDOPA PET image (early acquisition) shows focal uptake; b axial 18F-FDOPA PET/CT fusion image; c attenuation-corrected axial 18F-FDOPA PET image (delayed acquisition) is negative; d contrast-enhanced US (delayed image) shows a hypoechogenic nodule. Final diagnosis: insulinoma located in the isthmus
Considering all patients with HH, including five without imaging evidence of disease, the primary lesion detection rate on 18F-FDOPA PET/CT was 53 % (9/17 studies). In the 11 patients with histologically proven insulinoma (one malignant and ten benign tumours), 18F-FDOPA PET/CT showed concordant positive findings in six and no concordant negative findings when compared with the CT and MRI results. In the patient with malignant insulinoma, PET/CT was positive for the primary tumour and metastases but failed to identify millimetric liver metastases revealed by MRI. In the remaining five patients, the results of PET/CT and conventional imaging were discordant: PET/CT was negative and conventional imaging positive in three patients, and PET/CT was positive and conventional imaging negative in the two remaining patients. The primary tumour remained occult on imaging by all modalities and EUS in five patients.
In positive 18F-FDOPA PET/CT studies, the mean tumour/normal pancreas ratio was 3.0 ± 1.4 (range 1.3 – 4.9) and 2.7 ± 1.0 (range 1.4 – 4.5) in early and delayed PET acquisitions, respectively (not significant).
18F-FDOPA PET/CT findings in patients evaluated without carbidopa premedication
Before 2011, eight consecutive patients (five women, three men; age range 16 – 81 years) with HH were evaluated before any pancreatic surgery using 18F-FDOPA PET/CT without CD administration (Table 2). In all selected patients, the imaging work-up performed before PET/CT consisted of a variable combination of contrast-enhanced three-phase thoracoabdominal CT, pancreatic MRI and EUS.
The final diagnosis was benign insulinoma in four patients (50 %) and nesidioblastosis in one other patient. In the remaining three patients, no lesion was detected. Normal pancreatic parenchyma always showed a physiological, diffuse and intense 18F-FDOPA uptake (Fig. 3). PET/CT was positive in none of the four patients with histologically proven insulinoma, showing no pancreatic focal radiotracer uptake higher than normal pancreas. Moreover, no regional uptake was seen in patients with nesidioblastosis. Finally, 18F-FDOPA PET/CT failed to detect any tumour in the eight patients without CD premedication.
Patient 5 (33-year-old woman; Table 2). a, b Attenuation-corrected axial 18F-FDOPA PET (a) and PET/CT (b) images without carbidopa premedication acquired about 60 min after radiotracer injection show physiological, diffuse and intense pancreatic uptake. c T1-weighted gadolinium-contrast-enhancement axial MRI image (c) shows a 20-mm tumour at the body–tail pancreatic junction masked from the normally high pancreatic uptake on PET/MRI. d Fused image. Insulinoma was histologically confirmed after surgery
Comparison between 18F-FDOPA PET/CT protocols
The results can be summarized as follows:
-
1.
18F-FDOPA PET/CT with CD premedication was positive in 8 of 11 patients with histologically proven solitary insulinoma, a detection rate of 73 %. In the control patients who underwent PET/CT without CD premedication, none of the confirmed lesions (four insulinomas, one nesidioblastosis) was detected (p < 10−3).
-
2.
Among all patients with HH, including those without imaging evidence of disease, the detection rate of the primary lesions by 18F-FDOPA PET/CT with and without CD premedication was 53 % (9/17) and 0 %, respectively (p < 10−3).
Discussion
18F-FDOPA PET has been shown to be able to distinguish focal from diffuse β-cell hyperplasia in newborns with HH due to the lower tracer uptake by immature acinar cells (low AADC expression) [24–29]. The main limitation of the use of 18F-FDOPA PET/CT in adults is related to the intense and prolonged 18F-FDOPA uptake by the mature exocrine pancreas (decarboxylation properties of zymogen granules), resulting in a low tumour-to-background uptake ratio [13]. This drawback can be circumvented by the use of CD premedication that reduces pancreatic uptake via inhibition of AADC. Our study suggests that the combination of CD premedication and early acquisition results in a higher detection rate of insulinoma than delayed PET/CT images alone with or without CD premedication.
It is well established that CD (a peripheral AADC inhibitor) decreases whole pancreatic uptake [30]. Insulinomas are probably less sensitive to the CD inhibitory effect than acinar cells, resulting in an increased lesion-to-background uptake ratio. Negativization of 18F-FDOPA focal pancreatic hot spots has been reported after CD administration in patients with HH [18, 25, 29]. In the present series, we could not exclude the possibility that the use of CD may have induced negative findings in a few patients due to inhibition of tumoral AADC. However, in our opinion this possible drawback is counterbalanced by the increased sensitivity of PET imaging. The two patients with negative findings were treated with diazoxide at the time of the PET study. It is uncertain whether diazoxide interferes with 18F-FDOPA uptake, but PET studies are usually performed 72 h after drug withdrawal in newborns with HH [29]. If possible, this withdrawal period should also be applied in adults.
Because some insulinomas are known to overexpress somatostatin receptor 2, they have been investigated with somatostatin receptor scintigraphy (SRS) during the past decades and more recently with 68Ga-labelled somatostatin analogues (68Ga-SSTa). These radiopharmaceuticals should be used if 18F-FDOPA PET/CT imaging is not available, in patients with negative 18F-FDOPA PET/CT or in patients with malignant insulinoma due to its high expression of somatostatin. The indications for the use of 68Ga-SSTa will also rapidly evolve with the clinical use of peptide receptor radionuclide therapy to select good candidates for this therapy. Interestingly, insulinoma negative with 18F-FDOPA could be positive on SRS or with 68Ga-labelled somatostatin analogues [31]. This imaging pattern may correspond to a specific tumour phenotype since discordance between imaging based on somatostatin receptor and GLP1R has already been shown [32].
The timing of acquisition may also be important in the localization of insulinoma. In three patients, insulinoma was only detected on early images. In the population studied, early images showed 8 of 11 proved solitary insulinomas compared to delayed images that showed only 5 lesions, without reaching statistical significance. This could be attributed to the small size of our study population and to the fact that delayed acquisitions were performed earlier than in classical protocols for 18F-FDOPA PET imaging (30 min vs. 60 min). Based on the present study, we recommend combining pharmacological modulation by CD and early PET/CT acquisitions. In patients with no history of MEN1 or MEN1-related syndromic manifestations and small tumours detected on early acquisition, the role of whole-body acquisition remains questionable.
Based on our longstanding clinical experience, a decrease in tracer uptake is often seen in secreting and nonsecreting pancreatic NETs (unpublished observations). In our opinion, 18F-FDOPA PET/CT protocols need to be tailored to individual clinical situations. It has been shown that in medullary thyroid carcinoma (MTC), tumour uptake decreases by 40 % between early and delayed images (starting after 60 min). Early acquisition (during the first 15 min) in patients with MTC with persistent/recurrent hypercalcitoninaemia is therefore recommended [33]. The imaging phenotype of insulinoma differs from that of carcinoids or paragangliomas/phaeochromocytomas in that it usually exhibits prolonged tracer retention. Both tumour types overexpress both large neutral amino acid transporters (i.e. LAT1 or LAT2) and AADC, a key enzyme involved in both serotonin and catecholamine biosynthesis. Quantification of LAT expression and AADC expression and activity in insulinoma could be of particular interest in the understanding of 18F-FDOPA PET/CT findings.
We acknowledge several limitations to this study: its retrospective design, the involvement of two independent institutions with different patient populations, and the absence of a head-to-head comparison of the two protocols in the same patient. However, a clinical trial based on a dual-imaging protocol (with and without CD) in each patient could raise ethical issues related to the very low sensitivity of the classical 18F-FDOPA PET/CT protocol for detecting insulinomas. In the present study, we used the same radiopharmaceutical and the same imaging protocol in both institutions. The two groups were also evaluated by different imaging protocols (early image centred over the pancreas in the study group vs. delayed whole-body evaluation in the control group). However, we have previously shown that there is early and intense tracer uptake in normal pancreas [13]. Therefore, we consider that early images without CD would have also missed insulinomas. Besides these limitations, the present study is one of the largest studies investigating 18F-FDOPA PET/CT in patients with HH and CD premedication.
CD-assisted 18F-FDOPA PET/CT leads to a low residual pancreatic 18F-FDOPA activity with preservation of tumour uptake and enables detection of insulinoma in more than half of adult patients with HH. If 18F-FDOPA PET/CT is indicated, we strongly recommend combining CD premedication and early PET/CT acquisition centred over the pancreas. This approach should be performed in the absence of available GLP1R-based imaging. The role of CD-assisted 18F-FDOPA PET/CT in the detection of insulinoma will need to be compared to that of newly introduced and promising agents such as GLP-1 analogues.
References
Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709–28. doi:10.1210/jc.2008-1410.
Zimmer T, Stolzel U, Bader M, Koppenhagen K, Hamm B, Buhr H, et al. Endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localisation of insulinomas and gastrinomas. Gut. 1996;39:562–8.
Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–93. doi:10.1007/s00259-003-1184-3.
Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–98. doi:10.1016/j.bpg.2005.05.008.
Guettier JM, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009;94:1074–80.
Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458–511.
Kwekkeboom DJ, Krenning EP, Scheidhauer K, Lewington V, Lebtahi R, Grossman A, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: somatostatin receptor imaging with (111)In-pentetreotide. Neuroendocrinology. 2009;90:184–9. doi:10.1159/000225946.
Sharma P, Arora S, Karunanithi S, Khadgawat R, Durgapal P, Sharma R, et al. Somatostatin receptor based PET/CT imaging with 68Ga-DOTA-Nal3-Octreotide for localisation of clinically and biochemically suspected insulinoma. Q J Nucl Med Mol Imaging. 2014.
Christ E, Wild D, Forrer F, Brandle M, Sahli R, Clerici T, et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94:4398–405. doi:10.1210/jc.2009-1082.
Wild D, Macke H, Christ E, Gloor B, Reubi JC. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N Engl J Med. 2008;359:766–8.
Brom M, Oyen WJ, Joosten L, Gotthardt M, Boerman OC. 68Ga-labelled exendin-3, a new agent for the detection of insulinomas with PET. Eur J Nucl Med Mol Imaging. 2010;37:1345–55. doi:10.1007/s00259-009-1363-y.
Kauhanen S, Seppanen M, Minn H, Gullichsen R, Salonen A, Alanen K, et al. Fluorine-18-L-dihydroxyphenylalanine (18F-DOPA) positron emission tomography as a tool to localize an insulinoma or beta-cell hyperplasia in adult patients. J Clin Endocrinol Metab. 2007;92:1237–44.
Tessonnier L, Sebag F, Ghander C, De Micco C, Reynaud R, Palazzo FF, et al. Limited value of 18F-F-DOPA PET to localize pancreatic insulin-secreting tumors in adults with hyperinsulinemic hypoglycemia. J Clin Endocrinol Metab. 2010;95:303–7. doi:10.1210/jc.2009-1357.
Orlefors H, Sundin A, Lu L, Oberg K, Langstrom B, Eriksson B, et al. Carbidopa pretreatment improves image interpretation and visualisation of carcinoid tumours with 11C-5-hydroxytryptophan positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:60–5. doi:10.1007/s00259-005-1891-z.
Neels OC, Koopmans KP, Jager PL, Vercauteren L, van Waarde A, Doorduin J, et al. Manipulation of [11C]-5-hydroxytryptophan and 6-[18F]fluoro-3,4-dihydroxy-L-phenylalanine accumulation in neuroendocrine tumor cells. Cancer Res. 2008;68:7183–90. doi:10.1158/0008-5472.CAN-08-0095.
Imperiale A, Rust E, Gabriel S, Detour J, Goichot B, Duclos B, et al. 18F-fluorodihydroxyphenylalanine PET/CT in patients with neuroendocrine tumors of unknown origin: relation to tumor origin and differentiation. J Nucl Med. 2014;55:367–72. doi:10.2967/jnumed.113.126896.
Koopmans KP, Neels OC, Kema IP, Elsinga PH, Sluiter WJ, Vanghillewe K, et al. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J Clin Oncol. 2008;26:1489–95. doi:10.1200/JCO.2007.15.1126.
Kauhanen S, Seppanen M, Nuutila P. Premedication with carbidopa masks positive finding of insulinoma and beta-cell hyperplasia in [18F]-dihydroxy-phenyl-alanine positron emission tomography. J Clin Oncol. 2008;26:5307–8. doi:10.1200/JCO.2008.18.8581. author reply 8–9.
Kauhanen S, Seppanen M, Minn H, Nuutila P. Clinical PET imaging of insulinoma and beta-cell hyperplasia. Curr Pharm Des. 2010;16:1550–60.
Hoegerle S, Altehoefer C, Ghanem N, Koehler G, Waller CF, Scheruebl H, et al. Whole-body 18F dopa PET for detection of gastrointestinal carcinoid tumors. Radiology. 2001;220:373–80. doi:10.1148/radiology.220.2.r01au25373.
Beuthien-Baumann B, Strumpf A, Zessin J, Bredow J, Kotzerke J. Diagnostic impact of PET with 18F-FDG, 18F-DOPA and 3-O-methyl-6-[18F]fluoro-DOPA in recurrent or metastatic medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2007;34:1604–9. doi:10.1007/s00259-007-0425-2.
Koopmans KP, de Groot JW, Plukker JT, de Vries EG, Kema IP, Sluiter WJ, et al. 18F-dihydroxyphenylalanine PET in patients with biochemical evidence of medullary thyroid cancer: relation to tumor differentiation. J Nucl Med. 2008;49:524–31. doi:10.2967/jnumed.107.047720.
Koopmans KP, de Vries EG, Kema IP, Elsinga PH, Neels OC, Sluiter WJ, et al. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol. 2006;7:728–34. doi:10.1016/S1470-2045(06)70801-4.
Barthlen W, Blankenstein O, Mau H, Koch M, Hohne C, Mohnike W, et al. Evaluation of [18F]fluoro-L-DOPA positron emission tomography-computed tomography for surgery in focal congenital hyperinsulinism. J Clin Endocrinol Metab. 2008;93:869–75. doi:10.1210/jc.2007-2036.
de Lonlay P, Simon-Carre A, Ribeiro MJ, Boddaert N, Giurgea I, Laborde K, et al. Congenital hyperinsulinism: pancreatic [18F]fluoro-L-dihydroxyphenylalanine (DOPA) positron emission tomography and immunohistochemistry study of DOPA decarboxylase and insulin secretion. J Clin Endocrinol Metab. 2006;91:933–40. doi:10.1210/jc.2005-1713.
Hardy OT, Hernandez-Pampaloni M, Saffer JR, Suchi M, Ruchelli E, Zhuang H, et al. Diagnosis and localization of focal congenital hyperinsulinism by 18F-fluorodopa PET scan. J Pediatr. 2007;150:140–5.
Otonkoski T, Nanto-Salonen K, Seppanen M, Veijola R, Huopio H, Hussain K, et al. Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes. 2006;55:13–8.
Ribeiro MJ, Boddaert N, Bellanne-Chantelot C, Bourgeois S, Valayannopoulos V, Delzescaux T, et al. The added value of [18F]fluoro-L-DOPA PET in the diagnosis of hyperinsulinism of infancy: a retrospective study involving 49 children. Eur J Nucl Med Mol Imaging. 2007;34:2120–8. doi:10.1007/s00259-007-0498-y.
Ribeiro MJ, De Lonlay P, Delzescaux T, Boddaert N, Jaubert F, Bourgeois S, et al. Characterization of hyperinsulinism in infancy assessed with PET and 18F-fluoro-L-DOPA. J Nucl Med. 2005;46:560–6.
Timmers HJ, Hadi M, Carrasquillo JA, Chen CC, Martiniova L, Whatley M, et al. The effects of carbidopa on uptake of 6-18F-fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. J Nucl Med. 2007;48:1599–606.
Treglia G, Inzani F, Campanini N, Rindi G, Agnes S, Giordano A, et al. A case of insulinoma detected by 68Ga-DOTANOC PET/CT and missed by 18F-dihydroxyphenylalanine PET/CT. Clin Nucl Med. 2013;38:e267–70. doi:10.1097/RLU.0b013e31825b222f.
Wild D, Christ E, Caplin ME, Kurzawinski TR, Forrer F, Brandle M, et al. Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J Nucl Med. 2011;52:1073–8. doi:10.2967/jnumed.110.085142.
Soussan M, Nataf V, Kerrou K, Grahek D, Pascal O, Talbot JN, et al. Added value of early 18F-FDOPA PET/CT acquisition time in medullary thyroid cancer. Nucl Med Commun. 2012;33:775–9. doi:10.1097/MNM.0b013e3283543304.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imperiale, A., Sebag, F., Vix, M. et al. 18F-FDOPA PET/CT imaging of insulinoma revisited. Eur J Nucl Med Mol Imaging 42, 409–418 (2015). https://doi.org/10.1007/s00259-014-2943-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2943-z