Abstract
Purpose
Insulinomas are the most common functioning neuroendocrine neoplasms of the pancreas, typically diagnosed due to characteristic symptoms. In the vast majority, the treatment is surgical and curative, requiring accurate localization of the tumour; conventional imaging, including somatostatin receptor molecular imaging, is negative in up to 10 % of cases. Recently, labelled glucagon-like peptide receptor (GLP-1R) analogues were introduced as a sensitive diagnostic method for localization of insulinomas. The aim of this study was to assess the diagnostic accuracy of a Tc-99m-labelled GLP-1R agonist [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 for localization of occult insulinoma.
Procedures
Eight patients (all females; age range 35–75 years) with biochemically proven insulinoma and with negative or inconclusive conventional imaging (consisting of somatostatin receptor scintigraphy, computed tomography, endoscopic ultrasound and magnetic resonance imaging) were enrolled. Whole-body single-photon emission tomography/computed tomography (SPECT/CT) imaging was performed 4 h post-injection of 740 MBq of [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4. Surgical treatment was performed based on imaging findings. Histology of the removed lesions and biochemical and clinical symptom resolution was considered as the gold standard for analysis of the imaging results.
Results
Focal uptake of [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 was found in all patients, leading to successful removal of the offending lesion and complete biochemical and symptomatic resolution. Histological analysis confirmed insulinoma in all included patients.
Conclusions
[Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 SPECT/CT appears to be an excellent molecular imaging method for preoperative localization of an occult insulinoma, surpassing conventional imaging methods. If routinely available, it could be considered as a method of choice due to its favorable combination of imaging characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Insulinomas are the most common functioning neuroendocrine neoplasms of the pancreas (p-NENs) [1,2,3,4]. Patients characteristically develop symptoms due to hypoglycaemia. The vast majority (90–95 %) of insulinomas are benign, most often smaller than 2 cm in diameter and 95–100 % of these can be surgically cured [3, 5]. The main challenge is the accurate localization of the tumour, since in about 5–10 % of cases, all conventional imaging techniques (computed tomography, CT; magnetic resonance imaging, MRI) along with endoscopic ultrasound (EUS) do not detect the tumour [6,7,8]. Invasive techniques such as intraarterial calcium stimulation test have revealed better sensitivity (88–100 %) but provide information only on the anatomical region and not the exact location of the tumour [9, 10]. Accurate preoperative localization is essential to minimize unnecessary blind surgical resection and to enable pancreas-sparing surgery to preserve exocrine and endocrine function of the gland and full patient recovery [11].
Over the past few years, nuclear medicine imaging techniques based on peptide receptor targeting have been rapidly developing and have an important role in the diagnosis of p-NENs. The most established imaging method for staging and localization of p-NENs is somatostatin receptor scintigraphy (SRS), preferably performed as positron emission tomography with computed tomography (PET/CT) with Ga-68-labelled somatostatin analogues; according to the current ENETS guidelines, it is considered as the method of choice for this purpose [4]. The method is based on the overexpression of somatostatin receptors (SSTR) on the surface of p-NEN cells. Unfortunately, insulinomas frequently exhibit low expression and density of SSTR on the surface of tumour cells, and therefore, the sensitivity of PET/CT with Ga-68-labelled somatostatin analogues can be as low as 25 % [12]. However, in almost all insulinomas, another peptide receptor—glucagon-like peptide-1 (GLP-1) receptor (GLP-1R)—is expressed in high densities [13, 14]. As a result, GLP-1R-targeting probes were developed and scintigraphy of GLP-1R was successfully introduced as a sensitive diagnostic method for localization of even very small tumours (diameter below 1 cm), otherwise barely detectable or undetectable by conventional imaging techniques. GLP-1R agonists for use in humans were first labelled with In-111 [15] and subsequently with Tc-99m [8] and Ga-68 [16]; several promising positron-emitting probes labelled with F-18 [17] and Cu-64 [18] were also tested in animal models.
The aim of the present study was to assess the diagnostic accuracy of a 99mTc-labelled GLP-1 receptor agonist [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 for localization of occult insulinoma.
Materials and Methods
Patients
Eight patients (all females; age range 35–75 years) with suspected insulinoma were enrolled in the study. Seven patients were treated at the Department of Endocrinology, Diabetes and Metabolic Diseases, University Medical Centre Ljubljana; one foreign patient was referred for diagnostic imaging and surgery to our institution. The study was approved by the National Medical Ethics Committee of Republic of Slovenia (Protocol No. 140/10/12). Each patient provided a signed informed consent upon entering the study.
For the diagnosis of insulinoma, the diagnostic criteria proposed by the US Endocrine Society [19] and ENETS guidelines [4] were used: endogenous hyperinsulinism documented by appropriate symptoms, signs, or both with plasma concentrations of glucose < 3.0 mmol/l, insulin ≥ 3.0 mU/l, and C-peptide ≥ 0.2 nmol/l.
Conventional imaging techniques (CT, MRI, and EUS) and SRS with [99mTc]EDDA/HYNIC-TOC were performed ahead of GLP-1R scintigraphy.
Imaging Technique and Interpretation of GLP-1R Scintigraphy
The radiolabelling of [Lys40(Ahx-HYNIC)NH2]-exendin-4 with Tc-99m followed a two-vial kit formulation as previously published [8]. Between August 2012 and May 2018, examinations with [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 for localization of biochemically suspected insulinoma were performed at the Department for Nuclear Medicine, University Medical Centre Ljubljana, Slovenia.
Blood glucose values were monitored before and after the injection of 740 MBq of [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 at several time points (5 min, 15 min, 30 min, 1 h, 4 h post-injection). None of the patients required glucose infusion at the time of the procedure. Between 3 and 4 h after injection of the radiopharmaceutical, the images were acquired on a dual-head single-photon emission computed tomography/computed tomography (SPECT/CT) system Siemens Symbia T2. Whole-body SPECT/CT imaging was performed (32 projections per detector, matrix 256 × 256, zoom level 1, 20 s/frame; low-dose CT at 30 mAs and 120 kV). Reconstruction was performed using Siemens Flash3D proprietary software with the iterative OSEM method (8 iterations, 16 subsets), attenuation and scatter correction and resolution recovery.
Final Diagnosis
Surgical excision of the lesion identified on GLP-1R imaging (and other imaging modalities, where identified) was performed in all patients. Histopathological evaluation of the lesion and patient follow-up (symptom resolution, normalization of blood glucose levels) served as the gold standard.
Results
Clinical Characteristics
The patient history revealed symptoms suggestive of hypoglycaemia lasting from 1 month to up to several years prior to diagnostic testing. None of the patients were treated with oral antidiabetic agents. The clinical characteristics are summarized in Table 1.
A 72-h fast was performed in six patients (Table 2). During hospitalization, patient no. 4 (P4) frequently developed symptoms and signs of hypoglycaemia and plasma levels of glucose (2.2 mmol/l), insulin (8.9 mU/l), and C-peptide (0.9 nmol/l) were recorded, fitting diagnostic criteria. None of the patients were treated with oral antidiabetic agents. Patient no. 6 (P6) was admitted to our University Medical Centre Ljubljana for a confirmatory GLP-1R scintigraphy to be performed, which was not available in her country of origin. From the diagnostic workup of P6 which started in her home country, the insulin and C-peptide levels at calcium stimulation test were included in the discharge papers of the last hospitalization; 72-h fast performed at an earlier time was reported as positive by the referring physician in the accompanying documentation. Surgery was also performed in our centre.
Preoperative Localization
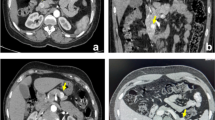
SRS and CT imaging were performed in all patients; EUS was performed in seven patients and MRI in four patients. The preoperative localization is summarized in Table 3. In all eight patients, focal uptake of the radiopharmaceutical in the pancreas was found and the results of GLP-1R scintigraphy were deemed positive. Images demonstrating insulinomas located in the body-tail transition (patient no. 1 (P1); Fig. 1) and pancreatic tail (patient no. 7 (P7); Fig. 2) are shown, in which other imaging techniques were inconclusive or negative; Fig. 3 demonstrates a case (patient no. 5 (P5)) with an insulinoma located at the neck of the pancreas with completely negative conventional imaging (somatostatin receptor scintigraphy, CT, MRI).
Patient 1: Increased uptake of [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 at the body-tail transition in the pancreas. a SPECT image. b CT image. c Fused SPECT/CT image. d Negative fused SPECT/CT image, somatostatin receptor scintigraphy ([99mTc]EDDA/HYNIC-TOC). e Suspicious lesion at the body-tail transition in the pancreas (arrow) on the MRI T2-weighted image, but negative on T1-weighted image (not shown). f Postoperative MRI T2-weighted image shows no residual disease.
Patient 7: Increased uptake of [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 in the tail of the pancreas. High image quality allows clear localization of the tumour despite high neighbouring physiological activity in the kidney. a SPECT image. b CT image. c Fused SPECT/CT image. d Negative fused SPECT/CT image, somatostatin receptor scintigraphy ([99mTc]EDDA/HYNIC-TOC). e Minimal perfusion anomaly in the tail of the pancreas in the arterial phase of contrast-enhanced CT (arrow).
Patient 5: Increased uptake of [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 in the neck of the pancreas. a SPECT image. b CT image. c Fused SPECT/CT image. d Negative-fused SPECT/CT image, somatostatin receptor scintigraphy ([99mTc]EDDA/HYNIC-TOC); e Negative arterial phase of contrast-enhanced CT. f Negative MRI T2-weighted image.
Surgical Procedure and Histology, Follow-up
All patients were eligible for surgery, and the offending lesion was successfully removed in all of them. Complete resolution of symptoms after operative management was observed in all patients. Histopathological analysis confirmed a well-differentiated insulin-secreting neuroendocrine tumour in all cases (Table 3).
During follow-up (duration between 1 and 75 months, median 24.5 months), all patients were asymptomatic; one patient died due to unrelated causes (sudden cardiac death, advanced heart failure).
Discussion
In this prospective study, we evaluated the diagnostic accuracy of [Lys40(AhxHYNIC-[99mTc]EDDA)NH2]-exendin-4 hybrid SPECT/CT imaging for the localization of occult insulinoma. Our results showed excellent sensitivity and specificity of this imaging modality, enabling an accurate and focused surgical approach and in accordance with the results of Sowa-Staszczak et al. using the same radiopharmaceutical [8], where sensitivity and specificity were reported to be 100 %. Even in patients where the tumour was located in the tail of the pancreas, the high physiological activity in the kidneys did not preclude the accurate confirmation and localization of the lesion. In most patients enrolled in the study, the conventional imaging was negative and inconclusive or confirmation of the suggested location of the tumour was requested, so this imaging technique was the decisive option for the precise preoperative localization of biochemically proven insulinoma.
Several studies have shown that GLP-1R scintigraphy is a superior method for the detection of insulinoma in comparison with CT, MRI, EUS, and SRS [8, 15, 16, 20]. In accordance with previous results, the sensitivity of CT in our series was 62.5 % (five out of eight patients). MRI was performed only in four patients, and inconclusive results were reported in two patients, whereas in the other two, the lesion was not detected. EUS was false-negative in three out of seven patients, possibly due to the size of the lesion, measuring only up to 10 mm. Another reason might be the location of the tumour, since three of them were located in the pancreatic tail, where the reported sensitivity is only 37–50 % [21]. In contrast, tumours in the tail of the pancreas were clearly visible in the present series (Fig. 2).
Only two-thirds of insulinomas show in vitro expression of somatostatin receptor (in particular subtype 2) [13, 22], whereas in in vivo studies, even lower detection rates were published, as low as 20 % [16]. In the current study, none of the patients was positive on SRS (one inconclusive result), confirming the low expression and/or low surface density of somatostatin receptors and also implying the benign status of evaluated insulinomas; malignant insulinomas were shown to express somatostatin receptors more frequently [8, 23]. In keeping with the benign status of the primary lesions, no metastases were found elsewhere in any of the patients. However, in contrast to conventional imaging modalities, nuclear medicine imaging routinely allows whole-body scanning for staging purposes, which may be required in advanced disease grade or at suspicion of an advanced stage.
Previous studies showed the significant impact of GLP-1R scintigraphy on modifying clinical management and permitting precise operative treatment, reducing surgical trauma and pancreatic tissue loss [20, 24]. Our results confirm these findings as in four out of eight patients only GLP-1R scintigraphy showed the lesion in the pancreas enabling focused access to the tumour and preserving as much of the normal pancreatic tissue as possible. Hypoglycaemia and nausea were reported as possible side effect of GLP-1R targeting radiopharmaceuticals in former studies [20, 23]. However, no side effects were observed in our study. Hypoglycaemia was mainly reported in malignant insulinoma [23]; as already stated, only benign insulinomas were found in the current study.
This study is subject to several important limitations. The most significant limitation is a small number of patients. However, insulinomas are a rare entity and the patient number reflects the expected incidence in the Slovenian population. The second important limitation is the modality used for somatostatin receptor molecular imaging conducted at the time the study: although [99mTc]EDDA/HYNIC-TOC is the best single-photon radiopharmaceutical for p-NEN imaging, the diagnostic performance of Ga-68-labelled somatostatin analogues is significantly higher, rendering them the molecular imaging modality of choice [4]; it is available in our institution from late 2018. However, data in the literature on the diagnostic performance of Ga-68-labelled somatostatin analogues for localization of insulinomas is limited and conflicting: whilst some workers report low sensitivities of around 25 % [12], others report far superior diagnostic performance with sensitivities around 90 % [25]; in the latter study, histological analysis confirmed a well-differentiated insulinoma in all patients, in keeping with our results. Further studies are warranted to clarify this issue. Finally, the radiopharmaceutical used in this study is not commercially available, which hampers confirmation of our results in other centres and in larger patient numbers. To our knowledge, apart from the present report, only one centre has the radiopharmaceutical available for investigational use. However, the same limitation applies to all GLP-1 analogues currently used in human studies, whether labelled with Tc-99m, In-111, or Ga-68. In comparison with In-111-labeled peptide receptor analogues, Tc-99m-labelled peptides provide superior imaging quality and diagnostic performance with the additional advantage of significantly lower radiation exposure [26]; the same advantage was also demonstrated in GLP-1 imaging [27, 28]. Ga-68-labelled analogues provide the potential advantage of superior spatial resolution inherent to PET: a recent study comparing [68Ga]DOTA-exendin-4 PET/CT with [111In]DOTA-exendin-4 SPECT/CT showed superior performance of PET imaging over SPECT imaging [29]. Several other PET radiopharmaceuticals were developed for GLP-1 receptor imaging, labelled either with F-18 [17, 30, 31] or Cu-64 [18]. These also offer the advantage of superior spatial resolution and—with longer half-life of Cu-64 (12.7 h)—the potential advantage of delayed imaging and increased contrast through higher target-to-background ratio. Although these tracers were only implemented in preclinical models with no experience in human studies and all radiometal-labelled exendin-4 probes still suffer from high kidney uptake, 18F-labelled derivatives like [18F]TTCO-Cys40-exendin-4 showed lower kidney uptake, faster clearance rate, and thus favorable tumour-to-kidney ratio [31], which in summary makes this derivative an interesting probe for further clinical translation. Nevertheless, SPECT/CT systems combining conventional tomographic gamma cameras with CT imaging still represent the most widely available nuclear medicine imaging modality. Therefore, a 99mTc-labelled GLP-1 receptor analogue represents the most suitable option for widespread adoption of GLP-1R imaging from the viewpoint of availability, image quality, diagnostic performance, possibility of whole-body staging, and radiation exposure.
Conclusion
[Lys40(AhxHYNIC-[99mTc]/EDDA)NH2]-exendin-4 hybrid SPECT/CT imaging for localization of biochemically proven insulinoma demonstrates excellent imaging characteristics and diagnostic accuracy. The limited availability of the GLP-1R targeting radiopharmaceuticals precludes the widespread adoption of this imaging approach. If commercially available in the future, these agents may be considered an imaging method of choice for this indication, not only in patients with negative or inconclusive results on conventional imaging. In particular, Tc-99m-labelled GLP-1R agonists may offer an optimal combination of relevant characteristics for introduction into clinical practice.
References
Oberg K (2010) Pancreatic endocrine tumors. Semin Oncol 37:594–618
Guettier J-M, Gorden P (2010) Insulin secretion and insulin-producing tumors. Expert Rev Endocrinol Metab 5:217–227
Vanderveen K, Grant C (2010) Insulinoma. Cancer Treat Res 153:235–252
Falconi M, Eriksson B, Kaltsas G et al (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103:153–171
Zhao Y-P, Zhan H-X, Zhang T-P, Cong L, Dai MH, Liao Q, Cai LX (2011) Surgical management of patients with insulinomas: result of 292 cases in a single institution. J Surg Oncol 103:169–174
Lewis MA, Thompson GB, Young WF (2012) Preoperative assessment of the pancreas in multiple endocrine neoplasia type 1. World J Surg 36:1375–1381
Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K (2013) Diagnosis and management of insulinoma. World J Gastroenterol 19:829–837
Sowa-Staszczak A, Pach D, Mikołajczak R, Mäcke H, Jabrocka-Hybel A, Stefańska A, Tomaszuk M, Janota B, Gilis-Januszewska A, Małecki M, Kamiński G, Kowalska A, Kulig J, Matyja A, Osuch C, Hubalewska-Dydejczyk A (2013) Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC- 99mTc/EDDA)NH2]-exendin-4 for the detection of insulinoma. Eur J Nucl Med Mol Imaging 40:524–531
Guettier J-M, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, Libutti SK, Pingpank JF, Gorden P (2009) Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab 94:1074–1080
Morganstein DL, Lewis DH, Jackson J, Isla A, Lynn J, Devendra D, Meeran K, Todd JF (2009) The role of arterial stimulation and simultaneous venous sampling in addition to cross-sectional imaging for localisation of biochemically proven insulinoma. Eur Radiol 19:2467–2473
Nikfarjam M, Warshaw AL, Axelrod L et al (2016) Improved contemporary surgical management of insulinomas: a 25-year experience at the Massachusetts General Hospital. Ann Surg 247:165–172
Sharma P, Arora S, Karunanithi S, Khadgawat R, Durgapal P, Sharma R, Kandasamy D, Bal C, Kumar R (2016) Somatostatin receptor based PET/CT imaging with 68Ga-DOTA-Nal3-octreotide for localization of clinically and biochemically suspected insulinoma. Q J Nucl Med Mol Imaging 60:69–76
Reubi JC, Waser B (2003) Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging 30:781–793
Körner M, Stöckli M, Waser B et al (2007) GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 48:736–743
Christ E, Wild D, Forrer F, Brändle M, Sahli R, Clerici T, Gloor B, Martius F, Maecke H, Reubi JC (2009) Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab 94:4398–4405
Luo Y, Pan Q, Yao S, Yu M, Wu W, Xue H, Kiesewetter DO, Zhu Z, Li F, Zhao Y, Chen X (2016) Glucagon-like peptide-1 receptor PET/CT with 68Ga-NOTA-exendin-4 for detecting localized Insulinoma: a prospective cohort study. J Nucl Med 57:715–720
Kiesewetter DO, Guo N, Guo J, Gao H, Zhu L, Ma Y, Niu G, Chen X (2012) Evaluation of an [(18)F]AlF-NOTA analog of exendin-4 for imaging of GLP-1 receptor in insulinoma. Theranostics 2:999–1009
Wu Z, Liu S, Nair I, Omori K, Scott S, Todorov I, Shively JE, Conti PS, Li Z, Kandeel F (2014) (64)Cu labeled sarcophagine exendin-4 for microPET imaging of glucagon like peptide-1 receptor expression. Theranostics 4:770–777
Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ, Endocrine Society (2009) Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 94:709–728
Christ E, Wild D, Ederer S, Béhé M, Nicolas G, Caplin ME, Brändle M, Clerici T, Fischli S, Stettler C, Ell PJ, Seufert J, Gloor B, Perren A, Reubi JC, Forrer F (2013) Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol 1:115–122
Ardengh JC, Rosenbaum P, Ganc AJ, Goldenberg A, Lobo EJ, Malheiros CA, Rahal F, Ferrari AP (2000) Role of EUS in the preoperative localization of insulinomas compared with spiral CT. Gastrointest Endosc 51:552–555
Bertherat J, Tenenbaum F, Perlemoine K, Videau C, Alberini JL, Richard B, Dousset B, Bertagna X, Epelbaum J (2003) Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in vivo and in vitro study. J Clin Endocrinol Metab 88:5353–5360
Wild D, Christ E, Caplin ME, Kurzawinski TR, Forrer F, Brandle M, Seufert J, Weber WA, Bomanji J, Perren A, Ell PJ, Reubi JC (2011) Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J Nucl Med 52:1073–1078
Wenning AS, Kirchner P, Antwi K, Fani M, Wild D, Christ E, Gloor B (2015) Preoperative glucagon-like peptide-1 receptor imaging reduces surgical trauma and pancreatic tissue loss in insulinoma patients: a report of three cases. Patient Saf Surg 9:23
Nockel P, Babic B, Millo C, Herscovitch P, Patel D, Nilubol N, Sadowski SM, Cochran C, Gorden P, Kebebew E (2017) Localization of insulinoma using 68Ga-DOTATATE PET/CT scan. J Clin Endocrinol Metab 102:195–199
Gabriel M, Decristoforo C, Donnemiller E, Ulmer H, Watfah Rychlinski C, Mather SJ, Moncayo R (2003) An intrapatient comparison of 99mTc-EDDA/HYNIC-TOC with 111In-DTPA-octreotide for diagnosis of somatostatin receptor-expressing tumors. J Nucl Med 44:708–716
Tomaszuk M, Sowa-Staszczak A, Lenda-Tracz W, Glowa B, Pach D, Buziak-Bereza M, Stefanska A, Janota B, Pawlak D, Mikolajczak R, Hubalewska-Dydejczyk AB (2011) Dosimetry of exendin-4 based radiotracer for glucagonlikepeptide-1 receptor imaging: an initial report. J Phys Conf Ser 317:012011
Selvaraju RK, Bulenga TN, Espes D, Lubberink M, Sörensen J, Eriksson B, Estrada S, Velikyan I, Eriksson O (2015) Dosimetry of [(68)Ga]Ga-DO3A-VS-Cys(40)-exendin-4 in rodents, pigs, non-human primates and human - repeated scanning in human is possible. Am J Nucl Med Mol Imaging 5:259–269
Antwi K, Fani M, Heye T, Nicolas G, Rottenburger C, Kaul F, Merkle E, Zech CJ, Boll D, Vogt DR, Gloor B, Christ E, Wild D (2018) Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imaging 45:2318–2327
Kiesewetter DO, Gao H, Ma Y, Niu G, Quan Q, Guo N, Chen X (2012) 18F-radiolabeled analogs of exendin-4 for PET imaging of GLP-1 in insulinoma. Eur J Nucl Med Mol Imaging 39:463–473
Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, Todorov I, Fox JM, Li Z, Shively JE, Conti PS, Kandeel F (2013) Development and evaluation of 18F-TTCO-Cys40-Exendin-4: a PET probe for imaging transplanted islets. J Nucl Med 54:244–251
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Senica, K., Tomazic, A., Skvarca, A. et al. Superior Diagnostic Performance of the GLP-1 Receptor Agonist [Lys40(AhxHYNIC-[99mTc]/EDDA)NH2]-Exendin-4 over Conventional Imaging Modalities for Localization of Insulinoma. Mol Imaging Biol 22, 165–172 (2020). https://doi.org/10.1007/s11307-019-01372-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01372-z