Abstract
Purpose
To compare the value of pretreatment functional and morphological imaging parameters for predicting survival in patients undergoing transarterial radioembolization using yttrium-90 (90Y-TARE) for unresectable hepatocellular carcinoma (uHCC).
Methods
We analysed data from 48 patients in our prospective database undergoing 90Y-TARE treatment for uHCC (31 resin, 17 glass). All patients underwent 18F-FDG PET/CT and morphological imaging (CT and MRI scans) as part of a pretherapeutic work-up. Patients did not receive any treatment between these imaging procedures and 90Y-TARE. Kaplan-Meier estimates of progression-free survival (PFS) and overall survival (OS) were used to assess the prognostic value of 18F-FDG PET/CT metabolic parameters, including SUVmax, tumour-to-liver (T/L) uptake ratio and SUVmean of healthy liver, and morphological data, including number and size of lesions, portal-venous infiltration (PVI). Relevant prognostic factors for HCC including Child-Pugh class, Barcelona Clinic Liver Cancer (BCLC) stage, tumour size, PVI and serum AFP level were compared with metabolic parameters in univariate and multivariate analyses.
Results
The median follow-up in living patients was 16.2 months (range 11.4–50.1 months). Relapse occurred in 34 patients (70.8%) at a median of 7.4 months (range 1.4–27.9 months) after 90Y-TARE, and relapse occurred in 24 of 34 patients (70.8%) who died from their disease at a median of 8.1 months (range 2.2–35.2 months). Significant prognostic markers for PFS were the mean and median lesion SUVmax (both P = 0.01; median PFS 10.2 vs. 7.4 months), and significant prognostic markers for OS were the first quarter (Q1) cut-off values for lesion SUVmax and T/L uptake ratio (both P = 0.02; median OS 30.9 vs. 9 months). The multivariate analysis confirmed that lesion SUVmax and T/L uptake ratio were independent negative predictors of PFS (hazard ratio, HR, 2.7, 95% CI 1.2–6.1, P = 0.02, for mean SUVmax; HR 2.6, 95% CI 1.1–5.9, P = 0.02, for median SUVmax:) and OS (HR 3.2, 95% CI 1–10.9, P = 0.04 for Q1 SUVmax; HR 3.7, 95% CI 1.1–12.2, P = 0.03, for Q1 T/L uptake ratio), respectively, when testing with either the BCLC staging system or serum AFP level.
Conclusion
Lesion SUVmax and T/L uptake ratio as assessed by 18F-FDG PET/CT, but not morphological imaging, were predictive markers of survival in patients undergoing 90Y-TARE for uHCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most frequent type of primary liver cancer and is considered the second leading cause of cancer death worldwide [1]. Surgery is often the most effective modality of treatment, but it is not always possible due to the anatomical location of lesions or the massive involvement at presentation. Transarterial radioembolization using 90Y microspheres (90Y-TARE) has recently been used in more patients diagnosed with locally advanced HCC. It involves the administration of 90Y-labelled resin microspheres (SIR-Spheres™; SIRTex Medical, Sydney, Australia) or glass microspheres (TheraSphere™; BTG Biocompatibles, Farnham, UK) directly into the hepatic arteries which supply the majority of the tumour cells, thus directing the injected microspheres selectively to the tumour [2].
Morphological assessment based on solid tumour size and Response Evaluation Criteria In Solid Tumors (RECIST) have been used as the gold standard in the initial evaluation and for determining response to treatment [3]. Tumour size alone has many limitations in predicting prognosis and assessing the response to treatment, mainly in the case of cytostatic treatments and 90Y-TARE where tumour necrosis may lead to an apparent increase in the size of the lesion. Analysing biomarkers of tumour metabolism has also been suggested as a possible prognostic factor, especially for assessing the response to therapy [4]. However, metabolic prognostic factors have not been well investigated in HCC. The aim of this study was to compare the prognostic value of pretreatment functional and morphological imaging staging variables including 18F-FDG PET/CT in patients undergoing 90Y-TARE for unresectable HCC (uHCC).
Materials and methods
Patient selection
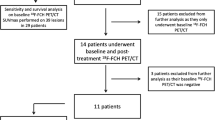
We analysed our prospective database and identified patients who had undergone 18F-FDG PET/CT and morphological imaging (CT, MRI scans) before 90Y-TARE for uHCC between December 2010 to December 2015. 18F-FDG PET/CT and morphological imaging were performed in all patients as part of pretherapeutic work-up. The American Association for the Study of Liver Diseases (AASLD) guidelines [5] were used to diagnose HCC. The BCLC staging system has been used to stage HCC [6]. Patients were not eligible for curative treatment if they had a locally advanced tumour, multifocal disease, poor liver reserve, portal-venous infiltration (PVI) or extrahepatic metastasis. Patients were included if they had liver-dominant or liver-only disease, a Child-Pugh score of ≤B7, adequate haematological, renal and hepatic function, an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and a life expectancy ≥3 months. Patients with an inadequate liver reserve (bilirubin ≥34 μmol/L, ascites), a Child-Pugh score ≥B8, an ECOG performance status ≥3, a lung shunt fraction of ≥20%, an estimated lung dose of ≥30 Gy per session and 50 Gy in total and an uncorrectable extrahepatic flow on the pretherapy 99mTc-MAA SPECT/CT scan were excluded. All patients underwent imaging procedures and 90Y-TARE as standard care. The local Ethics Research Committee of the State of Vaud, taking into account the retrospective nature of this study, approved the protocol (no. CER-VD 2016–00640) and waived the need for patient informed consent for the analysis.
18F-FDG PET/CT
Patients underwent 18F-FDG PET/CT on a Discovery D690 TOF (45 patients), a Discovery LS (GE HealthCare, Waukesha, WI; 2 patients) and a Gemini TF Big Bore (Philips, Da Best, The Netherlands; 1 patient) 50–70 min after a planned intravenous injection of 3.7 ± 0.5 MBq/kg of 18F-FDG. All patients fasted for at least 6 h and blood glucose levels were less than 140 mg/dL before administration of 18F-FDG. A low-dose helical CT scan (120–140 kV, 80–200 mA) was first performed for anatomical correlation and attenuation correction. Then whole-body emission images were acquired using seven to nine overlapping bed positions of 2 min each (starting from the top of skull and ending at the mid-thigh). Images were reconstructed using conventional oncological iterative protocols with body weight-normalized SUV computation.
Analysis of 18F-FDG PET/CT images
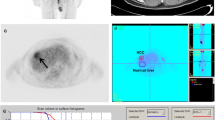
For each patient, lesion SUVmax (grams per millilitre), tumour-to-liver (T/L) uptake ratio (defined as the lesion SUVmax divided by the mean SUV, SUVmean, of a circular region of interest of diameter at least 3 cm drawn on the healthy liver), and SUVmean of healthy nonlesional liver were computed. Morphological parameters including tumour size (the longest diameter of all measurable tumours), the number of lesions, and the presence of PVI were analysed on contrast-enhanced CT and MRI scans.
90Y-TARE planning and procedure
Before 90Y-TARE, all patients underwent a pretherapy SPECT/CT scan with intraarterial administration of 120–180 MBq of 99mTc-MAA. Lung shunting was evaluated on whole-body and planar images. Abdominal SPECT/CT (Discovery 670/Infinia Hawkeye IV; GE Healthcare, Waukesha, WI) was performed (120 × 20 s, low-energy high-resolution collimator) and reconstructed with a Xeleris 3.1 workstation (GE Healthcare) using a 3D ordered-subsets expectation maximization algorithm (four iterations, ten subsets) with a Butterworth filter (cut-off 0.5 cycles/cm, order 10) and CT-based attenuation correction. According to the findings of the 99mTc-MAA SPECT/CT scan and clinical and hepatic biochemical parameters, the required 90Y administered activity in gigabecquerels was determined from a pretreatment partition dosimetry model [7, 8] and was administered into the selected hepatic artery. Patients with small tumour volumes were preferentially addressed to 90Y-labelled glass microspheres due to their higher specific 90Y activity and lower particle number with the aim of avoiding lesion saturation and consequent reflux to nontarget volumes. A post 90Y-TARE SPECT/CT or PET/CT scan was performed to confirm the optimal distribution of 90Y microspheres.
Statistical analyses
Continuous variables are reported as medians (25th – 75th interquartile range) and dichotomous data as percentages. The prognostic value of 18F-FDG PET/CT metabolic parameters was assessed using the endpoints progression-free survival (PFS) and overall survival (OS). PFS was defined as time from the date of 90Y-TARE to the date of the first occurrence of hepatic tumour progression based on imaging with contrast-enhanced CT or MRI (World Health Organization Bidimensional and Three-dimensional European Association for the Study of the Liver Response Criteria), distant recurrence, death or last known consultation (censored). OS was defined as the time from the date of 90Y-TARE to death from any cause or last known consultation (censored). Survival curves were obtained from Kaplan-Meier estimates and compared using the log-rank test. To date, given the absence of known prognostic thresholds, the mean, median, first quarter (Q1) and third quarter (Q3) values were determined for each metabolic parameter (lesion SUVmax, T/L uptake ratio and healthy liver SUVmean) in the entire cohort and served as cut-off values to create subgroups. Relevant prognostic factors in HCC including Child-Pugh class, Barcelona Clinic Liver Cancer (BCLC) stage, tumour size, PVI and serum AFP level were compared with metabolic parameters in univariate and multivariate analyses. All statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY). P values <0.05 were considered as statistically significant.
Results
Study population
Clinical, demographic and therapeutic data are given in Tables 1 and 2. The study included 48 consecutive patients (median age 68, range 61 to 72 years) undergoing 90Y-TARE for uHCC (31 resin, 17 glass) between December 2010 and December 2015. The median delay between 18F-FDG PET/CT and 90Y-TARE was 0.5 months (range 0.03–2.8 months). The median delay between the last morphological imaging (CT or, MRI scans) and 90Y-TARE was 1.6 months (range 0.2–3.9 months). Patients did not receive any treatment between 18F-FDG PET/CT, morphological imaging and 90Y-TARE. The median delay between the last treatment (including targeted therapy, embolization, transarterial chemoembolization, radiofrequency ablation, ethanol ablation, cryoablation, 90Y-TARE) and 90Y-TARE performed after 18F-FDG PET/CT was 2.3 months (range 0.4–6.6 months). Using the BCLC staging system, 3 patients (6.3%) were stage A, 19 (39.6%) stage B and 26 (54.1%) stage C. Three patients (6.3%) had a normal liver; all others (93.7%) had cirrhotic liver disease including 35 patients with Child-Pugh class A (72.9%) and 10 patients with Child-Pugh class ≤B7 (20.8%). Many patients underwent several other treatments (Table 3) with two or more treatment modalities in six patients (12.5%) before 90Y-TARE and in six patients (12.5%) after 90Y-TARE.
Survival analysis
The median follow-up in living patients was 16.2 months (range 11.4–50.1 months). Relapse occurred in 34 patients (70.8%) at a median of 7.4 months (range 1.4–27.9 months) after 90Y-TARE, and relapse occurred in 24 of 34 patients (70.8%) who died from their disease at a median of 8.1 months (range 2.2–35.2 months). As shown in Fig. 1 and Table 4, Significant prognostic markers for PFS were the mean and median lesion SUVmax (both P = 0.01; median PFS 10.2 vs. 7.4 months), and significant prognostic markers for OS were the Q1 cut-off values for lesion SUVmax and T/L uptake ratio (both P = 0.02; median OS 30.9 vs. 9 months). In the univariate analysis, the other variables including Child-Pugh class, BCLC stage, serum AFP level, SUVmean of healthy liver and morphological parameters (size of lesions considering limits of 3 cm or 5 cm and the presence of PVI) were not predictive of survival (Table 4). The multivariate analysis confirmed that lesion SUVmax and T/L uptake ratio were independent negative predictors of PFS (hazard ratio, HR, 2.7, 95% CI 1.2–6.1, P = 0.02 for mean SUVmax; HR 2.6, 95% CI 1.1–5.9, P = 0.02 for median SUVmax) and OS (HR 3.2, 95% CI 1–10.9, P = 0.04 for Q1 SUVmax; HR 3.7, 95% CI 1.1–12.2, P = 0.03 for Q1 T/L uptake ratio), respectively, when testing with either the BCLC staging system and serum AFP level (Table 5).
Discussion
Although 18F-FDG PET/CT is used for staging in many oncological conditions, few data are available addressing the potential utility of this imaging modality in treatment planning in patients with a hepatic tumour treated with 90Y microspheres [9]. To the best of our knowledge, this is the first study comparing the prognostic value of pretreatment functional and morphological imaging parameters for predicting survival in patients undergoing 90Y-TARE for uHCC. In this specific patient population predictive markers are highly valuable. Due to the underlying liver disease, patients need to be better selected for maximum benefit. In the current study lesion SUVmax and T/L uptake ratio as assessed by 18F-FDG PET/CT were the only predictive markers of survival in these patients. No correlations with survival after hepatic 90Y-TARE were found with 18F-FDG metabolism in the healthy liver tissue outside the tumour (in terms of SUVmean of nontumoral liver tissue), the size of the largest hepatic lesion or the presence of PVI.
Our findings are corroborated by those of a recent meta-analysis including a total of 1,721 HCC patients which showed that pretreatment 18F-FDG PET/CT is a useful tool in predicting the prognosis in HCC patients. Both high tumour SUV and T/L uptake ratio were strongly associated with poorer PFS (P = 0.000) and OS (P = 0.000) [10]. Riedl et al. investigated the correlation between 18F-FDG uptake on pretreatment PET and some cellular characteristics and the clinical behaviour of tumours in 90 patients with resectable colorectal cancer metastatic to the liver. SUVmax was significantly correlated with GLUT-1, Ki67 and p53, predicting a longer survival in patients with a low SUV than in those with a high SUV [11]. Other studies have concluded that 18F-FDG PET/CT is a more sensitive and accurate predictor of response to therapy compared to CT and MRI for liver metastases treated with 90Y-TARE [12–15]. In a retrospective study on 28 HCC patients undergoing 90Y-TARE, Soydal et al. demonstrated that the size of the largest liver lesion has a significant negative effect on survival [16]. Compared with our findings, these divergent results might be explained by a larger sample of patients with an advanced disease stage as tumour activity is an independent predictive factor.
The absence of prognostic value of tumour size and PVI may imply that the efficacy of 90Y-TARE is independent of morphological factors or local invasion. Furthermore, in 25 patients with liver-dominant metastatic colorectal cancer, Zerizer et al. [17] found that for the early assessment of the metabolic response to 90Y-TARE, 18F-FDG PET/CT is superior to contrast-enhanced CT using RECIST, predicting PFS and the responses of tumour markers after 90Y-TARE. The current study confirmed that metabolic parameters including lesion SUVmax and T/L uptake ratio have similar predictive value in uHCC, whereas morphological variables do not. In a cohort study investigating 31 patients with liver metastases from different histological types, Zalom et al. found no association between the decrease of 18F-FDG uptake in lesions after 90Y-TARE and survival. Correlation between pretreatment 18F-FDG uptake in the lesion and survival was not investigated in that study [18].
The role of 18F-FDG PET/CT in the primary assessment of uHCC is considered limited due to its low sensitivity in the overall HCC patient population [19]. 18F-FDG uptake can vary from low in well-differentiated uHCC to variable in poorly differentiated HCC [20, 21]. However, in a recent study, Na et al. showed that 18F-FDG uptake on pretreatment PET/CT has an incremental prognostic value for OS in both intrahepatic and extrahepatic HCC in patients with BCLC stage C disease [22]. Higher SUVmax and T/L uptake ratio in uHCC imply a higher absolute and relative lesion metabolism than in normal hepatic parenchyma resulting in higher aggressiveness of well-differentiated HCC, which may negatively affect the prognosis. Kucuk et al. [23] investigated the role of 18F-FDG PET/CT in the selection of uHCC patients before 90Y-TARE in 19 patients. Assessment of disease stage and metabolic activity of liver lesions showed that higher lesion SUVmax was unexpectedly associated with better PFS rates after 90Y-TARE. These findings appear divergent from those of the current study that showed better survival in patients with mean and median SUVmax below the cut-off values of 8.1 and 6.7 g/mL, respectively, for the hottest malignant lesion, and from those of Sabet et al. [24] who investigated the predictive value of 18F-FDG PET/CT in 33 HCC patients who underwent PET/CT at baseline and 4 weeks after 90Y-TARE. The 12 18F-FDG PET-negative patients had a significantly longer OS than 18F-FDG PET-positive patients (P = 0.01). Among the 21 18F-FDG-positive patients, metabolic responders on follow-up survived significantly longer than metabolic nonresponders (P = 0.003). The current study confirms previous suggestions that quantitative 18F-FDG PET/CT is useful for predicting survival after 90Y-TARE for HCC as the results are in line with those of Sabet at al. suggesting that lower lesion SUVmax can positively predict the final outcome after 90Y-TARE.
Our study had several limitations. The main limitation the relatively small but uniform patient cohort. Secondly, a significant proportion of patients had undergone treatments before (12.5%) and after (12.5%) 90Y-TARE. However, this limitation might be considered as a standard limitation due to the growing treatment landscape of HCC and its confounding impact in OS. This shortcoming was limited by evaluating PFS. In our analysis, functional imaging (SUVmax and T/L uptake ratio), but not size of the lesion, BCLC stage or Child-Pugh score, was predictive. This contradicts the findings of phase II studies, which have shown that these parameters are predictive prior to 90Y-TARE [25]. Adopting personalized dosimetry based on hepatic volumetric analysis may explain the absence of impact of tumour size, BCLC stage and Child-Pugh score on post-TARE survival. Nevertheless, further larger studies with a prospective design are warranted to confirm our results.
Conclusion
Lesion SUVmax and T/L uptake ratio as assessed by 18F-FDG PET/CT, but not morphological imaging, were identified as negative predictive factors for survival in patients undergoing 90Y-TARE for uHCC. These findings highlight the prognostic role of metabolic imaging in addition to morphological imaging in the pretherapeutic work-up of patients undergoing 90Y-TARE for uHCC.
References
Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765–779.
Taylor I, Bennett R, Sherriff S. The blood supply of colorectal liver metastases. Br J Cancer. 1978;38:749–756.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216.
Larson SM, Schwartz LH. 18F-FDG PET as a candidate for “qualified biomarker”: functional assessment of treatment response in oncology. J Nucl Med. 2006;47:901–903.
Murray KF, Carithers RL, AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432.
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338.
Gnesin S, Canetti L, Adib S, Cherbuin N, Silva Monteiro M, Bize P, et al. Partition model-based 99mTc-MAA SPECT/CT predictive dosimetry compared with 90Y TOF PET/CT posttreatment dosimetry in radioembolization of hepatocellular carcinoma: a quantitative agreement comparison. J Nucl Med. 2016;57:1672–1678.
Van Der Gucht A, Jreige M, Denys A, Blanc-Durand P, Boubaker A, Pomoni A, et al. Resin versus glass microspheres for yttrium-90 transarterial radioembolization: comparing survival in unresectable hepatocellular carcinoma using pretreatment partition model dosimetry. J Nucl Med. 2017. doi:10.2967/jnumed.116.184713.
Bagni O, Filippi L, Schillaci O. The role of 18F-FDG positron emission tomography in the follow-up of liver tumors treated with 90Yttrium radioembolization. Am J Nucl Med Mol Imaging. 2015;5:220–232.
Sun DW, An L, Wei F, Mu L, Shi XJ, Wang CL, et al. Prognostic significance of parameters from pretreatment (18)F-FDG PET in hepatocellular carcinoma: a meta-analysis. Abdom Radiol (NY). 2016;41:33–41.
Riedl CC, Akhurst T, Larson S, Stanziale SF, Tuorto S, Bhargava A, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48:771–775.
Wong CO, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F] FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29:815–820.
Pöpperl G, Helmberger T, Münzing W, Schmid R, Jacobs TF, Tatsch K. Selective internal radiation therapy with SIR-Spheres in patients with nonresectable liver tumors. Cancer Biother Radiopharm. 2005;20:200–208.
Szyszko T, Al-Nahhas A, Canelo R, Habib N, Jiao L, Wasan H, et al. Assessment of response to treatment of unresectable liver tumours with 90Y microspheres: value of FDG PET versus computed tomography. Nucl Med Commun. 2007;28:15–20.
Haug AR, Heinemann V, Bruns CJ, Hoffmann R, Jakobs T, Bartenstein P, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2011;38:1037–1045.
Soydal C, Keskin O, Kucuk ON, Ozkan E, Bilgic S, Idilman R, et al. Prognostic factors for prediction of survival of hepatocellular cancer patients after selective internal radiation therapy. Ann Nucl Med. 2015;29:426–430.
Zerizer I, Al-Nahhas A, Towey D, Tait P, Ariff B, Wasan H, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging. 2012;39:1391–1399.
Zalom M, Yu R, Friedman M, Bresee C, Waxman A. FDG PET/CT as a prognostic test after 90Y radioembolization in patients with metastatic hepatic disease. Clin Nucl Med. 2012;37:862–865.
Izuishi K, Yamamoto Y, Mori H, Kameyama R, Fujihara S, Masaki T, et al. Molecular mechanisms of [18F]fluorodeoxyglucose accumulation in liver cancer. Oncol Rep. 2014;31:701–706.
Pant V, Sen IB, Soin AS. Role of 18F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749–757.
He Y, Guo Q. Clinical applications and advances of positron emission tomography with fluorine-18-fluorodeoxyglucose (18F-FDG) in the diagnosis of liver neoplasms. Postgrad Med J. 2008;84:246–251.
Na SJ, Oh JK, Hyun SH, Lee JW, Hong IK, Song BI, et al. 18F-FDG PET/CT can predict survival of advanced hepatocellular carcinoma patients: A multicenter retrospective cohort study. J Nucl Med. 2016. doi:10.2967/jnumed.116.182022.
Kucuk ON, Soydal C, Araz M, Bilgic S, Ibis E. Prognostic importance of 18F-FDG uptake pattern of hepatocellular cancer patients who received SIRT. Clin Nucl Med. 2013;38:e283–e289.
Sabet A, Ahmadzadehfar H, Bruhman J, Sabet A, Meyer C, Wasmuth J-C, et al. Survival in patients with hepatocellular carcinoma treated with 90Y-microsphere radioembolization. Prediction by 18F-FDG PET. Nuklearmedizin. 2014;53:39–45.
Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona Clinic Liver Cancer stages: a European evaluation. Hepatology. 2011;54(3):868–878.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The procedure followed was in accordance with the ethical standards and guidelines of the responsible committee on human experimentation.
Conflicts of interest
None.
Additional information
All authors approved the manuscript, and agree with its submission to the EJNMMI.
Rights and permissions
About this article
Cite this article
Jreige, M., Mitsakis, P., Van Der Gucht, A. et al. 18F-FDG PET/CT predicts survival after 90Y transarterial radioembolization in unresectable hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 44, 1215–1222 (2017). https://doi.org/10.1007/s00259-017-3653-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3653-0