Abstract
Objective
To use T2 relaxation time distribution profiles to assess inter-group regional differences along articular surfaces and to evaluate the feasibility of this analysis for comparison of cartilage insufficiency.
Materials and methods
Twelve pairs matched according to age and gender (12 healthy volunteers and 12 patients after anterior cruciate ligament reconstruction (ACLR)) underwent 3-T MRI. T2 maps were calculated from six time echo images of the mid-sagittal slice in the lateral and medial compartment. The femoral and tibial cartilage was analyzed by measuring T2 distribution profiles along the articular surfaces.
Results
T2 distribution profiles were generated along the length of the articular surface in the femorotibial compartments. Differences in the T2 distribution profiles between the tibial and femoral cartilage as well as between the cartilage of the femoral condyles were identified in healthy individuals. T2 distribution profiles clearly demonstrated cartilage insufficiency in the weight-bearing areas for subjects in the ACLR group.
Conclusions
T2 distribution profiles can identify regional differences in femoral and tibial cartilage. The T2 distribution profile pattern is preserved with cartilage insufficiency, however, with important differences in T2 values for the ACLR group in weight-bearing areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging is the ideal modality to assess cartilage degeneration due to its ability to non-invasively quantify the structural and biochemical changes associated with the onset and progression of osteoarthritis [1, 2]. Novel MRI sequences such as T2 mapping and T1rho have been developed in order to provide a quantitative assessment of cartilage degeneration [3,4,5]. T2 mapping is the most widely utilized technique, and has been known to be sensitive to both collagen matrix organization and the water content of cartilage [3,4,5].
Quantitative evaluations of cartilage T2 texture may provide further insight into cartilage matrix properties reflective of injury and degeneration [6]. Given the complex relationship between cartilage T2 relaxation time and disease severity, recent evidence suggests that mean T2 values alone may be inadequate for determining cartilage health over time [6]. Furthermore, the definition of “healthy” cartilage and the sub-compartments lacks consistency across centers and the specific values associated with healthy cartilage are variable within the literature [7]. To overcome these limitations, recent image analysis techniques have been introduced to evaluate the spatial distribution of cartilage T2 values [6]. Our study aimed to further develop this concept by creating a T2 distribution profile along the length of the femorotibial compartment articular surfaces in order to assess regional differences. Additionally, we aimed to evaluate the feasibility of this type of analysis for intergroup comparisons of cartilage insufficiency.
Materials and methods
Subjects
The National Medical Ethics Committee approved the study and written informed consent was obtained from all participants. Twelve matched pairs (12 healthy volunteers and 12 patients with prior anterior cruciate ligament repair) were recruited for the study.
A total of 247 patients underwent anterior cruciate ligament repair (ACLR) at the Orthopaedic Department at our institution in the years 2008 and 2009. The inclusion criteria for the ACLR group were (1) 16–45 years at time of ACLR, (2) transtibial ACLR technique performed by a single senior surgeon using the same graft fixation, (3) quadruple stranded semitendinosus-gracilis tendon graft, (4) intact lateral meniscus at ACLR according to arthroscopy records (5) body mass index (BMI) of 18.5 to 30, (6) preoperative sports activity of at least 4 on the Tegner scale. All ACLR procedures were performed by a senior orthopedic surgeon (O.Z.); in the tibial tunnel graft was fixed with Milagro interference screw (DePuyMitek, Raynham, MA, USA) and in the femoral tunnel graft was fixed with RIGIDfix (Mitek, Johnson & Johnson, Norwood, MA, USA) [8]. All patients underwent an identical rehabilitation program. Based on the arthroscopy record and patient’s history, the following exclusion criteria were established (1) known chondropathy, (2) concomitant collateral ligament injury, (3) concomitant posterior cruciate ligament injury, (4) MR contraindication, and (5) meniscal tear at ACLR. Twenty-three patients met the study inclusion criteria, however only 15 were successfully contacted. In three patients, graft rupture was identified on MR examination, and thus were excluded from the study. Thus, 12 patients (nine male, three female) were included in the study (Table 1). The average time from the ACLR to the MR examination was 6.3 ± 0.5 years.
Twelve healthy volunteers were recruited to a control group, with each participant matched to an ACLR patient according to age and sex. The inclusion criteria for the control group were (1) International Knee Documentation Committee Subjective Knee Form (IKDC) score of at least 95 and (2) no history indicative of any knee joint disorder.
Subjective clinical evaluation
Two questionnaires were presented to the study subjects at the MR examination; IKDC and Tegner scale.
MR protocol
MR examinations were performed as single time-interval captures using a 3.0-T imager (Magnetom®Trio, Siemens, Erlangen, Germany) with an eight-channel transmit-receive knee coil (Invivo, Gainesville, FL, USA). To minimize the changes of different loading conditions, subjects were instructed to avoid sport activities for at least 24 h prior to the MR examination and were scanned after resting at least 30 min. The imaging protocol included proton density (PD) turbo spin-echo (TSE) fat-saturation (FS) images in the sagittal plane (2230/29 [TR msec/TE msec], 16 cm field of view [FOV], 3 mm/1 mm [slice thickness/interslice gap], 512 × 512 matrix, 120° flip angle [FA], two signals acquired) and in the coronal plane (2540/35 [TR msec/TE msec], 15 cm FOV, 3 mm/1 mm [slice thickness/interslice gap], 384 × 384 matrix, 150° FA, two signals acquired) [8]. T2 maps were calculated from obtained six images in the sagittal plane (1000/13.8; 27.6; 41.4; 55.2; 69.0; 82.8 [TR msec/TE msec], 16 cm FOV, 3 mm/1 mm [slice thickness/interslice gap], 384 × 384 matrix, 180° FA, one signal acquired).
Quantitative cartilage evaluation
For quantitative analysis, T2 maps were first calculated from six TE images at TEs by the corresponding ImageJ macro program, an open-source image-processing program designed for scientific multidimensional images (NIH programs, USA). T2 maps were calculated on a pixel-by-pixel basis by fitting the recorded signal (S) to a first-order model described by:
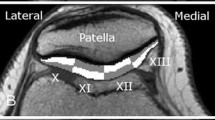
where t is n∙TE, n is the echo number, and TE is the inter-echo time. After computation of relaxation times, color-coded maps were created to allow better visual discrimination of cartilage T2 maps. Analysis was performed on three consecutive mid-sagittal slices in both femorotibial compartments. Femoral and tibial cartilages of each compartment were analyzed separately by measuring T2 distribution profiles along the articular surfaces, i.e., from an anterior to posterior direction within the cartilage. All distribution profiles were generated by manual segmentation of the femoral and tibial cartilage. Due to the relatively large voxel dimensions, the distribution of T2 relaxation times plot profiles were obtained from T2 maps by calculating the average T2 values of the all visible cartilage pixels, avoiding strict layer distribution. In order to compare the T2 profiles of different individuals (with variable articular surface lengths), the profiles were considered as functions of relative articular lengths (x), i.e., distances from a starting point and normalized to the articular length (Fig. 1). The maps were filtered in order to reduce the impact of noise and partial-volume effects of bone and synovial fluid on cartilage T2 values. Due to the image acquisition plane, we measured the femoral cartilage only in the anterior and central sub-compartments. Measurements were repeated on the same slice by the same reader at a 1-month time interval. As illustrated in Fig. 1, borders of the menisci were used to define the margins of the covered femoral and tibial cartilage. To determine regional differences of all visible cartilage, a subset analysis was performed for the medial and lateral compartment. Cartilage segmentation was performed in accordance with previous work: subdivided into sub-compartments with regard to the menisci, consistent with the regional subdivision used in Whole-Organ Magnetic Resonance Imaging Score (WORMS) [10,11,12,13]. In our study, the medial compartment was subdivided into the following sub-compartments: medial central femoral (cFM), medial posterior femoral (pFM), medial anterior tibial (aTM), medial central tibial (cTM), and medial posterior tibial (pTM). The lateral compartment was subdivided into the sub-compartments: lateral anterior femoral (aFL), lateral central femoral (cFL), lateral anterior tibial (aTL), lateral central tibial (cTL), and lateral posterior tibial (pTL) sub-compartment.
Menisci assessment
To assess the meniscal status, the meniscal feature from the WORMS system was used [9]. The anterior horn, body, and posterior horn of meniscus were graded separately and a cumulative grade for each meniscus was determined according to paper by Peterfy et al. [10]. The ACLR group was divided according to the meniscal status in two groups: menisci intact and menisci insufficient. In the menisci insufficient group, all patents with signs of meniscal injury were included regardless of the lesion location or meniscal total score.
Statistical analysis
Unpaired t test was used to compare groups with respect to the demographic characteristics. The Mann–Whitney U test was used to compare groups according to the Tegner scale and was used to identify any discrepancy between the mean ranks of two groups in relation to the T2 distribution profiles. We used a non-parametric, distribution-free, Kolmogorov–Smirnov (KS) test to evaluate differences between the control and the ACLR groups in order to reject the null hypothesis of no difference between groups. The results of KS test were expressed as the maximum difference between the cumulative distributions, D, with a corresponding p value. Cronbach’s α coefficient was used to express intra-reader variability. Continuous variables were expressed as mean ± SD and categorical values were expressed as median. Significance was set at p < 0.05. Statistical analysis was performed with SPSS v.17.0 (SPSS Inc., Chicago, IL, USA).
Results
Subjective clinical evaluation
The control group showed significantly higher scores than the ACLR group for the subjective clinical evaluation with IKDC (Table 1). No difference between the groups was observed according to sports activity (Table 1).
Quantitative cartilage evaluation
The T2 distribution profiles along the length of the articular surface in the lateral femorotibial compartment are shown in the Figs. 2, 3 and 4. The result of the KS test comparison of the T2 relaxation times of the femoral and tibial cartilage between the control and the ACLR group shows that datasets do not follow a normal distribution; p < 0.01.
Lateral compartment
The maximum difference between the cumulative distributions for the femoral cartilage was D (200) = 0.23, p < 0.01, with mean 48 ± 6 ms (range, 37–58 ms) and median 50 ms in the control group, while in the ACLR group cartilage mean was 47 ± 5.5 ms (range, 33–58 ms) and median 48 ms (Fig. 2). For the tibial cartilage, the maximum difference between the cumulative distributions D (50) was 0.62, p < 0.01, with mean 39 ± 5 ms (range, 33–48 ms) and median 38 ms in control group, while the ACLR group cartilage mean value was 32 ± 6 ms (range, 27–45 ms) and median 29 ms (Fig. 2). The difference in the median values of the tibial cartilage between the two groups is greater than would be expected by chance; with a statistically significant difference observed (p < 0.01). However, there was no significant difference noted for the femoral cartilage (p = 0.03) (Fig. 4).
Medial compartment
The maximum difference between the cumulative distributions for the femoral cartilage was D (202) = 0.11, p = 0.14, with mean 48 ± 11 ms (range, 39–55 ms) and median 47 ms in the control group, while in the ACLR group cartilage mean was 47 ± 10 ms (range, 36–57 ms) and median 49 ms Fig. 3). For the tibial cartilage, the maximum difference between the cumulative distributions D (182) was 0.39, p < 0.01, with mean 41 ± 10 ms (range, 36–50 ms) and median 49 ms in control group, while in the ACLR group cartilage mean was 34 ± 8 ms (range, 28–48 ms) and the median 34 ms (Fig. 3). The difference in the median between the two groups of tibial cartilage was statistically significant (p < 0.01), however, no significant difference was found for the femoral side cartilage (p = 0.03) (Fig. 4).
Sub-compartment analysis
The results for inter-group comparisons for the sub-compartments are shown in Table 2. The intra-reader reliability for assessments of the femoral-sided cartilage was α = 0.77 and α = 0.80 for the tibial-sided cartilage.
Meniscal status subdivision
Four patients showed lateral meniscal lesions at MR (one patient had a total score of 2; two patients had a total score of 3; one patient had a total score of 4). Five patients showed medial meniscal lesions at MR (two patients had a total score of 2; one patient had a total score of 3; two patients had a total score of 4). No significant difference was found between the menisci insufficient and menisci intact group with either the subjective clinical evaluation or the quantitative cartilage evaluation.
Discussion
In the presented study, analysis of cartilage T2 relaxation time distribution profiles were used in order to assess regional differences along the articular surfaces between the ACLR and control group. We assessed the cartilage regional variations along two adjacent articular surfaces between two observed groups with presumed difference in cartilage status. Similar approach with T2 relaxation time distribution profiles has been previously applied, however only femoral cartilage analysis of healthy individuals was performed [1].
The femoral T2 distribution profiles have a more pronounced sinusoidal pattern compared to profiles seen along the tibial articular surface. The sub-compartmentation ratio was in accordance with previous studies revealing different ratios for the tibial plateaus and comparable ratios for the femoral condyles [10,11,12,13]. When adjusting the tibial plateau ratio, it was shown that the T2 distribution profiles are comparable for the medial and lateral plateau. On both sides, a similar up-sloping pattern was observed towards central and posterior sub-compartment. Conversely, the T2 distribution profiles appear to be different for the femoral condyles. The most apparent difference was observed in weight-bearing areas of the femoral condyles, with an up-sloping pattern in the lateral and a down-sloping pattern in the medial compartment. This finding is consistent with a previous study by Kaneko et al. and may be explained by the fact that the medial condyle has wider weight-bearing area than the lateral condyle, and that weight-bearing areas have lower T2 values than non-weight-bearing areas [1].
Group comparison revealed a relative preservation of the T2 distribution profile patterns with reasonable fitting for the sinusoidal femoral distribution profiles, however, an apparent profile offset was seen in the ACLR group over the entire tibial cartilage length. In sub-compartmental analysis, anterior femoral lateral and posterior femoral medial sub-compartment showed no group differences, thus important differences were only observed in weight-bearing areas. Previous studies have shown insufficiency of the femoral cartilage, with the most pronounced changes in weight-bearing areas of the medial condyle [14, 15]. In our study, weight-bearing areas of both condyles were significantly affected. Interestingly, despite the posterior lateral tibial plateau experiencing the most substantial impact during initial ACL injury, the T2 distribution profiles showed cartilage insufficiency over the entire tibial articular surface [16]. It has been previously shown that in the first year after an injury, a dynamic process of cartilage regeneration takes place [17]. A possible explanation for the observed T2 distribution profile in the ACLR group is the weighted balance between regeneration processes and biomechanical alteration in a prolonged follow-up after the ACLR.
It is well established that despite ACLR, the knee biomechanics are altered and ACLR patients have a high risk of developing post-traumatic osteoarthritis, even following successful reconstruction [18, 19]. The post-ACLR alteration of knee biomechanics even leads to significant bone remodeling, most prominent in the weight-bearing areas [18]. The ACLR group showed important T2 value decreases in weight-bearing areas, in contradistinction to the majority of previous studies in this field [11, 12, 17]. However, few of these studies have evaluated or discussed T2 shortening times in relation to cartilage degeneration [20,21,22,23]. Despite histological validation, the explanation is not straightforward and authors have hypothesized that loss of water trapped within collagen fibrils results in a relative increase of the shorter T2 components, which captures contributions from all measured T2 components [20].
An important factor that also needs to be considered for the relative T2 shortening in ACLR group is the follow-up period. The majority of post-ACLR studies showed early cartilage insufficiency and cartilage loss at up to 3 years follow-up [11, 12, 14, 17]. Contrary to previous studies, a longer follow-up of 6 years was selected in our study due to accelerated cartilage degradation at 5–7 years post ACL injury [16]. In the early follow-up period, T2 values are increasing due to collagen matrix fragmentation and increasing water content [24]. However, when advanced cartilage morphological defects are present, there appears to be a limit to further cartilage degradation as demonstrated by T2 mapping [24]. In addition, when correlating T2-dependent relaxation times with Outerbridge classification, it appears that in lower Outerbridge grades prolongation is seen, and with higher Outerbridge grades shortening is seen, even below the values of healthy cartilage [25]. In proven anterior cruciate ligament deficient, knee T2 values tend be lower than in controls, with authors attributing this to advanced cartilage degeneration [26]. Furthermore, decreased T2 relaxation times were observed in patients with osteoarthritis and in cartilage lesions over time [27, 28]. The explanation for T2 shortening is supported by the fact that the cartilage volume is depleting in osteoarthritis, particularly the superficial cartilage layer with prolonged T2 relaxation times, and we speculate that this may contribute to the shorter relaxation T2 values, as the superficial layer would denudate over time [29]. Further studies are needed to elucidate how T2 changes occur longitudinally, and whether additional phenomena may impact T2 values in the long term [29].
Cartilage regional variations have been demonstrated in previous studies, however cartilage distribution profiling has been described in only one [1, 30]. Two important views need to be considered in interpretation of cartilage regional variations observed with cartilage quantitative imaging. Firstly, the arrangement of the cartilage layer biochemical composition and extracellular matrix macroscopic architecture organization are unevenly distributed along the articular surfaces between compartments and sub-compartments [1, 7]. The second is the importance of the magic angle effect in T2 cartilage representation [1, 30]. The effect of angular orientation and cartilage layer on T2 values has already been established for the femoral condyles, and the observed sinusoidal pattern in femoral cartilage may be attributed to this [1]. The anatomical curvature of the femoral condyles is an important factor, however, the magic angle effect also appears to be important for the relatively flat tibial cartilage [30]. With qualitative T2 assessment, it has been shown that T2 values are higher towards the joint periphery as organization of the extracellular matrix appears to differ between the periphery and the center of the tibial plateau [30]. In our study, we observed higher T2 values towards the posterior tibial sub-compartments, however not the anterior tibial sub-compartments. To minimize the magic angle effect in our analysis, full-thickness cartilage was analyzed.
One of the main benefits of using cartilage T2 distribution profiles is that this method enables continuous assessment of cartilage structural changes along the anatomical surfaces. T2 mapping of cartilage along with segmentation and regions of interest relevant to the particular pathology enables quantitative analysis of cartilage structural changes [7, 10, 12, 31]. However, an important advantage of using cartilage T2 distribution profiles is that they enable comparison between studied groups for each point along the articular surface, thus potentially depicting smaller cartilage defects as well as enabling assessment of the entire cartilage along the measured articular surface. Comparison of cartilage T2 distribution profiles could also enable easier visual differentiation of cartilage quality, since assessments based on color encoding may be difficult to interpret. Furthermore, generation of cartilage T2 distribution profiles may have a great application in overcoming the comparative issues of absolute cartilage values between different MRI machines and software programs.
The current study has several limitations. First, subjects were limited to comparatively young individuals aged 22–47 years. Second, the sample size is small, but this was due to the strict selection criteria and creation of matched pairs. Third, T2 relaxation times were assessed manually, however we believe that the measurements were sufficiently reliable due to the high signal contrast between cartilage and adjacent subchondral bone or joint fluid. Finally, cartilage T2 mapping was evaluated in a limited area of the knee cartilage. Although the midsagittal planes display the primary load bearing regions of the knee joint and are most likely to have the greatest degeneration, it has been shown that articular cartilage injury can occur over the entire femoral condyle [32]. Future studies with a larger number of patients, including other joints and defining cartilage profiles for entire compartments are needed in order to test the proposed applicability.
In conclusion, good depiction of regional differences can be made with T2 distribution profiles, showing different patterns for femoral and tibial cartilage. Furthermore, the T2 distribution profile pattern is preserved with cartilage insufficiency, however with important differences in T2 values between ACLR and the control group in weight-bearing areas.
Change history
27 December 2019
Unfortunately in Volume 49, Issue 1 had been published online with an incorrect date (2001 instead of 2020).
References
Kaneko Y, Nozaki T, Yu H, Chang A, et al. Normal T2 map profile of the entire femoral cartilage using an angle/layer-dependent approach. J Magn Reson Imaging. 2015;42:1507–16.
Plut D, Faganel Kotnik B, Preložnik Zupan I, et al. Diagnostic accuracy of haemophilia early arthropathy detection with ultrasound (HEAD-US): a comparative magnetic resonance imaging (MRI) study. Radiol Oncol. 2019. https://doi.org/10.2478/raon-2019-0027.
Kim T, Min BH, Yoon SH, et al. An in vitro comparative study of T2 and T2* mappings of human articular cartilage at 3-Tesla MRI using histology as the standard of reference. Skelet Radiol. 2014;43:947–54.
Nieminen MT, Rieppo J, Toyras J, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487–93.
Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartil. 2007;15:789–97.
Williams A, Winalski CS, Chu CR. Early articular cartilage MRI T2 changes after anterior cruciate ligament reconstruction correlate with later changes in T2 and cartilage thickness. J Orthop Res. 2017;35(3):699–706.
Surowiec RK, Lucas EP, Fitzcharles EK, et al. T2 values of articular cartilage in clinically relevant subregions of the asymptomatic knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:1404–14.
Snoj Z, Zupanc O, Salapura V. Effect of anterior cruciate ligament reconstruction technique on graft signal intensity at midterm follow up. Iran J Radiol. 2018;15(1):e14060. https://doi.org/10.5812/iranjradiol.14060.
Snoj Ž, Zupanc O, Stražar K, et al. A descriptive study of potential effect of anterior tibial translation, femoral tunnel and anterior cruciate ligament graft inclination on clinical outcome and degenerative changes. Int Orthop. 2017;41:789–96.
Peterfy CG, Guermazi A. Zaim S et. al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12:177–90.
Theologis AA, Haughom B, Liang F, et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc. 2014;22:298–307.
Li H, Chen S, Tao H, Chen S. Quantitative MRI T2 relaxation time evaluation of knee cartilage: comparison of meniscus-intact and -injured knees after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(4):865–72.
Snoj Ž, Zupanc O, Salapura V. Retrospective quantitative cartilage and semi-quantitative morphological evaluation at 6 years after ACL reconstruction. Arch Orthop Trauma Surg. 2016;136:967–74.
Bae JH, Hosseini A, Wang Y, et al. Articular cartilage of the knee 3 years after ACL reconstruction. A quantitative T2 relaxometry analysis of 10 knees. Acta Orthop. 2015;86:605–10.
Su F, Hilton JF, Nardo L, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthr Cartil. 2013;21:1058–67.
Potter HG, Jain SK, Ma Y. Black BR et. al.. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40:276–85.
Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology. 2011;258:505–14.
Zhong Q, Pedoia V, Tanaka M, et al. 3D bone-shape changes and their correlations with cartilage T1ρ and T2 relaxation times and patient-reported outcomes over 3-years after ACL reconstruction. Osteoarthr Cartil. 2019. https://doi.org/10.1016/j.joca.2019.01.017.
Mueske NM, Patel AR, Pace JL. Improvements in landing biomechanics following anterior cruciate ligament reconstruction in adolescent athletes. Sports Biomech. 2018. https://doi.org/10.1080/14763141.2018.1510539.
Bittersohl B, Miese FR, Hosalkar HS, et al. T2* mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthr Cartil. 2012;20(7):653–60.
Bittersohl B, Hosalkar HS, Miese FR, et al. Zonal T2* and T1Gd assessment of knee joint cartilage in various histological grades of cartilage degeneration: an observational in vitro study. BMJ Open. 2015;5(2):e006895. https://doi.org/10.1136/bmjopen-2014-006895.
Bittersohl B, Hosalkar HS, Hughes T, Kim YJ, Werlen S, Siebenrock KA, et al. Feasibility of T2* mapping for the evaluation of hip joint cartilage at 1.5T using a three-dimensional(3D), gradient-echo (GRE) sequence: a prospective study. Magn Reson Med. 2009;62(4):896–901. https://doi.org/10.1002/mrm.22096.
Wirth W, Maschek S, Roemer FW, Eckstein F. Layer-specific femorotibial cartilage T2 relaxation time in knees with and without early knee osteoarthritis: data from the osteoarthritis initiative (OAI). Sci Rep. 2016;6:34202.
Jungmann PM, Kraus MS, Nardo L, et al. T(2) relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: longitudinal data from the osteoarthritis initiative. J Magn Reson Imaging. 2013;38(6):1415–24. https://doi.org/10.1002/jmri.24137.
Williams AA, Qian Y, West RV, Fu FH, Chu CR. Clinical Ultrashort TE-Enhanced T2* Mapping of Knee Cartilage at 3T. Poster No. 1445, ORS 2012 Annual Meeting.
Hovis KK, Alizai H, Tham SC, et al. Non-traumatic anterior cruciate ligament abnormalities and their relationship to osteoarthritis using morphological grading and cartilage T2 relaxation times: data from the osteoarthritis initiative (OAI). Skelet Radiol. 2012;41(11):1435–43.
Juras V, Schreiner M, Laurent D, et al. The comparison of the performance of 3 T and 7 T T2 mapping for untreated low-grade cartilage lesions. Magn Reson Imaging. 2019;55:86–92.
Stahl R, Blumenkrantz G, Carballido-Gamio J, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthr Cartil. 2007;15:1225–34.
Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr Cartil. 2015;23(10):1639–53.
Goodwin DW, Wadghiri YZ, Zhu H, et al. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182:311–8.
Zhu J, Hu N, Liang X et al. T2 mapping of cartilage and menisci at 3T in healthy subjects with knee malalignment: initial experience. Skeletal Radiol. 2019. https://doi.org/10.1007/s00256-019-3164-0.
Slauterbeck JR, Kousa P, Clifton BC, et al. Geographic mapping of meniscus and cartilage lesions associated with anterior cruciate ligament injuries. J Bone Joint Surg Am. 2009;91:2094–103.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Snoj, Ž., Vidmar, J., Gergar, M. et al. T2 distribution profiles are a good way to show cartilage regional variabilities and cartilage insufficiency. Skeletal Radiol 49, 137–145 (2020). https://doi.org/10.1007/s00256-019-03256-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03256-3