Abstract
Purpose
The goal of this study is to compare the cartilage of anterior cruciate ligament (ACL)-reconstructed and uninjured contralateral knees using T 1ρ MRI 12–16 months after ACL reconstructions.
Methods
Eighteen patients with ACL-reconstructed knees (10 women, 8 men, mean age = 38.3 ± 7.8 years) were studied using 3T MRI. Injured and contralateral knee MR studies were acquired 12–16 months post-operatively. Cartilage sub-compartment T 1ρ values of each injured knee were compared with the contralateral knee’s values. Subgroup analysis of sub-compartment T 1ρ values in both knees was performed between patients with and without meniscal tears at the time of ACL reconstruction using a paired Student’s t test.
Results
In ACL-injured knees, the T 1ρ values of the medial tibia (MT) and medial femoral condyle (MFC) were significantly elevated at 12–16 months follow-up compared to contralateral knees. Patients with a medial meniscal tear had higher MFC and MT T 1ρ values compared to respective regions in contralateral knees. Patients with lateral meniscal tears had higher lateral femoral condyle and LT T 1ρ values compared to respective regions in contralateral knees. There were no differences between the injured and contralateral knees of patients without meniscal tears.
Conclusions
T 1ρ MRI can detect significant changes in the medial compartments’ cartilage matrix of ACL-reconstructed knees at 1 year post-operatively compared to contralateral knees. The presence of a meniscal tear at the time of ACL reconstruction is a risk factor for cartilage matrix degeneration in the femorotibial compartments on the same side as the meniscal tear.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The anterior cruciate ligament (ACL) is one of the most commonly injured ligaments in the knee. Patients with ACL-injured knees are at an increased risk of post-traumatic osteoarthritis [13, 20, 36]. Over 40 % of patients with ACL ruptures develop radiographic changes of osteoarthritis 10–20 years after the injury, primarily in the medial compartment of the injured knee [16, 22, 27, 30]. This increased rate of osteoarthritis was initially attributed to additional traumatic and progressive deterioration of the joint as a result of abnormal laxity and abnormal loading patterns in knees without ACL reconstructions [4, 23, 26]. However, recent studies have shown that 50–60 % of patients with functionally stable ACL-reconstructed knees continue to develop degenerative changes in the knee, suggesting an alternative aetiology in the development of post-traumatic osteoarthritis [9, 14].
Advanced MRI techniques have been developed to detect macromolecular changes within cartilage matrix at the early stages of osteoarthritis. Among them, T 1ρ imaging is an attractive candidate relative to standard MRI because it probes the interactions between water molecules and the cartilage extracellular matrix and can be sensitive to changes in proteoglycans [1, 11, 12, 21, 24, 28]. A change, specifically an increase, in T 1ρ values represents damage to the extracellular matrix. While both T 1ρ and T 2 values increase with the degree of osteoarthritis, studies suggest that T 1ρ is more sensitive than T 2 for detecting early cartilage degeneration [18, 29].
T 1ρ quantitative MRI is capable of detecting cartilage matrix changes in the cartilage overlying bone marrow edema-like lesions (BMEL) compared to healthy cartilage surrounding BMELs and detecting changes of the weight-bearing medial femorotibial cartilage matrix as early as 1 year after ACL reconstruction compared to age-matched, healthy control subjects [19, 33]. No study has used non-invasive quantitative MRI to compare the cartilage matrix properties of the ACL-injured knee to the contralateral healthy knee. A study comparing a patient’s own ACL-injured knee’s quantitative MRI cartilage values to their contralateral, uninjured knee is important, as it will help to provide an evaluation of the pathologic changes to a patient’s injured knee, as the contralateral knee is the most appropriate internal control.
The objective of this study was to evaluate damage and potential early degeneration of cartilage in ACL-reconstructed knees by comparing cartilage T 1ρ values of defined sub-compartments in ACL-reconstructed knees and the patient’s own contralateral knee at 12 to 16 months after ACL reconstructions and to explore a potential effect of meniscal tears at the time of injury on cartilage T 1ρ at 1 year after ACL reconstruction. The two hypotheses of this study are (1) the cartilage in the medial compartments of the injured knee will have significantly higher T 1ρ signal changes compared to the respective compartments in the contralateral knee, and (2) patients with a meniscal tear will have higher T 1ρ signal changes in the femoral and tibial compartments on the side of the meniscal tear compared to respective cartilage regions in contralateral knees.
Materials and methods
The study was approved by the Committee on Human Research at our institution. All patients with ACL injuries were referred by one orthopaedic surgeon (CBM) at our institution’s Sports Medicine clinic. The inclusion criteria were clinically diagnosed acute complete ACL rupture using an increased anterior–posterior laxity scale (Lachman grade 2–3) [35] and confirmation by MRI, willingness to have an ACL reconstruction, and capability to undergo the standard pre- and post-injury/operative rehabilitation. The exclusion criteria include prior history of osteoarthritis, inflammatory arthritis, previous injury and surgery on either knee, and repeated injuries to either knee during the follow-up period. In addition, patients who required surgical intervention for ligamentous injuries, including collateral ligament and posterior cruciate ligament tears, were excluded from the study. All eligible patients provided written informed consent for their enrolment in the study.
Eighteen patients (10 women and 8 men, mean age = 38.3 ± 7.8 years, age range = 28–53 years) with acute ACL injuries were studied. All patients underwent anatomic ACL reconstruction, which was performed by one orthopaedic surgeon (CBM). Anatomic reconstruction of the ACL was performed using standard surgical steps: sizing and preparation of the graft, arthroscopic takedown of the previously ruptured ACL, femoral tunnel drilling and measurement through an anteromedial portal, tibial drilling using a tibial guide, passage of the graft through the tunnels and subsequent graft fixation on the femur with an endobutton or a metal interference screw and on the tibia with a BioIntrafix screw. Post-operatively, patients were scanned between 12 and 16 months (12.8 ± 1.7 months) after their ACL reconstructions. Patients with meniscal tears were identified by chart review of each patient’s post-operative dictation and note (arthroscopic diagnosis). Each patient’s characteristics and graft type are summarized in Table 1. Information that was available regarding descriptive characteristics of each meniscal tear (i.e. location, size, type) is also presented in Table 1.
Magnetic resonance imaging
MR data were acquired with 3 Tesla GE MR scanners (Signa HDx, General Electric Healthcare, Milwaukee, WI, USA). The patients included in this study are sub-cohorts of two other ongoing studies in our group, and as a result, 7 patients were scanned using a transmit/receive quadrature knee coil (Clinical MR Solutions, Brookfield, WI, USA) on scanner #1 and 12 patients were scanned using an eight-channel phased array knee coil (Invivo, Orlando, FL, USA) on scanner #2. The imaging protocols for all subjects included sagittal 3D fat-suppressed high-resolution spoiled gradient-echo (SPGR) images, sagittal T 2-weighted fat-saturated fast spin-echo (FSE) images and sagittal 3D T 1ρ quantification sequences. For scans with the 8-channel knee coil, parallel imaging was performed with an array spatial sensitivity technique (ASSET) using an acceleration factor of 2. The detailed parameters for each sequence are listed in Table 2.
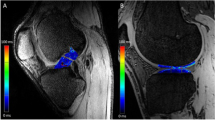
There is an excellent agreement in quantification of cartilage morphology and relaxation times between measurement with ASSET (acceleration factor = 2) and without ASSET [38]. In this study, cross-validation experiments in three healthy controls were performed. The controls were scanned twice within a week using the quadrature and 8-channel knee coil on the two scanners, respectively, with the protocol listed in Table 2. T 1ρ relaxation times were quantified in five compartments of the knee, the lateral and medial femoral condyles (LFC/MFC), the lateral and medial tibias (LT/MT), and the patella (Fig. 1a), resulting in 15 compartments with 2 repeated measures in each compartment. From these compartments, the root-mean-square coefficients of variation (%), defined as 100 times the standard deviation divided by the mean, were calculated for the T 1ρ values.
Representative sagittal 3D water excitation high-resolution spoiled gradient-echo images with segmented cartilage of ACL-reconstructed knees. a To evaluate global compartmental T 1ρ values, the patella (red spline), femoral condyles (blue spline) and tibias (yellow spline) are segmented. b For regional analysis, the cartilage of the femur and tibia is subdivided with respect to the meniscal horns. The femoral condyles are divided into 5 sub-compartments (FC-1,2,3,4,5), and the tibia is divided into three sub-compartments (T-1,2,3)
Furthermore, during analysis of this study (as detailed in the section of statistical analysis), T 1ρ values in the injured knees were compared to the uninjured contralateral knee, which was used as an internal control for each patient. Such analysis will minimize any potential bias to the results introduced by potential difference in T 1ρ quantification between the two scanners.
Cartilage processing
Cartilage segmentation
Semi-automatic cartilage segmentation was performed on the sagittal SPGR images using proprietary software developed with Matlab (Mathworks, Natick, MA, USA) based on Bezier splines and edge detection [6]. The medial and lateral femoral condyles and tibias were also divided into sub-compartments with regard to the meniscus (Fig. 1b). The medial and lateral femoral condyles were divided into 5 regions. MFC/LFC-1 and MFC/LFC-5 were cartilage regions anterior and posterior to the anterior and posterior horns of the meniscus, respectively (Fig. 1b). MFC/LFC-2 and MFC/LFC-4 were the cartilage regions overlying the anterior and posterior horns of the meniscus, respectively (Fig. 1b). MFC-3 was the cartilage region between the anterior and posterior horns of the meniscus (Fig. 1b). The tibia was divided into three sub-compartments. MFC/LFC-1 and MFC/LFC-3 were the cartilage regions overlying the anterior and posterior horns of the meniscus, respectively (Fig. 1b). MT-2 was the cartilage region between the anterior and posterior horns of the meniscus (Fig. 1b). The segmentation was corrected manually to avoid synovial fluid or other surrounding tissue. The mean cartilage thickness of all segmented cartilage compartments and sub-compartments was calculated using an in-house developed software [6]. The root-mean-square CV for intra-observer reproducibility of cartilage thickness quantification using this algorithm was between 2.4 and 3.7 %, as reported previously [5].
Cartilage T 1ρ relaxation quantification
T 1ρ maps were reconstructed using a proprietary developed fitting algorithm. T 1ρ-weighted image intensities obtained for different time of spin-lock were fitted pixel by pixel to the following equation:
T 1ρ maps were registered to SPGR images, and cartilage contours generated from SPGR images after segmentation were overlaid to the registered T 1ρ maps. The average T 1ρ values of the full-thickness of each cartilage compartment and its sub-compartments were quantified for the injured and contralateral knees. The T 1ρ values of the weight-bearing and non-weight-bearing regions of the femoral condyles were determined by averaging the average T 1ρ values of the sub-compartments overlying and between the anterior and posterior horns of the meniscus (LFC/MFC-2,3,4). To reduce artefacts caused by partial volume effects with synovial fluid, regions with relaxation time >150 ms in T 1ρ maps were manually removed from the data used for quantification. The scan–rescan coefficients of variation (CV) of cartilage T 1ρ quantification using the same technique have been reported to range between 1.7 and 8.7 % [17, 18], indicating excellent in vivo reproducibility of the MR T 1ρ technique.
Statistical analysis
Overall statistical analysis included the two major statistical comparisons. The average T 1ρ values of the five knee compartments (LFC, LT, MFC, MT and patella; Fig. 1a) were compared between injured and uninjured knees using a paired, one-tailed Student’s t test. The average T 1ρ values of the defined sub-compartments (LFC/MFC-1,2,3,4,5; LT/MT-1,2,3, Fig. 1b) were compared between injured and uninjured knees using a paired, one-tailed Student’s t test. A sub-analysis of patients with and without meniscal tears included three statistical comparisons. The average T 1ρ values of the MFC and MT and defined sub-compartments (MFC-1,2,3,4,5; MT-1,2,3) of patients with medial meniscal tears were compared between injured and uninjured knees using a paired, one-tailed Student’s t test. The average T 1ρ values of the LFC and LT and defined sub-compartments (LFC-1,2,3,4,5; LT-1,2,3) of patients with lateral meniscal tears were compared between injured and uninjured knees using a paired, one-tailed Student’s t test. The average T 1ρ values of the five knee compartments (LFC, LT, MFC, MT and patella) and of the defined sub-compartments (LFC/MFC-1,2,3,4,5; LT/MT-1,2,3) in patients without meniscal tears were compared between injured and uninjured knees using a paired, one-tailed Student’s t test. Identical analysis was used for cartilage thickness in knee compartments and respective sub-compartments between injured and uninjured knees. All statistical analyses were calculated using Microsoft Excel. A p value <0.05 was considered statistically significant for all comparisons.
Results
Cross-validation analysis of the two knee coils used to scan patients in this study showed a 4.6 % root-mean-square coefficients of variation of the T 1ρ values of cartilage in knees. This value is within the in vivo reproducibility of the T 1ρ quantification using the 3D MAPSS sequence as previously reported [17].
The medial tibia and medial femoral condyle in ACL-injured knees 12–16 months post-reconstruction had T 1ρ values that were significantly elevated compared to respective regions in contralateral knees (Fig. 2a); the cartilage thickness in the medial compartments was not different between injured and uninjured knees (Table 3). While no differences were found between the injured and uninjured knees with respect to global T 1ρ values of the LFC, LT and patella (Fig. 2a), the cartilage thicknesses of the LFC and LT were significantly less in the injured knee compared to the uninjured knee (Table 3).
a Average global articular cartilage T 1ρ values of the lateral femoral condyle (LFC), lateral tibia (LT), medial femoral condyle (MFC), medial tibia (MT) and patella in ACL-injured knees and uninjured, contralateral knees. Note that the T 1ρ values in the MFC and MT of injured knees are significantly elevated compared to the values of respective regions in uninjured knees. The T 1ρ values of the LFC, LT and MFC of injured knees are also elevated relative to the respective sub-compartments’ values of uninjured knees. b Average articular cartilage T 1ρ values in sub-compartments of the LFC, MFC, LT, MFC and MT in ACL-injured and contralateral, uninjured knees. Note that the injured knees have significantly higher T 1ρ values in LFC-2,5, MFC-1,2,3,4 and MT-2
Sub-compartment analysis of the medial tibia revealed the weight-bearing contact region (MT-2) in the injured knee had greater T 1ρ values and less thick cartilage compared to the MT-2 of the uninjured knee (Fig. 2b; Table 3). It was also found that the MFC’s most anterior cartilage compartment (MFC-1) and weight-bearing regions (MFC-2,3,4) had greater T 1ρ values compared to the respective sub-compartments in the uninjured knee (Fig. 2b). The cartilage thickness of MFC-3 in injured knees was significantly thinner than the respective compartment in uninjured knees (Table 3). On the lateral side of the knee, the cartilage thicknesses of LT-1 and LT-3 in injured knees were significantly thinner than the respective compartments in uninjured knees (Table 3). LFC-2 and LFC-5 were also found to have significantly greater T 1ρ values compared to the respective regions in the uninjured, contralateral knee (Fig. 2b). No significant difference was noted in the individual sub-compartments of the LFC with respect to cartilage thickness (Table 3).
To analyse the effect of a meniscal tear in the ACL-injured knee, a sub-analysis between injured and uninjured knees in patients with meniscal tears and a separate sub-analysis of injured and uninjured knees in patients without meniscal tears were performed. Ten patients had meniscal tears, of which 5 patients had lateral and medial meniscal tears, 3 patients each had an isolated lateral meniscal tear and 2 patients each had an isolated medial meniscal tear (Table 1). All meniscal tears were located in the posterior horn of the medial and lateral menisci. There were a variety of tears, including 1 root tear, 1 longitudinal tear, 1 radial tear and 2 complex tears (Table 1), and treatment of each was tailored based on the tear’s location, size and type.
Patients without meniscal tears were found to have no significant differences in global T 1ρ values in all five cartilage compartments (Fig. 3a). The 7 patients with medial meniscal tears were found to have significantly higher global T 1ρ values in the MFC and MT compared to respective regions in contralateral knees (Fig. 3b). The 8 patients with lateral meniscal tears were found to have elevated global T 1ρ values in the LFC and LT compared to respective regions in contralateral knees (Fig. 3c; p = 0.06).
Analysis of global articular T 1ρ values in LFC, LT, MFC and MT in patients with and without medial and/or lateral meniscal tears. a Note that there are no differences in T 1ρ values in all cartilage compartments between injured and uninjured knees in patients without meniscal tears. b In patients with a medial meniscal tear, the MFC and MT have significantly higher global T 1ρ values compared to the respective regions in uninjured, contralateral knees. c Patients with lateral meniscal tears have elevated global T 1ρ values in the LFC and LT compared to respective regions in uninjured, contralateral knees, with the difference approaching significance
With regard to cartilage sub-compartments, patients without meniscal tears were found to have no significant difference in T 1ρ values in all sub-compartments between injured and contralateral knees (Fig. 4a). Patients’ injured knees with associated medial meniscal tears were found to have significantly greater T 1ρ values in the MFC-1,3,4 and MT-3 compared to respective regions in the contralateral knees (Fig. 4b). The T 1ρ values in MFC-2,5 and MT-2 of patients with medial meniscal tears were greater than the values in contralateral knees (Fig. 4b; p = 0.07–0.08). Patients with associated lateral meniscal tears were found to have significantly greater T 1ρ values in the LFC-1 and LFC-2 compared to respective regions in the contralateral knees (Fig. 4c). The T 1ρ values in LFC-5 and LT-3 of patients with lateral meniscal tears were greater than the values in contralateral knees (Fig. 4c; p = 0.07–0.08).
Analysis of articular T 1ρ values in sub-compartments of the LFC, LT, MFC and MT in patients with and without medial and/or lateral meniscal tears. a Note that there are no differences in T 1ρ values in all cartilage sub-compartments between injured and uninjured knees in patients without meniscal tears. b In patients with a medial meniscal tear, the entire MFC and the femorotibial contact region and posterior cartilage region in the MT (MT-2,3) have higher global T 1ρ values compared to the respective regions in uninjured, contralateral knees. c Patients with lateral meniscal tears have elevated T 1ρ values in the anterior and posterior cartilage regions of the LFC (LFC-1,2,5) and the posterior LT (LT-3) compared to respective regions in uninjured, contralateral knees
Discussion
The most important finding of this study is that medial femorotibial compartments in ACL-injured knees 12–16 months after surgical reconstruction had significantly greater T 1ρ values compared to the medial compartments in the contralateral, uninjured knee. In particular, the contact region between the medial femur and tibia (not protected by meniscus; MT-2) and the weight-bearing regions of the MFC in the injured knee showed significantly elevated T 1ρ values compared to the uninjured, contralateral knee. As T 1ρ is sensitive to proteoglycan loss, and that loss of proteoglycan is suggested as an initiating event of osteoarthritis [10], the observed T 1ρ elevations in the medial compartments suggest early cartilage matrix degeneration and thus ultimately may represent “hot spots” in the cartilage at which post-traumatic osteoarthritis develops after ACL injury. This is consistent with a previous study that found significantly elevated T 1ρ values in the weight-bearing sub-compartments of medial femorotibial cartilage in ACL-injured knees at 1-year post-operative follow-up compared with values in knees of a healthy control cohort [19]. They also suggested that the degeneration in the medial compartments starts from the superficial layer of the cartilage, as they observed significantly greater T 1ρ values in superficial layers of the medial compartment’s contact region at 1-year follow-up in ACL-injured knees compared with values in control knees [19]. No significant differences were observed in the deep layers [19]. Interestingly, while there were no significant differences noted in cartilage thickness between the whole medial compartments of injured and uninjured knees, the cartilage of areas not protected by menisci, MFC-3 and MT-2, was significantly thinner in injured knees. While minor, these changes in thickness may represent early cartilage loss.
The changes presented herein of the knees’ medial compartments are consistent with previous studies by Carpenter et al. [7] and Seon et al. [31] who suggested that ACL reconstruction does not fully restore normal knee kinematics, particularly in the compartments of the medial knee. Specifically, Carpenter et al. [28] found that normal motion on the lateral side of the knee but not on the medial side was restored after ACL reconstruction, which resulted in increased internal tibial rotation when moving from full extension to 40° of flexion. In addition to reconstructing the ACL, addressing other injuries about the knee, including posterolateral corner tears, is critically important to improving knee kinematics. Griffith et al. [15] found that only medial compartment cartilage matrix injury, as determined by increased T 1ρ relaxation times, was detectable when Grade III posterolateral knee injuries were created in a canine knee model and tested under different biomechanical loads. This change in biomechanics due to missed injuries or after ACL reconstruction subsequently may cause joint loads to be shifted to infrequently loaded areas of the cartilage and lead to early cartilage degeneration [8].
As altered biomechanics and joint loads in the ACL-injured knee play important proposed roles in the development of post-traumatic injury, this study addressed whether an additional mechanical injury, such as a meniscal tear, further increases one’s risk for cartilage matrix damage. This question was addressed by performing a sub-analysis comparing the injured and uninjured knees of patients with an ACL injury and an associated meniscal tear. The injured knees of patients with medial meniscal tears had significantly elevated T 1ρ values throughout the MFC and in the MT, particularly in the contact region of the medial tibia (MT-2) and the cartilage underlying the posterior meniscal horn (MT-3). Additionally, the injured knees of patients with lateral meniscal tears had significantly elevated T 1ρ values in the cartilage underlying the posterior meniscal horn (LT-3) and in the most anterior and posterior cartilage sub-compartments of the LFC (LFC-1,2,5). Importantly, no differences were found between sub-compartments of the injured knee and uninjured, contralateral knee in patients without meniscal tears (data not shown). The data presented herein suggest that the presence of meniscal damage at the time of ACL injury is a risk factor for cartilage degeneration in the femorotibial compartments on the side of the injured meniscus. These results are consistent with reports from long-term observational cohort studies [20, 32, 34, 37]. For example, von Porat et al. [32] found that 59 % of subjects with a meniscus tear at the time of ACL tear had radiographic changes equivalent to Kellgren and Lawrence grade 2 or worse compared to 1 % of patients without a meniscal tear. Wu et al. [33] also found that patients who had undergone any degree of meniscal resection had worse subjective and objective functional outcomes 10.4 years after ACL reconstruction compared to those without a meniscal tear.
This study has limitations, which include its cross-sectional design, the relatively few number of patients evaluated, the absence of correlation between MRI findings and post-operative clinical laxity, histologic cartilage samples, arthroscopic evaluations or functional outcome scores, that is, KOOS, IKDC and no comparison to a control cohort with two “normal” knees. Additionally, the heterogeneity of each meniscal tear (i.e. location, size, type) is an important limitation of the study, as different types of tears in different locations and subsequent treatments are known to result in significantly different effects on tibiofemoral contact forces and overall knee biomechanics [2, 3, 25]. As the study evaluates patients at only the 12–16-month post-operative time point, conclusions cannot be drawn as to whether the observed T 1ρ values represent lower, stable or higher values relative to the time of injury and early post-operative follow-up time points. However, the observed increased T 1ρ values in the medial compartment of the knee likely represent a change from earlier post-operative time points, as previous studies have found that the medial compartment’s cartilage T 1ρ values are not elevated initially after the injury and subsequently increase overtime [33]. A significant question that should be addressed is whether the observed T 1ρ values remain elevated at later time points following ACL injury and whether the observed radiographic properties correlate with histologic cartilage samples and/or whether they can predict current or future functional status. Answers to these questions are critical, as they may elucidate whether these regions represent cartilage “hot spots” for post-traumatic osteoarthritis following ACL injury in reconstructed knees. In order to address all these pressing questions, future studies ideally should be prospective in nature, include longer follow-up time points and correlate radiographic findings to histologic samples, arthroscopic findings and/or functional outcome scores.
Conclusion
Quantitative T 1ρ MRI is capable of detecting cartilage matrix changes in the medial compartments of ACL-injured knees compared to uninjured, contralateral knees as early as 12 months post-ACL reconstruction. The presence of a meniscal tear may be a significant risk factor for cartilage matrix injury in the femorotibial compartments on the side of the meniscal tear. Thus, T 1ρ MRI holds great potential as a modality for detection of early, asymptomatic cartilage damage in ACL-reconstructed knees. Such capability of non-invasive and early detection of cartilage matrix changes will be helpful clinically in stratifying patients, monitoring early joint degeneration and allowing potential early pharmacologic and/or rehabilitation interventions to ultimately improve patient management after acute injuries.
References
Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, Leigh JS, Reddy R (2001) Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med 46(3):419–423
Allaire R, Muriuki M, Gilbertson L, Harner CD (2008) Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am 90(9):1922–1931
Baratz ME, Fu FH, Mengato R (1986) Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med 14(4):270–275
Barrack RL, Bruckner JD, Kneisl J, Inman WS, Alexander AH (1990) The outcome of nonoperatively treated complete tears of the anterior cruciate ligament in active young adults. Clin Orthop Relat Res 259:192–199
Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM, Steinbach LS, Majumdar S (2004) A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthr Cartil 12(12):997–1005
Carballido-Gamio J, Bauer J, Lee KY, Krause S, Majumdar S (2005) Combined image processing techniques for characterization of MRI cartilage of the knee. Conf Proc IEEE Eng Med Biol Soc 3:3043–3046
Carpenter RD, Majumdar S, Ma CB (2009) Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy 25(7):760–766
Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP (2008) Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc 40(2):215–222
Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR (1994) Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med 22(5):632–644
Dijkgraaf LC, de Bont LG, Boering G, Liem RS (1995) The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg 53(10):1182–1192
Dijkgraaf LC, Liem RS, de Bont LG (1998) Temporomandibular joint osteoarthritis and crystal deposition diseases: a study of crystals in synovial fluid lavages in osteoarthritic temporomandibular joints. Int J Oral Maxillofac Surg 27(4):268–273
Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS (1997) T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med 38(6):863–867
Feagin JA Jr (1979) The syndrome of the torn anterior cruciate ligament. Orthop Clin North Am 10(1):81–90
Ferretti A, Conteduca F, De Carli A, Fontana M, Mariani PP (1991) Osteoarthritis of the knee after ACL reconstruction. Int Orthop 15(4):367–371
Griffith CJ, Wijdicks CA, Goerke U, Michaeli S, Ellermann J, LaPrade RF (2011) Outcomes of untreated posterolateral knee injuries: an in vivo canine model. Knee Surg Sports Traumatol Arthrosc 19(7):1192–1197
Hawkins RJ, Misamore GW, Merritt TR (1986) Followup of the acute nonoperated isolated anterior cruciate ligament tear. Am J Sports Med 14(3):205–210
Li X, Han ET, Busse RF, Majumdar S (2008) In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med 59(2):298–307
Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S (2005) In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med 54(4):929–936
Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, Ma CB, Majumdar S (2011) Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2–initial experience with 1-year follow-up. Radiology 258(2):505–514
Lohmander LS, Ostenberg A, Englund M, Roos H (2004) High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum 50(10):3145–3152
Makela HI, Grohn OH, Kettunen MI, Kauppinen RA (2001) Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun 289(4):813–818
Marks PH, Donaldson ML (2005) Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy 21(11):1342–1347
McDaniel WJ Jr, Dameron TB Jr (1980) Untreated ruptures of the anterior cruciate ligament. A follow-up study. J Bone Joint Surg Am 62(5):696–705
Menezes NM, Gray ML, Hartke JR, Burstein D (2004) T2 and T1rho MRI in articular cartilage systems. Magn Reson Med 51(3):503–509
Muriuki MG, Tuason DA, Tucker BG, Harner CD (2011) Changes in tibiofemoral contact mechanics following radial split and vertical tears of the medial meniscus an in vitro investigation of the efficacy of arthroscopic repair. J Bone Joint Surg Am 93(12):1089–1095
Noyes FR, McGinniss GH, Mooar LA (1984) Functional disability in the anterior cruciate insufficient knee syndrome. Review of knee rating systems and projected risk factors in determining treatment. Sports Med 1(4):278–302
Noyes FR, Mooar PA, Matthews DS, Butler DL (1983) The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am 65(2):154–162
Redfield AG (1969) Nuclear spin thermodynamics in the rotating frame. Science 164(3883):1015–1023
Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R (2006) T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging 23(4):547–553
Roos H, Adalberth T, Dahlberg L, Lohmander LS (1995) Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthr Cartil 3(4):261–267
Seon JK, Gadikota HR, Kozanek M, Oh LS, Gill TJ, Li G (2009) The effect of anterior cruciate ligament reconstruction on kinematics of the knee with combined anterior cruciate ligament injury and subtotal medial meniscectomy: an in vitro robotic investigation. Arthroscopy 25(2):123–130
Shelbourne KD, Gray T (2000) Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med 28(4):446–452
Theologis AA, Kuo D, Cheng J, Bolbos RI, Carballido-Gamio J, Ma CB, Li X (2011) Evaluation of bone bruises and associated cartilage in anterior cruciate ligament-injured and -reconstructed knees using quantitative t(1rho) magnetic resonance imaging: 1-year cohort study. Arthroscopy 27(1):65–76
von Porat A, Roos EM, Roos H (2004) High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis 63(3):269–273
Werier J, Keating JF, Meek RN (1998) Complete dislocation of the knee—the long-term results of ligamentous reconstruction. Knee 5(4):255–260
Wright V (1990) Post-traumatic osteoarthritis—a medico-legal minefield. Br J Rheumatol 29(6):474–478
Wu WH, Hackett T, Richmond JC (2002) Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med 30(6):845–850
Zuo J, Li X, Banerjee S, Han E, Majumdar S (2007) Parallel imaging of knee cartilage at 3 Tesla. J Magn Reson Imaging 26(4):1001–1009
Acknowledgments
This research was supported by the National Institutes of Health grants K25 AR053633 and R01 AR46905.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theologis, A.A., Haughom, B., Liang, F. et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc 22, 298–307 (2014). https://doi.org/10.1007/s00167-013-2397-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2397-z