Abstract
Purpose
In order for T2 mapping to become more clinically applicable, reproducible subregions and standardized T2 parameters must be defined. This study sought to: (1) define clinically relevant subregions of knee cartilage using bone landmarks identifiable on both MR images and during arthroscopy and (2) determine healthy T2 values and T2 texture parameters within these subregions.
Methods
Twenty-five asymptomatic volunteers (age 18–35) were evaluated with a sagittal T2 mapping sequence. Manual segmentation was performed by three raters, and cartilage was divided into twenty-one subregions modified from the International Cartilage Repair Society Articular Cartilage Mapping System. Mean T2 values and texture parameters (entropy, variance, contrast, homogeneity) were recorded for each subregion, and inter-rater and intra-rater reliability was assessed.
Results
The central regions of the condyles had significantly higher T2 values than the posterior regions (P < 0.05) and higher variance than the posterior region on the medial side (P < 0.001). The central trochlea had significantly greater T2 values than the anterior and posterior condyles. The central lateral plateau had lower T2 values, lower variance, higher homogeneity, and lower contrast than nearly all subregions in the tibia. The central patellar regions had higher entropy than the superior and inferior regions (each P ≤ 0.001). Repeatability was good to excellent for all subregions.
Conclusion
Significant differences in mean T2 values and texture parameters were found between subregions in this carefully selected asymptomatic population, which suggest that there is normal variation of T2 values within the knee joint. The clinically relevant subregions were found to be robust as demonstrated by the overall high repeatability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most common type of arthritis and is the leading cause of disability among adults [1]. The noninvasive quantification of structural and biochemical properties related to the onset and progression of OA is critical for studies of this condition. Early detection of OA may make it a possibility to implement treatment strategies to slow or stop progression of the disease. Quantitative MRI cartilage mapping has demonstrated potential for detecting the early biochemical changes, which include a decrease in proteoglycans, a disruption of the cartilage matrix, and an influx of chondrocytes and water as a response to the area of damage, within cartilage with the ability to detect damage not visualized on conventional MRI [9, 26]. T2 mapping is the most commonly used noninvasive mapping technique as it is widely available, less time-consuming than most techniques, and has been reported to be sensitive to collagen matrix organization and water content of cartilage [11, 23, 37–40].
Researchers have reported T2 mapping values of the knee in healthy populations including reporting global values, values from the tibiofemoral regions, and larger subregions or the entire cartilage plate [25, 35, 41]. However, the specific values associated with healthy cartilage are variable within the literature, and thus, the reproducibility among reports is uncertain. Moreover, the definition of “healthy” cartilage and the subregions used for analysis of T2 cartilage are inconstant across centres [18, 26, 41]. The International Cartilage Repair Society (ICRS) has developed a standardized method of evaluating cartilage damage which focuses on the lesion depth and the area of damage [4]. To aid in the arthroscopic assessment, the ICRS has described an articular cartilage mapping system which divides the knee into sections, allowing for a method of describing lesion location. A modified version of these regions may be appropriate to describe and detect small regions of cartilage variation in T2 mapping, because a set of comprehensive and standardized subregions would allow for the long-term assessment of cartilage health following treatment and allow for comparisons to be made across multiple institutions.
While mean T2 values are typically reported, important information may also be imbedded in the grey values of the MR image. Grey values in an image carry intensity information which is expressed as a range between black and white. Using information from grey values, the spatial pattern of T2 values (known as matrix-based texture analysis) has also been analysed and reported to correlate with varying stages of OA [6, 16, 20]. Texture analysis parameters (such as entropy, contrast, variance, and homogeneity) characterize the underlying structure of a given tissue present in an image and may provide a more comprehensive understanding of the cartilage structure than solely mean T2 values.
The purpose of this study was to define clinically relevant subregions of knee cartilage based on the ICRS articular cartilage mapping system using bone landmarks identifiable in both MRI and arthroscopy and to determine standardized, asymptomatic T2 parameter values (T2 values and texture analysis) for these subregions. It was hypothesized that T2 parameters would significantly differ between the proposed subregions based on the ICRS articular cartilage mapping system within the patellar, femoral, and tibial cartilage, and the proposed subregions would have excellent inter-rater and intra-rater repeatability among three raters with varying medical backgrounds.
Materials and methods
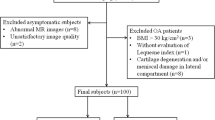
Twenty-five asymptomatic volunteers (age 18–35) were enrolled. Volunteers were deemed asymptomatic by a self-administered subjective scoring form (pain/swelling/stiffness visual analogue score), an objective clinical examination performed by a sports medicine orthopaedic surgeon, and by morphological MRI examination by a musculoskeletal radiologist. Clinical examination included evaluation of limb alignment, pain/tenderness, and the following tests: Lachman test, anterior, anterolateral, posterior, and posterolateral drawer, pivot, and reverse pivot shift test, and evaluation of the medial and lateral joint opening. Exclusion criteria included symptoms (e.g. pain, stiffness, and swelling exceeding mild levels) in the knee and/or hip of the imaged side, prior injury or surgery in the knee and hip, history of inflammatory arthritis or infection within the joint of interest, and evidence of cartilage lesions, meniscal pathology, or bone marrow signal changes from a conventional morphological MRI examination using a semi-quantitative modified whole-organ magnetic resonance imaging score (WORMS).
Image acquisition
Magnetic resonance imaging was performed with a Siemens Magnetom Verio 3.0 T scanner (Siemens Medical Solutions, Erlangen, Germany) with a gradient strength of 40 mT/m, using a 15-channel multi-element phased-array knee coil (Quality Electrodynamics, LLC, Mayfield Village, OH, USA). The time delay between lying down for the MR session and the beginning of the first sequence was limited to <5 min.
The scanning protocol consisted of: (1) a 3D fat-suppressed Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (FS SPACE) scan, (2) a multi-echo spin-echo T2 mapping scan in the sagittal plane (MESE T2 Map Sag), (3) a proton density turbo spin-echo scan in the coronal plane (PD with TSE Cor), and (4) a T2-weighted turbo spin-echo scan in the axial plane (T2w TSE Ax). The SPACE scan was reformatted in all three planes (Table 1). T2 mapping was performed at the end of the examination following morphological scans, approximately 16 min after entering the scanner, because it has been suggested that the cartilage should be unloaded prior to obtaining T2 values [2]. It was theorized that 16 min would be an appropriate time to unload the subject because the length of the imaging session must be kept relatively short for patient comfort, the avoidance of movement artefacts, and to allow for the entire examination to fit into a typical workflow timeslot to be clinically applicable.
Data analysis
MRI scoring
To investigate pathology within the joint, MR images were graded using a reduced version of the whole-organ magnetic resonance scoring system (WORMS) [20]. The WORMS evaluation consists of a semi-quantitative score for 14 features in the knee [28]. Here, only five compartments (patella, medial tibia, medial femur, lateral tibia, and lateral femur) were evaluated because fewer findings were expected in an asymptomatic population [20]. A board certified radiologist with 13 years of experience graded cartilage lesions with scores between 1 and 6 where scores of 1 represented increased T2 signal and a sum of 6 represented lesions >75 % of thickness. Bone marrow signal changes were graded between 1 and 3 where scores of 1 represented <25 % increased T2 signal and a sum of 3 represented >50 % increase in T2 signal within the five compartments [28]. Meniscal pathology was analysed in three compartments: anterior/posterior horn and the body of each meniscus. The meniscal signal was graded between 1 and 4 where scores of 1 represented intermeniscal degeneration and a sum of 4 represented an absence of meniscal tissue [20]. Subjects with a score of two or greater in any category were excluded from analysis to reduce the potential of including subjects with early asymptomatic cartilage degeneration.
Segmentation and subregion division
Manual segmentations were performed to create segmentation masks by tracing the contours of the patellar, femoral, and tibial cartilage (Fig. 1) with a stylus and touchscreen monitor using Mimics software (Materialise, Plymouth, MI, USA) by three raters: a musculoskeletal radiologist with 13 years of experience [Rater 1], a biomedical engineer with 5 years of medical image analysis experience [Rater 2], and an orthopaedic surgeon with 6 years of experience [Rater 3]. Segmentations were performed on the second echo of the sagittal T2 mapping sequence on a slice-by-slice basis spanning all slices within the knee joint. To exclude synovial fluid and chemical shift artefact from the segmentations, the raters simultaneously examined the corresponding sagittal fat-suppressed SPACE sequence on a neighbouring monitor. To assess the intra-rater and inter-rater reproducibility, all raters performed manual segmentations twice for all subjects, with a delay of 1 month between segmentations.
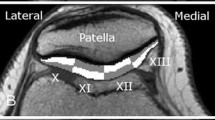
For the selection of bone landmarks, bone contours from the T2 mapping sequence were manually segmented based on the subchondral/cortical bone dark signal, and using the segmentations, the 3D bone geometries of the femur, tibia, and patella were reconstructed in Mimics. Using a split screen view, the 3D bone reconstructions which triangulated to the second echo of the T2 image were used to choose 22 anatomical bone landmarks (Table 2), identifiable both during arthroscopy and in MRI sequences. For the eight landmarks used to describe the femur, two were placed at the most inferior and superior portion of the deepest part of the femoral trochlear groove. Two landmarks were chosen at the deepest point in the trochlea relative to the mid-plane between the condyles slightly lateral and the sulcus terminalis, at the most medial and lateral aspects. Two landmarks were chosen at the most medial and lateral aspect of the widest and most anterior portion of the intercondylar notch apex. From the posterior aspect of the femur, landmarks were placed on the medial and lateral condyle at the most posterior aspect of the cartilage margin. Eight landmarks were used to describe the tibia. A landmark was placed at the apex of both the medial and lateral tibial eminences. A landmark was placed at the most medial and lateral aspect of the tibia at the end of the cartilage margin. The remaining tibial landmarks were placed on the most posterior and anterior portion of the cartilage margin on both the medial and lateral tibial plateau. For example, on the lateral side, this point was roughly half the distance between the lateral tibial eminence landmark and the lateral tibial landmark. Patellar landmarks included a landmark at the most inferior, superior, medial, and lateral bony portion at the cartilage margin of the bone. Two additional landmarks were placed within the line of the median patellar ridge: an inferior landmark and a superior landmark. The coordinates (x, y, z) of these landmarks were used in the custom MATLAB program (Mathworks, Natick, MA) to divide the cartilage (in detail below) into 21 clinically relevant subregions (six patellar, nine femoral, and six patellar) adapted from the ICRS Articular Cartilage Mapping System (Fig. 2) [4].
Modified ICRS Articular Cartilage Mapping System based on landmarks and bone landmarks. Landmarks are depicted on one subject’s bone model. Subregions within the femoral, tibial, and patellar cartilage were divided based on the bone landmarks (red circles; also Table 2) identified by the raters. The landmarks can be seen arthroscopically as well as within MR images (left knee). S superior, I inferior, A anterior, P posterior, M medial, L lateral, C central, Fem femoral, Tib tibia, Pat patella
The custom MATLAB program utilized the bone landmark coordinates to divide the cartilage segmentations as follows. The femoral trochlea was separated from the lateral condyle by a line passing through two landmarks placed within the terminalis sulcus. The medial condyle was separated from the trochlea at the superior point of the intercondylar notch. The borders of the central trochlea started at the most medial and lateral points of the intercondylar notch and were parallel with the line determined by two landmarks designating the deepest part of the trochlear groove. The condyles were divided into thirds (anterior, central, and posterior). For division of the tibia, the medial and lateral tibial plateaus were divided into thirds in the anterior–posterior direction using the eminence, anterior, posterior, medial, and lateral landmarks. For the patella, the ridge landmarks were used to separate the medial and lateral facets. The facets were divided into thirds in the superior–inferior direction using the superior border and the most inferior landmark of the patella located at the change in the slope of the patella.
Although the methodology was presented using a T2 mapping sequence acquired at 3.0 T MRI, the techniques described can be extrapolate to other quantitative techniques and acquisition vendors and magnet strengths.
T2 map quantification
T2 values were calculated using a Siemens WIP (work in progress) algorithm, modified from the Siemens MapIt software algorithm (Siemens Medical Solutions, Erlangen Germany). The cartilage masks were exported as binary images and imported into a custom MATLAB program with the coordinates of the landmarks and the T2 maps. The software divided the segmentation masks with the T2 overlay into the proposed 21 subregions using the 3D coordinates of the landmarks, and T2 parameters were calculated and exported to an Excel document. Only T2 values between 1.0 and 250.0 ms were included in the analysis to exclude outliers such as synovial fluid (T2 values >250.0 ms) and T2 values rejected due to poor fit (T2 values of 0 ms).
Further subanalysis included textural analysis of the T2 values was performed using MATLAB by calculating the grey level co-occurrence matrices for each of the subregions [16]. Texture parameters of entropy, variance, contrast, and homogeneity were calculated at orientations of 0°, 45°, 90°, and 180°, with an offset of one pixel [17, 32]. The texture values at each orientation were then averaged together for each texture parameter [20].
Institutional review board (IRB) approval was obtained from the Vail Valley Medical Center (IRB# 2011-03) prior to conducting this study. All subjects provided informed consent.
Statistical analysis
Statistical analyses were performed using SPSS, version 20 (IBM Corporation, Armonk, NY, USA). Median T2 values for each patient and in each subregion were compiled and used for further analysis. A repeated measures ANOVA was used to compare means (of median T2 values) between subregions separately. Bonferroni corrections were applied to post hoc comparisons between subregions. The mean texture parameters of entropy, variance, contrast, and homogeneity were compared between subregions. To assess the repeatability of the segmentations in a manner that could be generalized to a single future rater from the population of qualified raters, a two-way random effects model calculated the single measures intra-class correlation coefficient (ICC) for each subregion’s median T2 value. The ICC values were graded using the scoring method described by Fleiss et al.: 0.75–1.00 = excellent reliability, 0.40–0.75 = fair to good reliability, 0–0.40 = poor reliability [10]. The root mean square coefficient of variation (RMSCV) was also calculated to assess rater reliability [14].
Results
Seven subjects had a WORMS score of two or greater in at least one region and thus were not considered entirely healthy and were excluded from analysis.
T2 values
The averages (±STD) of the median T2 values for each subregion are demonstrated in each subregion (Fig. 3). Within each subregion, the median T2 value was chosen to summarize the T2 measurements. Subsequent comparison testing between subregions utilized these median values as data (“Appendix”). The median T2 values, averaged across subjects, ranged from 43.2 to 54.2 ms in the femur, 40.3 to 48.0 ms in the tibia, and 40.3 to 47.2 ms in the subregions of the patella.
Averages of median T2 values (±STD) in subregions within the femoral, tibial, and patellar cartilage in asymptomatic volunteers. S superior, I inferior, A anterior, P posterior, M medial, L lateral, C central. Stars indicate significance (P ≤ 0.05) between subregions. Significant differences between femoral, tibial, and patellar subregions were not analysed
Texture analysis: subanalysis of T2 values
Mean contrast, entropy, homogeneity, and variance for the T2 measurements in each subregion are presented (Fig. 4a–d). Further subregion analysis is described in detail below (“Appendix”).
Mean contrast of cartilage T2 (±STD) (a), mean entropy of cartilage T2 (±STD) (b), mean homogeneity of cartilage T2 (±STD) (c), and mean variance of cartilage T2 (±STD) (d) in subregions within the femur, tibia, and patella in asymptomatic volunteers. S superior, I inferior, A anterior, P posterior, M medial, L lateral, C central. Star indicates significance (P ≤ 0.05) between subregions. Significant differences between femoral, tibial, and patellar subregions were not analysed
Within the femoral cartilage, the central trochlea had higher variance (P < 0.05) and entropy (P < 0.001) than the medial trochlea. Both the central and lateral trochlea had higher entropy than all other regions (P < 0.05) except the central lateral condyle. The central medial subregion of the femoral condyles had significantly higher variance than both the medial (P < 0.001) and lateral (P < 0.05) posterior regions of the condyles.
Within the tibial plateaus, the central regions had significantly higher entropy than the anterior and posterior regions (P < 0.001), except for the lateral posterior region which was not significantly different. The central lateral region also had lower variance (P < 0.05), higher homogeneity (P < 0.05), and lower contrast (P < 0.05) than each of the other tibial subregions.
For the patellar subregions, both the medial and lateral central regions had higher entropy (P < 0.001) than the superior and inferior regions. Homogeneity was significantly higher in the central lateral facet when compared to the superior lateral facet (P < 0.05) and the inferior lateral facet (P < 0.001).
Intra-class correlation coefficients (ICC)
The inter-rater and intra-rater ICC values are reported in Table 3. Inter-rater and intra-rater ICC values showed either “excellent” or “fair to good” agreement (13 subregions with excellent agreement and eight subregions with fair to good agreement) between and within the three raters.
Discussion
The most important finding of the present study was that significant differences in median T2 values and texture parameters were observed within clinically relevant subregions of the femoral, tibial, and patellar cartilage, confirming our hypothesis by demonstrating that there are normal variations of T2 values within the screened asymptomatic knee joint. This implies that there is not just one single T2 value for asymptomatic cartilage within the knee, confirming our hypothesis that subregions are an important consideration for analysis to locate natural variations of T2 values within a joint. The overall high inter-rater and intra-rater repeatability supports the robustness of the manual segmentation, selection of bone landmarks, and the resulting division based on our proposed clinically relevant ICRS-based subregions. Defining subregions based on an accepted method, such as the ICRS articular cartilage mapping system, may make the data more universally applicable to better integrate quantitative mapping from theory to practise in the clinical workflow.
Rather than evaluating the mean of the T2 values for the entire knee, this study summarized the central tendency of the values within each individual subregion by evaluating the median T2 values and averaging the medians within each subregion across subjects. While previous studies have calculated the mean within subregions, we advocate using the statistical median because it is more robust against outliers, such as areas of image misregistration between fat and water on MRI (i.e. chemical shift artefact) and synovial fluid that may have been manually segmented into the subregions. Additionally, for analysis, a T2 value cutoff of 1.0–250.0 ms was chosen to further exclude synovial fluid and chemical shift artefact which have high T2 values, and T2 values rejected due to poor fit (0 ms). This range may be appropriate for future studies, including patients with chondral damage who may as a result of pathology have increased fluid and inherently higher T2 values [15].
It is theorized that some of the observed differences in T2 values between subregions had clinical relevance because of the high T2 values observed in areas of joint loading and regions which commonly develop OA. Elevated T2 values in the central femoral condyles were consistent with what has been described in the literature and are a reported common area for development of OA [12, 13, 27, 33]. The central trochlea also had significantly elevated T2 values than nearly all femoral subregions and is another common reported area for OA development [30]. On the lateral plateau of the tibia, the anterior and posterior regions had significantly higher T2 values than the central lateral plateau region. The lower values in the central lateral tibial plateau were unexpected because this was a subregion we thought would have higher T2 values because this is another common area for OA development. We can hypothesize that perhaps this is the result of the different loading conditions from the contact between two convex surfaces causing a change in biochemical structure within the cartilage. Moreover, during arthroscopy, the central lateral plateau commonly appears soft to manual probing and there is commonly signal change reported in MRI images within this region [27, 36]. Using the ovine model, it has been reported that the lateral tibial plateau experienced an increase in joint contact stress which led to cartilage fibrillation, proteoglycan loss, and collagen breakdown in the lateral subregions [24]. Likewise, Maher et al. [24] observed an increase in T2 values in these subregions which corresponded to the higher contact pressures.
In the patella, the T2 values were higher and had larger standard deviations, especially in the medial facet, perhaps due to partial volume averaging with adjacent tissue and synovial fluid. The raters reported some difficulty segmenting the bone–cartilage interface on the sagittal scan which appeared thickened. In imaging, partial volume averaging occurs when multiple tissue types are encompassed within a single voxel, such as fat and water, or can occur when the structure is oriented obliquely to the imaging plane. The patella tends to normally tilt laterally causing the medial facet to align more obliquely within the sagittal plane of the scan, probably causing this to occur [19].
Texture analysis using grey level co-occurrence matrix texture features may be more sensitive to early changes in cartilage degeneration than the absolute mean T2 value because it analyses the distribution of each value in a subregion, using the grey values that make up the image, rather than combining the values into one mean value allowing for detection of slight changes. No studies were found in the literature which compared texture analysis results within subregions in a healthy cohort. The observed differences in texture parameters were in regions that have been reported to commonly develop OA such as the central trochlea, the medial femoral condyles, and the central tibia plateaus. The central trochlea had higher variance and entropy than the medial trochlea. High entropy signifies more conformity in neighbouring pixels. Blumenkrantz et al. [3] demonstrated that the measure of entropy was significantly higher in osteoarthritic cartilage compared to age-matched controls in the knee. High variance demonstrates a high dispersion of grey level pixel values around the mean, whereas high contrast signifies that many pixels with different values are detected to be neighbouring, each of which has been shown to be elevated in osteoarthritic cartilage [5, 22]. The findings may be due to the known anisotropy of cartilage. Anisotropy, or having different values in different directions, is lessened on a biochemical level with the early damage due to OA, making the cartilage more heterogeneous and may lend an explanation to the higher entropy, variance, and contrast values [5]. Interestingly, the central lateral tibial plateau had lower variance, higher homogeneity, and lower contrast than the remaining tibial subregions which was consistent with the finding of lower median T2 values in this subregion potentially inferring that in our healthy population, the cartilage was more heterogeneous even in this highly loaded subregion.
While several studies have reported T2 values using larger, gross subregions than presented in the present study, the different subregions make direct comparison to other findings difficult [7, 8, 31]. A standardized set of clinically relevant subregions for analysis, such as our proposed subregions, is necessary for comparison between research centres and implementation of T2 mapping into standard clinical practice. The subregions proposed in this study were modified from the ICRS articular cartilage mapping system, which is used for documenting arthroscopic findings. The main modification to this system was that the patella was separated into medial and lateral along the patellar ridge, rather than dividing it into medial, central, and lateral subregions. We theorized that this would increase reproducibility due to the ease of detecting the patellar ridge, whereas the central region is difficult to assign because of significant known anatomical variation of the patella between subjects.
While T2 mapping was used, it is possible that other quantitative cartilage mapping techniques may be more sensitive to early cartilage degeneration. In particular, T2 mapping is not sensitive to proteoglycan (PG) concentration, which is one of the earliest biochemical changes that occur with cartilage degeneration [29]. T1-weighted mapping sequences have been reported to be sensitive to changes in PG concentration thus may be more sensitive to early cartilage degeneration [21]. However, T2 mapping is currently the most widely available noninvasive mapping technique as most MRI manufacturers offer the sequence, and the image is acquired without the use of contrast agents. Moreover, the methodology presented in this study with the subregion definition based on bone landmarks reproducibly identifiable by arthroscopy, and imaging can be applied to any quantitative mapping parameter that should prove ultimately to be optimal.
The clinical relevance of this work is that it defines that there are differences in subregions of articular cartilage in biochemical composition, structure, and content of the knee joint. This indicates that all subregions of the knee are unique in their make-up and that further study is warranted as to the implications on both MRI evaluation and possibly resurfacing procedures. Limitations of this study include difficulty in assuring that asymptomatic volunteers have no early cartilage degeneration. Arthroscopy remains the gold standard in cartilage evaluation but is not reasonable to use in this population. Conventional methods for detecting cartilage damage are not sensitive enough for detecting early OA, which makes determining specific T2 values for healthy cartilage complicated. For the present study, inclusion/exclusion criteria were very rigorous and WORMS assessment was used to further exclude volunteers with bone oedema and degeneration in the cartilage and intra-substance meniscal degeneration and tears of the menisci.
The study was limited by the manual nature of the methodology. Manual segmentation and manual landmark selection were used which is too time intensive for inclusion clinically. However, automated cartilage segmentation and registration onto T2 maps may be available in the near future, and reproducible T2 mapping can be implemented into standard clinical care workflow [34].
Conclusion
This study demonstrated characteristic patterns of cartilage T2 mapping in clinically relevant articular cartilage subregions of the femur, tibia, and patella. While T2 mapping was used in the present study, the methodology proposed can be easily implemented in any other imaging biomarker of interest. The proposed subregions based on the ICRS articular cartilage mapping system may increase the efficacy and reproducibility of quantitative mapping and may make the data transferrable across centres and among orthopaedists and radiologists to better integrate quantitative mapping from theory to practise in the clinical routine.
References
(CDC) CfDCaP (2009) Prevalence and most common causes of disability among adults-United States, 2005. Morb Mortal Wkly Rep 58(16):421–426
Apprich S, Mamisch TC, Welsch GH, Stelzeneder D, Albers C, Totzke U, Trattnig S (2010) Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 81(4):438–443
Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, Hellio Le Graverand-Gastineau MP, Jain SK, Link TM, Majumdar S (2008) The feasibility of characterizing the spatial distribution of cartilage T2 using texture analysis. Osteoarthritis Cartilage 16(5):584–590
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A(Suppl 2):58–69
Burstein D, Gray M, Mosher T, Dardzinski B (2009) Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am 47(4):675–686
Carballido-Gamio J, Link TM, Majumdar S (2008) New techniques for cartilage magnetic resonance imaging relaxation time analysis: texture analysis of flattened cartilage and localized intra- and inter-subject comparisons. Magn Reson Med 59(6):1472–1477
Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM (2009) Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys 36(9):4059–4067
Chang G, Wiggins GC, Xia D, Lattanzi R, Madelin G, Raya JG, Finnerty M, Fujita H, Recht MP, Regatte RR (2011) Comparison of a 28-channel receive array coil and quadrature volume coil for morphologic imaging and T2 mapping of knee cartilage at 7T. J Magn Reson Imaging 35(2):441–448
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, Baum T, Mosher TJ, Carrino JA, Guermazi A (2011) Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 31(1):37–61
Fleiss JL (1986) The design and analysis of clinical experiments. Wiley series in probability and mathematical statistics Applied probability and statistics. Wiley, New York, pp 1–35
Fragonas E, Mlynarik V, Jellus V, Micali F, Piras A, Toffanin R, Rizzo R, Vittur F (1998) Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthritis Cartilage 6(1):24–32
Friedrich K, Shepard T, Chang G, Wang L, Babb J, Schweitzer M, Regatte R (2010) Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur J Radiol 20(6):1532–1538
Friedrich KM, Shepard T, de Oliveira VS, Wang L, Babb JS, Schweitzer M, Regatte R (2009) T2 measurements of cartilage in osteoarthritis patients with meniscal tears. AJR Am J Roentgenol 193(5):W411–W415
Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK (1995) Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int 5(4):262–270
Hannila I, Nieminen MT, Rauvala E, Tervonen O, Ojala R (2007) Patellar cartilage lesions: comparison of magnetic resonance imaging and T2 relaxation-time mapping. Acta Radiol 48(4):444–448
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Biomed Eng SMC-3(6):610–621
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Biomed Eng SMC-3:610–618
Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, McCulloch C, Lynch JA, Link TM (2011) Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum 63(8):2248–2256
Islam K, Duke K, Mustafy T, Adeeb SM, Ronsky JL, El-Rich M (2013) A geometric approach to study the contact mechanisms in the patellofemoral joint of normal versus patellofemoral pain syndrome subjects. Comput Methods Biomech Biomed Eng. doi:10.1080/10255842.2013.803082
Joseph G, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, Lynch J, McCulloch C, Majumdar S, Link T (2011) Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls-data from the osteoarthritis initiative. Arthritis Res Ther 13(5):R153
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S (2007) In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 15(7):789–797
Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, Majumdar S (2009) Spatial distribution and relationship of T1ρ and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med 61(6):1310–1318
Lusse S, Claassen H, Gehrke T, Hassenpflug J, Schunke M, Heller M, Gluer CC (2000) Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging 18(4):423–430
Maher SA, Rodeo SA, Potter HG, Bonassar LJ, Wright TM, Warren RF (2011) A pre-clinical test platform for the functional evaluation of scaffolds for musculoskeletal defects: the meniscus. HSS J 7(2):157–163
Mamisch TC, Trattnig S, Quirbach S, Marlovits S, White LM, Welsch GH (2010) Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading—initial results. Radiology 254(3):818–826
Nishioka H, Hirose J, Nakamura E, Okamoto N, Karasugi T, Taniwaki T, Okada T, Yamashita Y, Mizuta H (2013) Detecting ICRS grade 1 cartilage lesions in anterior cruciate ligament injury using T1rho and T2 mapping. Eur J Radiol 82(9):1499–1505
Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, Beary JF, Cline GA, Meyer JM, Martel-Pelletier J (2007) Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther 9(4):R74
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12(3):177–190
Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R (2002) Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol 9(12):1388–1394
Riegger-Krugh C, Gerhart TN, Powers WR, Hayes WC (1998) Tibiofemoral contact pressures in degenerative joint disease. Clin Orthop Relat Res 348:233–245
Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, Cahue S, Marshall M, Hudelmaier M, Dunlop D (2008) Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 58(6):1716–1726
Soh L, Tsatsoulis C (1999) Texture analysis of SAR sea ice imagery using gray level co-occurrence matrices. IEEE Trans Geosci Remote Sens 37(2):780–795
Souza RB, Baum T, Wu S, Feeley BT, Kadel N, Li X, Link TM, Majumdar S (2012) Effects of unloading on knee articular cartilage T1rho and T2 magnetic resonance imaging relaxation times: a case series. J Orthop Sports Phys Ther 42(6):511–520
Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, Majumdar S, Link TM (2011) A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging—data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 19(8):984–989
Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM (2010) Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage 18(6):776–786
Vasara AI, Jurvelin JS, Peterson L, Kiviranta I (2005) Arthroscopic cartilage indentation and cartilage lesions of anterior cruciate ligament–deficient knees. Am J Sports Med 33(3):408–414
Watrin-Pinzano A, Ruaud JP, Olivier P, Grossin L, Gonord P, Blum A, Netter P, Guillot G, Gillet P, Loeuille D (2005) Effect of proteoglycan depletion on T2 mapping in rat patellar cartilage. Radiology 234(1):162–170
Wayne JS, Kraft KA, Shields KJ, Yin C, Owen JR, Disler DG (2003) MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology 228(2):493–499
Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, White LM, Trattnig S (2008) Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures–initial experience. Radiology 247(1):154–161
Xia Y, Moody JB, Alhadlaq H (2002) Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med 48(3):460–469
Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, Majumdar S (2010) Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage 18(11):1408–1416
Acknowledgments
The authors would like to gratefully acknowledge Bill Brock for his time and expertise in acquiring the MR images. This research was funded by Siemens Medical Solutions, USA. The funding source did not contribute to the study design, collection, or interpretation of the data. The funding source did supply the algorithm for calculating T2 values which was their only contribution to the data analysis.
Disclaimer
The Steadman-Philippon Research Institute is a 501(c)(3) non-profit institution supported financially by private donations and corporate support from the following entities: Smith & Nephew Endoscopy, Inc., Arthrex, Inc., Siemens Medical Solutions USA, Inc., ConMed Linvatec, Inc., Össur Americas, Inc., Small Bone Innovations, Inc., Opedix, Inc., Evidence Based Apparel, and Sonoma Orthopedics, Inc.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Surowiec, R.K., Lucas, E.P., Fitzcharles, E.K. et al. T2 values of articular cartilage in clinically relevant subregions of the asymptomatic knee. Knee Surg Sports Traumatol Arthrosc 22, 1404–1414 (2014). https://doi.org/10.1007/s00167-013-2779-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2779-2