Abstract
Soft tissue sarcomas (STS) are rare tumours that require prompt diagnosis and treatment at a specialist centre. Magnetic resonance imaging (MRI) has become the modality of choice for identification, characterisation, biopsy planning and staging of soft tissue masses. MRI enables both the operating surgeon and patient to be optimally prepared prior to surgery for the likelihood of margin-negative resection and to anticipate possible sacrifice of adjacent structures and consequent loss of function. The aim of this review is to aid the radiologist in performing and reporting MRI studies of soft tissue sarcomas, with particular reference to the requirements of the surgical oncologist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas are a rare and heterogeneous group of lesions derived from mesenchymal cells, accounting for approximately 1% of all adult cancers [1]. They have recently been re-classified by The World Health Organisation (WHO) [2, 3]. Between 59–75% arise in the extremities and the majority present as an insidious, painless, soft tissue mass [4, 5], only becoming painful when reaching a significant size or locally invading. Some present with an incidental history of trauma. Size, including recent growth, is one of the most important clinical features suggesting malignancy, and any soft tissue lesion over 5 cm in maximum diameter has a 20% likelihood of being malignant [4, 6]. In a study conducted by Bauer et al. 35% of tumours were subcutaneous, 32% intramuscular and 32% deep, extramuscular [7]. The most recent NICE guidelines state that if a patient presents with a lump > 5 cm, deep to the fascia, fixed, immobile, painful and increasing in size, they should be referred promptly to a specialist centre [8]. As a general rule, any indeterminate soft tissue mass is a sarcoma until proven otherwise and requires referral to the local sarcoma multidisciplinary team (MDT) [6].
Prognosis is better in extremity STS than head and neck or torso STS, in part because of easier access for local resection and radiotherapy [9, 10]. The status of resection margins, local extension of the tumour, age at diagnosis and grade all correlate with risk of local recurrence, whereas distant metastases are associated with high-grade and larger tumours [9]. Haematogenous metastases are the most common cause of death [4].
Both ultrasound and CT can be useful for STS imaging but have been largely replaced by MRI [6, 10,11,12,13,14]. The superior soft tissue contrast and multiplanar imaging capabilities of MRI enable delineation of muscle groups, fascial planes and compartments, differentiate clearly among bone, vascular structures and tumour, and demonstrate necrosis and haemorrhage [4, 5, 14]. In addition to suggesting that a lesion may be benign or malignant [6], the reporting radiologist can (1) further characterise and suggest possible histological diagnoses, (2) describe local tumour margins and assess distant spread allowing planning of surgery, radiotherapy and chemotherapy, (3) guide needle biopsy planning, (4) perform the biopsy and (5) identify recurrence and guide further treatment. The value of the radiologist is not only in enabling early diagnosis and prompt referral to a specialist centre where the patient has the best chance of survival, but also at the specialist centre as part of a sarcoma MDT [8].
Technical factors for MR imaging
Imaging must precede biopsy, not only because biopsy can change the features, but also to help plan the optimal biopsy route [5, 15].
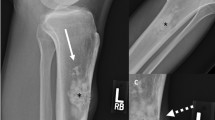
Positioning of the patient and choice of radiofrequency coil and pulse sequences are key to optimal imaging of soft tissue tumours. The aim is to create as homogeneous a field as possible. The patient should be positioned comfortably to minimise movement artefact with the lesion close to the isocentre of the magnet. Skin markers should be used, particularly for small or clinically indiscrete lesions (Fig. 1) [6]. The coil selected and the sequences should cover the entire lesion and closest joint (Fig. 1), but preferably the nearest joints both proximal and distal to the tumour. A reduced field of view focused on the lesion can also be obtained in the case of small lesions to optimise lesion characterisation and staging [14]. The lesion should be imaged in all three planes and typically with a combination of standard spin echo (SE) and fast spin echo (FSE) sequences [6, 14, 16]. Our routine protocol includes: coronal T1 W turbo spine echo (TSE) (TR-580 ms; TE-20 ms) and STIR (TR-4252 ms; TE-30 ms; TI-190 ms) sequences, a sagittal T2 W TSE sequence (TR-3061 ms; TE-90 ms), and axial PDW TSE (TR-3766 ms; TE-30 ms) and PDW SPAIR (spectral attenuated inversion recovery) TSE (TR-3766 ms; TE-30 ms) sequences covering the whole of the lesion.
Nevertheless, the use of axial T2-weighted images has been highlighted by Holzapfel et al. when assessing for encasement of arteries, veins and nerves [17]. When gadolinium is not primarily added to the protocol (for example in some departments the examination is unsupervised) to distinguish between a cystic or myxoid lesion, an ultrasound examination performed sometime after the MRI often solves the issue, and when necessary the US could be combined with needle biopsy. In many departments, the patient is called back for contrast (gadolinium) enhanced MRI. [10, 14, 18,19,20]. Advanced techniques such as chemical shift imaging, diffusion-weighted imaging and proton MR spectroscopy may also be of value in STS [14, 21, 22].
Histological characterisation on MRI: When is biopsy needed and where should it be performed?
MRI can suggest an accurate diagnosis in a large variety of benign neoplastic and non-neoplastic soft tissue lesions, including lipomas, vascular malformations, benign nerve sheath tumours, ganglia, haematomas, myositis ossificans and fat necrosis [23,24,25]. With regard to malignant lesions, liposarcoma sub-types (myxoid, pleomorphic and dedifferentiated), myxoid sarcomas and soft tissue lymphoma can demonstrate some characteristic MRI features [26,27,28]. However, the majority of soft tissue extremity masses have no specific differentiating features, being isointense to muscle on T1, intermediate to hyperintense to muscle on T2 and hyperintense on STIR. Features suggestive of malignant tumours include an irregular margin and inhomogeneous signal intensity due to haemorrhage and necrosis. The most sensitive markers of malignancy are size (>3.3 cm) and signal heterogeneity, while the most specific features are tumour necrosis, bone and neurovascular involvement and a diameter >6.6 cm [18]. However, imaging characteristics cannot always differentiate benign from malignant lesions and only suggest the correct diagnosis in one-third of soft tissue masses. Therefore, in a large proportion of cases, expert histological assessment is required for both cell type and grade, a crucial part of staging [10, 18, 29], [30].
Core needle biopsy is safe and accurate with a complication rate of less than 1% and a coaxial technique might be favourable using CT guidance [4]. The biopsy should be performed at a specialist centre, not only because tissue must be analysed by an experienced pathologist, but also since radiologically guided percutaneous biopsy planning must be co-ordinated with the likely operating surgeon. A multidisciplinary approach has been recognised for some time to improve outcome, including local control, functional preservation and limb salvage [10, 31]. The biopsy approach is discussed at MDT with the operating surgeon and indicated with an arrow on an axial MR image (Fig. 2a). The biopsy site is then marked with India ink, which aids in identifying the biopsy scar at the time of surgery, particularly if excision is delayed by either neoadjuvant chemotherapy or radiotherapy [32], thus allowing the surgical track to be included in the subsequent excision to avoid tumour seeding [10]. Viable areas of tissue can be identified with gadolinium enhancement or colour Doppler US, so that necrotic areas are avoided and there is a likely higher yield from biopsy (Fig. 2b,c). At this centre, soft tissue tumours are generally biopsied under ultrasound guidance and local anaesthesia, with at least four cores of tissue from a 14G soft tissue cutting needle.
Technique: (a) Axial T1 W SE MR image through the distal thigh in a patient with a myxoid liposarcoma showing the direction of biopsy as marked at the MDT. Use of contrast: (b) Sagittal T2 W FSE and (c) axial post-contrast fat suppressed T1 W SE MR images of the thigh in a patient with a high-grade pleomorphic sarcoma showing extensive necrosis (arrowheads) and enhancing intermediate SI tissue (arrows) consistent with viable tumour
Local staging of soft tissue tumours
Local staging of soft tissue sarcomas is primarily achieved with MR imaging, although contrast-enhanced CT is valuable if there are contraindications to MRI. Surgical staging of STS is achieved using the TGNM system of The American Joint Committee for Cancer. The new 2017 AJCC system for soft tissue sarcomas has changed from the previous version regarding the status of the primary tumour (T); superficial and deep location has been removed, and the tumour is now graded from T1 to T4 based on size criteria alone (T1 = 5 cm or less, T2 > 5 < 10 cm, T3 > 10 < 15 cm or T4 > 15 cm). There has been no change regarding the nodal status (N0 = no LN metastases or unknown LN status and N1 = regional LN metastases) or distant metastases (M0 = no distant metastases while M1 = distant metastases) [33]. Staging techniques conform to the ESMO/ESSR guidelines [3].
Table 1 lists features that should be explicitly visualised and detailed in the report of any MRI study of a soft tissue mass [6]. Tumour dimensions should be recorded in three orthogonal planes and the location in relation to the fascia (superficial or deep) noted. Anatomical compartments are classified according to standard divisions [34] and the extent that each compartment is occupied by the tumour is noted [10]. Soft tissue tumours have unique growth features, tending to grow within compartments and along fascial planes on a path of least resistance [18]. Also, a lesion arising in the superficial compartment that extends through the fascia is more likely to be malignant [35].
A common and relatively specific feature of both myxofibrosarcoma and undifferentiated sarcoma is tumour extension from the main mass into the surrounding tissues for a substantial distance along normal anatomical planes, particularly fascial planes. This results in distant microscopic tumour deposits that may predispose to an increased risk of local recurrence. On MRI, this infiltrative spread may manifest as T2 W hyperintense, enhancing, curvilinear projections termed the ‘tail sign’ (Fig. 3a, b). Such tumour extensions must be brought to the attention of the surgeon to facilitate their complete resection [36, 37]. The presence of prominent intratumoral vessels should also be noted since it may lead to performance of pre-operative embolisation.
The relationship of a sarcoma to the neurovascular bundle is of fundamental importance in determining the possibility of limb salvage, whether in contact, adherent or invasive [10, 13, 38]. A recent study identified intra-operative and/or histological encasement of the neurovascular structures in 25.3% of cases of STS [17]. This study determined neurovascular encasement to be present when greater than 1800 of the artery, vein or nerve was involved by the tumour on pre-operative MRI. Sensitivity for neurovascular encasement ranged from 72.2–84.6% and specificity from 93.2–97.3%. The tumour may be completely separated from the vessels and nerves by either fat or muscle (Fig. 4a), in which case the neurovascular bundle is not involved. Commonly, the tumour abuts and/or displaces the neurovascular bundle but the fat plane is preserved, and in such cases limb salvage is still possible, but may result in a marginal resection requiring post-operative radiotherapy. If the fat plane is obliterated but the nerve is not circumferentially surrounded, it may be preserved by splitting the epineurium and “shelling it out” from the tumour. When a nerve is completely encased it needs to be sacrificed with resulting functional loss, but in most cases limb salvage is still preferable to amputation. When vessels are partially or fully encased (Fig. 4b) they should be sacrificed with the tumour and either reconstructed with a biological or synthetic bypass graft or simply tied off depending on the anatomical location. For example, the anterior tibial vessels in the lower leg can almost always be tied off with the leg being adequately perfused by the posterior tibial vessels. However, the presence of direct extension of the tumour into the neurovascular structures is associated with decreased survival [9]. Tumour thrombus often enhances as it maintains a vascular supply, making it possible to differentiate between tumour and bland thrombus [39].
Neurovascular involvement: (a) Axial PDW FSE MR image of the thigh in a patient with grade 3 myxofibrosarcoma showing the tumour separate from vessels (black arrow) and nerves (white arrow). (b) Axial PDW FSE MR image of the distal thigh in a patient with a grade 2 leiomyosarcoma, which is completely encasing the popliteal artery (arrow)
Although histological evidence of bone involvement is uncommon, occurring in between 5–9% of soft tissue sarcomas, it is an independent poor prognostic factor associated with an increased risk of metastases, amputation and decreased survival even when the tumour grade, size and presence of metastases are controlled for [9, 12, 13, 38, 40]. The overall 5-year survival rate is 79% in patients without bone involvement, but only 47% in patients with bone involvement [41]. While cortical involvement may be identified on plain radiography or CT, MRI optimally demonstrates whether there is a preserved soft tissue interface, direct abutment, encasement and extent of cortical or medullary involvement (Fig. 5) based on signal alteration [10, 18]. The key feature to search for is preservation of a soft tissue interface between the tumour and bone on T1 W SE images. If this is the case, even in the presence of peri-tumoral oedema and reactive changes extending to the bone surface, this has a negative predictive value of 100% for osseous invasion. The observation that tumour abuts bone is highly sensitive (100% sensitivity) but not specific (27% specificity) for osseous involvement [12]. Importantly, the diameter of abutment and degree of circumferential encasement do not predict osseous invasion. The presence of cortical and medullary signal change and cortical destruction indicate likely osseous involvement with an overall sensitivity of 100% and specificity of 93%. T1 W SE images have greater specificity than T2 W images for intramedullary involvement [12].
As they grow, STSs develop a surrounding zone of compressed reactive tissue forming a capsule comprising fibrous connective tissue, inflammatory reaction and vascularisation, which often has microscopic tumour extensions within it that might be addressed by neoadjuvant treatment (Fig. 6) [4, 18]. This needs to be identified pre-operatively and completely removed with a margin of normal tissue to reduce the rate of local recurrence. Differentiating between peri-tumoral oedema/reactive change and tumour tissue can be difficult but peri-tumoral oedema has no mass effect, produces no distortion of soft tissues and has relatively ill-defined margins when compared to tumour. Furthermore, peri-tumoral oedema tends to have high signal on fat-suppressed PDW or T2 W FSE images but less appreciable signal change on T1 W SE images [12].
Skin involvement by superficial tumours needs to be identified pre-operatively to enable adequate surgical planning and avoid wound breakdown [42]. Post-resection plastic surgical input can be anticipated by both the site and extent of tumoral skin involvement, which appears as direct tumour invasion resulting in skin thickening (Fig. 7a,b).
Distant staging depends on the expected nature of the primary lesion, but includes CT of the thorax in all cases of sarcoma other than atypical lipomatous tumours (formerly well-differentiated liposarcoma), while nuclear medicine bone scintigraphy, abdominal/pelvic CT, PET-CT and whole spine/brain MRI are recommended with certain histological sub-types [6]. For example, abdominal and pelvic CT and MRI of the spine are added for myxoid liposarcoma, while brain CT/MRI may be added for alveolar soft part sarcoma, clear cell sarcoma and angiosarcoma [43]. Lymph node involvement in soft tissue tumours is rare (Fig. 8 a,b), but most commonly seen in rhabdomyosarcoma, clear cell sarcoma and epithelioid sarcoma. If involved, lymph nodes are managed aggressively with resection and radiotherapy [10].
The International Union against Cancer-American Joint Committee on Cancer stratifies patients with STS into low-, medium- and high-risk based on tumour grade, size, depth to fascia and presence of metastases. The grade is the most dominant feature in staging. Low-grade tumours are stage 1, independent of size and depth [10].
Surgery and adjuvant therapy
Surgery is the main treatment of soft tissue sarcomas [44, 45]. A marginal negative resection is the primary surgical aim with complete removal of the tumour. After this has been achieved, reconstruction of the remaining soft tissues and, if required, bone and neurovascular structures is performed [10].
The surgical margin status predicts risk of local recurrence [31]. A pathological margin classification encompasses both qualitative and quantitative margin data. The reactive zone of oedema around a mass is considered to contain microscopic disease and there is a risk of up to 40% local recurrence if the tumour alone is scooped out from the reactive oedema around it [10]. A wide excisional margin of 2 cm of skin, fat or muscle to include the microscopic disease within the reactive zone of transition is ideal [46]. This results in local recurrence rates of less than 8% [41], but as the fascia is an excellent barrier to tumour spread, 1 mm of fascia, periosteum or adventitia while quantitatively close is considered to represent a ‘good quality marginal margin’ [31]. Marginal resection of an infiltrative tumour growth pattern in muscle, such as a myxofibrosarcoma, can raise concerns about the margin quality. The planned margin dictates the resection, which is determined by the MR imaging.
A vein or artery can be displaced (pushed aside), effaced (thinned) and/or partially or totally encased by tumour. The presence of intra-luminal disease (Fig. 9 a,b), which is very rare with STS, also alerts the surgeon that vessel resection and reconstruction may be necessary. The degree of encasement is important not just in the axial plane, but also in longitudinal extent. Involvement of the vascular surgeon may be warranted with further angiographic studies of both the tumour and potential donor sites to plan segmental vascular resection with subsequent reconstruction.
Complete or partial nerve resection can be anticipated with detailed MR studies. Epineural dissection is used to safely separate a tumour from a nerve [41], but should complete neural encasement warrant resection, nerve grafting or transfer is considered [47, 48]. Fuchs et al. found that tumour involving the sciatic nerve can be treated by limb-sparing surgery, including complete nerve resection, as an alternative to hip disarticulation or hindquarter amputation because the limb salvage option provides an acceptable functional outcome [49].
Post-resection or subsequent tendon transfers can also be considered to compensate for a post-nerve resection deficit [31].
Regarding bone involvement, it is very important to make appropriate pre-operative plans and to counsel patients about the possibility of either bony resection and/or endoprosthetic reconstruction with subsequent rehabilitation if radiographs, CT or MRI demonstrate cortical or intramedullary spread. MRI combined with intra-operative assessment of periosteal adhesion enables safe resection of the tumour without compromising the margin. Reactive scalloping can be differentiated from bone infiltration by assessing the tumour’s mobility on the periosteal layer intra-operatively. This approach is associated with a high rate of local control [41].
Amputation has become much less common over the last 30 years now that wide local excision and RT are used to control local recurrence rates, but it remains unavoidable in 5% of patients [4, 50]. The risk of tumour recurrence following limb salvage surgery and radiotherapy is similar to that for amputation and has obvious functional advantages [41]. The outcome after major amputation is very variable but can give palliation to patients with distressing symptoms such as pain, bleeding and fungation. It can also give long-term disease-free survival and reasonable function in some patients [50].
Skin involvement mandates resection and may prevent primary wound closure. Surgical resection of peri-tumoral oedema, as demonstrated on MRI, can leave large soft tissue defects that may require skin grafting, local or free flap coverage. The presence of impending fungation, as well as absence of skin mobility on the tumour, makes wide skin resection important. Consideration must be given to the risk of wound breakdown due to heavy irradiation of local tissues [42].
If marginal excision (i.e. without a wide margin/cuff of normal tissue) is planned, then radiotherapy is considered to sterilise the surgical field around the tumour. Radiotherapy can be administered in either the pre- or post-operative context. Although the irradiated volume is smaller in the pre-operative setting, the risk of subsequent wound breakdown is greater [51]. The timing of radiotherapy (whether pre- or post-operative) is reflected in the extent of tissue fibrosis, joint stiffness and oedema secondary to irradiating either just the tumour site or to completely including the operative and surrounding field. This has additional implications for the development of secondary radiation-induced fractures [52]. Myxoid liposarcoma is exquisitely sensitive to radiotherapy and undergoes significant volume reduction following radiotherapy administration (Fig. 10 a,b) [53]. The phenomenon of increased post-radiotherapy and post-neoadjuvant chemotherapy tumour volume due to haemorrhage and necrosis is also well recognised (Figs. 11a,b) but is not associated with an adverse prognosis [54].
Post-radiotherapy increased tumour dimension: (a) Axial T1 W SE MR image through the thigh in a patient with a high-grade pleomorphic sarcoma (arrows) measuring 7.9 × 4.3 cm in transverse dimensions. (b) Corresponding axial PDW FSE MR image following neoadjuvant radiotherapy. The tumour now measures 9.2 × 5.4 cm and shows extensive T2-fluid signal with fluid-fluid levels consistent with tumour necrosis
An unplanned positive margin has a high risk of local recurrence (37%) [31] but in certain circumstances the surgical approach may involve a planned positive margin along a critical anatomical structure such as a blood vessel, bone or nerve. A positive margin is not associated with an increased risk of poor local control as long as the remaining margins are adequate and adjuvant radiotherapy is included in the treatment regimen. The planned positive margin is akin to a margin-negative marginal resection. In all cases, ongoing performance monitoring ensures that a low rate of inadequate resection can be maintained [55].
The role of chemotherapy in the management of soft tissue sarcoma is variable and should be limited to certain patient sub-groups [56]. It is used widely in the US but less so in Europe, as its role in improving overall survival is limited [57].
Conclusions
All patients with a suspected soft tissue tumour should be managed by a specialist sarcoma MDT as specified in the NICE guidance. Ultrasound scan by a musculoskeletal radiologist should be considered as the first-line investigation. A percutaneous ultrasound or CT-guided core needle biopsy remains the gold standard for diagnosis. However, key surgical management options for malignant masses can be decided from the MRI appearances alone. We have presented a reporting checklist of the MRI appearances of soft tissue sarcoma for assisting the surgical oncologist in the planning of patient management.
References
Hajdu SI. Soft tissue sarcomas: classification and natural history. CA Cancer J Clin. 1981;31:271–80.
Fletcher C, Bridge J, Hogendoorn P, Mertens F, editors. World Health Organization classification of tumours of soft tissue and bone: pathology and genetics of tumours of soft tissue and bone. 4th ed. Lyon, France: IARC Press; 2013.
ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3:iii):102–12.
Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109.
van Vliet M, Kliffen M, Krestin GP, Van Dijke CF. Soft tissue sarcomas at a glance: clinical, histological, and MR imaging features of malignant extremity soft tissue tumors. Eur Radiol. 2009;19(6):1499–511.
Noebauer-Huhmann IM, Weber MA, Lalam RK, et al. Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging. Semin Musculoskelet Radiol. 2015;19:475–82.
Bauer HC, Trovik CS, Alvegård TA, Berlin O, Erlanson M, Gustafson P, et al. Monitoring referral and treatment in soft tissue sarcoma: study based on 1,851 patients from the scandinavian sarcoma group register. Acta Orthop Scand. 2001, Apr;72:150–9.
Grimer R, Athanasou N, Gerrand C, et al. UK guidelines for the management of bone sarcomas. Sarcoma. 2010;2010:317462.
LeVay J, O’sullivan B, Catton C, et al. Outcome and prognostic factors in soft tissue sarcoma in the adult. Int J Radiat Oncol Biol Phys. 1993;27:1091–9.
Robinson E, Bleakney RR, Ferguson PC, O’Sullivan B. Oncodiagnosis panel: 2007: multidisciplinary management of soft-tissue sarcoma. Radiographics. 2008;28:2069–86.
Dalinka MK, Zlatkin MB, Chao P, Kricun ME, Kressel HY. The use of magnetic resonance imaging in the evaluation of bone and soft-tissue tumors. Radiol Clin N Am. 1990;28:461–70.
Elias DA, White LM, Simpson DJ, et al. Osseous invasion by soft-tissue sarcoma: assessment with MR imaging. Radiology. 2003;229:145–52.
Panicek DM, Go SD, Healey JH, Leung DH, Brennan MF, Lewis JJ. Soft-tissue sarcoma involving bone or neurovascular structures: MR imaging prognostic factors. Radiology. 1997;205:871–5.
Kransdorf MJ, Murphey MD. Imaging of soft-tissue musculoskeletal masses: fundamental concepts. Radiographics. 2016;36:1931–48.
Khoo MM, Saifuddin A. The role of MRI in image-guided needle biopsy of focal bone and soft tissue neoplasms. Skelet Radiol. 2013;42:905–15.
Manaster BJ. Soft-tissue masses: optimal imaging protocol and reporting. AJR Am J Roentgenol. 2013;201:505–14.
Holzapfel K, Regler J, Baum T, et al. Local staging of soft-tissue sarcoma: emphasis on assessment of neurovascular encasementvalue of MR imaging in 174 confirmed cases. Radiology. 2015;275:501–9.
Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175:575–87.
Verstraete KL, Lang P. Bone and soft tissue tumors: the role of contrast agents for MR imaging. Eur J Radiol. 2000;34:229–46.
Belli P, Costantini M, Mirk P, Maresca G, Priolo F, Marano P. Role of color doppler sonography in the assessment of musculoskeletal soft tissue masses. J Ultrasound Med. 2000;19:823–30.
Khoo MM, Tyler PA, Saifuddin A, Padhani AR. Diffusion-weighted imaging (DWI) in musculoskeletal MRI: a critical review. Skelet Radiol. 2011;40:665–81.
Teixeira PA, Beaumont M, Gabriela H, et al. Advanced techniques in musculoskeletal oncology: perfusion, diffusion, and spectroscopy. Semin Musculoskelet Radiol. 2015;19:463–74.
Wu JS, Hochman MG. Soft-tissue tumors and tumorlike lesions: a systematic imaging approach. Radiology. 2009;253:297–316.
Crundwell N, O’Donnell P, Saifuddin A. Nonneoplastic conditions presenting as soft-tissue tumors: pictorial review. Clin Radiol. 2007;62:18–27.
Goodwin RW, O’Donnell P, Saifuddin A. MRI appearances of common benign soft-tissue tumours. Clin Radiol. 2007;62:843–53.
O’Regan KN, Jagannathan J, Krajewski K, et al. Imaging of liposarcoma: classification, patterns of tumor recurrence, and response to treatment. AJR Am J Roentgenol. 2011;197:W37–43.
Baheti AD, Tirumani SH, Rosenthal MH, et al. Myxoid soft-tissue neoplasms: comprehensive update of the taxonomy and MRI features. AJR Am J Roentgenol. 2015;204:374–85.
Suresh S, Saifuddin A, O’Donnell P. Lymphoma presenting as a musculoskeletal soft tissue mass: MRI findings in 24 cases. Eur Radiol. 2008;18:2628–34.
Crim JR, Seeger LL, Yao L, Chandnani V, Eckardt JJ. Diagnosis of soft-tissue masses with MR imaging: can benign masses be differentiated from malignant ones? Radiology. 1992;185:581–6.
Berquist TH, Ehman RL, King BF, Hodgman CG, Ilstrup DM. Value of MR imaging in differentiating benign from malignant soft-tissue masses: study of 95 lesions. AJR Am J Roentgenol. 1990;155:1251–5.
Gerrand CH, Wunder JS, Kandel RA, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br. 2001;83:1149–55.
Jalgaonkar A, Dawson-Bowling SJ, Mohan AT, et al. Identification of the biopsy track in musculoskeletal tumour surgery: a novel technique using india ink. Bone Joint J. 2013;95-B:250–3.
Amin MB, Edge S, Greene F, et al (eds). 2017 AJCC cancer staging manual, 8th edition.
Anderson MW, Temple HT, Dussault RG, Kaplan PA. Compartmental anatomy: relevance to staging and biopsy of musculoskeletal tumors. AJR Am J Roentgenol. 1999;173:1663–71.
Galant J, Martí-Bonmatí L, Soler R, et al. Grading of subcutaneous soft tissue tumors by means of their relationship with the superficial fascia on MR imaging. Skelet Radiol. 1998;27:657–63.
Yoo HJ, Hong SH, Kang Y, et al. MR imaging of myxofibrosarcoma and undifferentiated sarcoma with emphasis on tail sign; diagnostic and prognostic value. Eur Radiol. 2014;24:1749–57.
Lefkowitz RA, Landa J, Hwang S, et al. Myxofibrosarcoma: prevalence and diagnostic value of the "tail sign" on magnetic resonance imaging. Skelet Radiol. 2013;42(6):809–18.
Panicek DM, Gatsonis C, Rosenthal DI, et al. CT and MR imaging in the local staging of primary malignant musculoskeletal neoplasms: report of the radiology diagnostic oncology group. Radiology. 1997;202:237–46.
Khosa F, Otero HJ, Prevedello LM, Rybicki FJ, Di Salvo DN. Imaging presentation of venous thrombosis in patients with cancer. AJR Am J Roentgenol. 2010;194:1099–108.
Ferguson PC, Griffin AM, O’Sullivan B, et al. Bone invasion in extremity soft-tissue sarcoma: impact on disease outcomes. Cancer. 2006;106:2692–700.
Ferguson PC, Kulidjian AA, Jones KB, Deheshi BM, Wunder JS. Peripheral nerve considerations in the management of extremity soft tissue sarcomas. Recent Results Cancer Res. 2009;179:243–56.
Stotter A, McLean NR, Fallowfield ME, Breach NM, Westbury G. Reconstruction after excision of soft tissue sarcomas of the limbs and trunk. Br J Surg. 1988;75:774–8.
Schwab JH, Boland PJ, Antonescu C, Bilsky MH, Healey JH. Spinal metastases from myxoid liposarcoma warrant screening with magnetic resonance imaging. Cancer. 2007;110:1815–22.
Mendenhall WM, Indelicato DJ, Scarborough MT, Zlotecki RA, Gibbs CP, Mendenhall NP, et al. The management of adult soft tissue sarcomas. Am J Clin Oncol. 2009;32:436–42.
Crago AM, Lee AY. Multimodality management of soft tissue tumors in the extremity. Surg Clin North Am. 2016;96:977–92.
Cable MG, Randall RL. Extremity soft tissue sarcoma: tailoring resection to histologic subtype. Surg Oncol Clin N Am. 2016;25:677–95.
Clarkson PW, Griffin AM, Catton CN, et al. Epineural dissection is a safe technique that facilitates limb salvage surgery. Clin Orthop Relat Res. 2005;438:92–6.
Lee GW, Mackinnon SE, Brandt K, Bell RS. A technique for nerve reconstruction following resection of soft-tissue sarcoma. J Reconstr Microsurg. 1993;9:139–44.
Fuchs B, Davis AM, Wunder JS, Bell RS, Masri BA, Isler M, et al. Sciatic nerve resection in the thigh: a functional evaluation. Clin Orthop Relat Res. 2001;382:34–41.
Clark MA, Thomas JM. Major amputation for soft-tissue sarcoma. Br J Surg. 2003;90:102–7.
O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–41.
Dickie CI, Parent AL, Griffin AM, et al. Bone fractures following external beam radiotherapy and limb-preservation surgery for lower extremity soft tissue sarcoma: relationship to irradiated bone length, volume, tumor location and dose. Int J Radiat Oncol Biol Phys. 2009;75:1119–24.
Pitson G, Robinson P, Wilke D, et al. Radiation response: An additional unique signature of myxoid liposarcoma. Int J Radiat Oncol Biol Phys. 2004;60:522–6.
Delisca GO, Alamanda VK, Archer KR, Song Y, Schwartz HS, Holt GE. Tumor size increase following preoperative radiation of soft tissue sarcomas does not affect prognosis. J Surg Oncol. 2013;107:723–7.
Biau DJ, Weiss KR, Bhumbra RS, et al. Monitoring the adequacy of surgical margins after resection of bone and soft-tissue sarcoma. Ann Surg Oncol. 2013:1858–64.
Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016;122:2952–60.
Woll PJ, Reichardt P, Le Cesne A, et al. EORTC soft tissue and bone sarcoma group and the NCIC clinical trials group sarcoma disease site committee. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045–54.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
De La Hoz Polo, M., Dick, E., Bhumbra, R. et al. Surgical considerations when reporting MRI studies of soft tissue sarcoma of the limbs. Skeletal Radiol 46, 1667–1678 (2017). https://doi.org/10.1007/s00256-017-2745-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2745-z