Abstract
Objectives
To assess the prevalence of the tail sign in soft tissue sarcomas and determine whether the local recurrence rate differed based on the presence of the tail sign.
Methods
In our retrospective study, myxofibrosarcoma (MFS, n = 25) and undifferentiated sarcoma (US, n = 38) comprised group 1, and the remaining tumours (n = 115) were assigned to group 2. Location, size, and imaging features of the tumours were assessed on MRI. The radiological-pathological correlation of the tail sign was analysed.
Results
The tail sign, thick fascial enhancement extending from the tumour margin, was more common and significantly thicker in group 1. In the subgroup analysis between MFS and US, there was no significant difference in the presence of a tail sign. Histological examination revealed extensive tumour cell infiltrations along the deep fascia from the main mass. Patients with a tail sign had a worse local recurrence-free survival than patients without it, not only in all tumours (p < 0.01), but also in group 1 (p = 0.019)
Conclusions
The tail sign was a common MRI feature of both MFS and US, and was also associated with worse local recurrence-free survival. Radiologists should be aware of these MRI findings and inform the surgeon preoperatively in order to obtain a sufficient surgical margin to minimise the risk of local tumour recurrence.
Key Points
• The tail sign was a common MRI feature of myxofibrosarcoma and undifferentiated sarcoma.
• The tail sign may be associated with worse local recurrence-free survival
• Radiologists should be aware of this MRI finding and inform the surgeon
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Malignant fibrous histiocytoma (MFH) was first described in the early 1960s and is generally accepted as one of the most common types of soft tissue sarcoma in the extremities of elderly patients [1–3]. The concept and classification of MFH have changed with the introduction of more stringent morphological and immunohistochemical criteria. In the 2002 World Health Organisation (WHO) classification of tumours, the myxoid variant of MFH with a predominant myxoid component (>50 %) was renamed " myxofibrosarcoma (MFS)" and moved into the fibroblastic/myofibroblastic tumour section [4]. In the latest WHO classification published in 2013, which we followed for this study, the malignant counterpart of so-called fibrohistiocytic tumours, formerly known as “malignant fibrous histiocytoma” and its subtypes, was renamed “undifferentiated sarcoma (US)” and reclassified under the undifferentiated/unclassified sarcomas section [5, 6].
Although these tumours are pathologically distinct, an unencapsulated infiltrative margin and the infiltrative growth pattern of the tumour were well described for both US and MFS in the literature [7–14]. A completely infiltrative growth pattern along the fascial planes without the formation of a discrete nodular lesion has also been described [11, 12]. The infiltrative growth pattern of the tumour can result in anatomically unclear boundaries at the time of surgery. This attribute often makes it difficult to obtain adequate surgical margins and is possibly associated with a high rate of local recurrence. Recognition of the infiltrative tumour spread extending from the primary mass on preoperative magnetic resonance imaging (MRI) is essential for planning adequate surgical margins and facilitating complete resection of the tumour. On MRI, the infiltrative spread of the tumour along the fascial plane could be manifested as a tail-like margin or tapered thick fascial enhancement extending from the primary mass, which has anecdotally been reported in several studies [8–12, 14]. Because some of these reports were made before the reclassification of MFH, both MFS and US may be included in these studies. In a recent study, a “tail sign” on MRI, which is a curvilinear projection from the primary mass-like portion of the MFS, was described as a sign representing tumour extension along the fascial plane and was reported as a moderately specific and sensitive sign for the diagnosis of MFS relative to other myxoid-containing tumours [10]. As they only focussed on myxoid-appearing tumours, most USs would not have been eligible for that study and thus they did not assess how many non-myxoid tumours had tails. However, based on our experience and previous reports, both MFS and US could show the unusual characteristic of extending along the fascial planes beyond the epicentre of the primary tumour. This is not surprising because US also has an infiltrative growth pattern like MFS.
In this study, we assessed the prevalence and diagnostic values of the tail sign in both MFS and US and determined the radiological-pathological correlation of the tail sign when possible. We also determined whether the local recurrence rate differed based on the presence of the tail sign.
Materials and methods
Study population
This retrospective study was approved by the institutional review board, and the need for informed consent was waived. A total of 281 adult patients, who had undergone wide excisions of soft tissue sarcomas in their extremities between January 2007 and July 2013 at our institution, were initially identified through our institutional database. Of these, 129 patients were excluded from our study for one or more of the following reasons: (1) no available preoperative MRI (n = 47), (2) no gadolinium-enhanced image (n = 18), (3) poor MR image quality (n = 6), (4) a time interval of less than 2 weeks between MRI and radiation therapy or biopsy (n = 5), and (5) deep location without contact to the deep fascial plane (n = 80). Finally, 178 tumours from 152 patients (age range, 15-93; average age standard deviation, 56.2 ± 19.3 years) were included. The group contained 79 men (17-93; 57.6 ± 19.3 years) and 73 women (15-91; 56.4 ± 18.7 years).
Histological diagnosis of soft tissue sarcomas
Histological diagnoses of the tumours were as follows: MFS (n = 25), US (n = 38), liposarcoma (n = 38), synovial sarcoma (n = 18), leiomyosarcoma (n = 14), extraskeletal osteosarcoma (n = 4), extraskeletal chondrosarcoma (n = 3), epithelioid sarcoma (n = 4), dermatofibrosarcoma protuberans (n = 2), malignant peripheral nerve sheath tumour (n = 6), fibrosarcoma (n = 3), low-grade fibromyxoid tumour (n = 6), malignant rhabdoid tumour (n = 1), rhabdomyosarcoma (n = 1), extraskeletal Ewing’s sarcoma (n = 2), alveolar soft part sarcoma (n = 1), angiosarcoma (n = 1), clear cell sarcoma (n = 6), malignant haemangiopericytoma (n = 1), and soft tissue metastatic tumours (n = 4).
MRI
Seventy-two patients underwent MRI studies at outside institutions before they were referred to our institution; therefore, the MRI systems and pulse sequences varied. However, all MRIs were performed with a 1.5- or 3-T system. Spin-echo T1-weighted images (SE T1WI), fast spine-echo T2-weighted images (FSE T2WI) with or without fat-suppression, and gadolinium-enhanced T1-weighted images with fat suppression (Gd-T1WI with FS) were available in all cases. Gadolinium-enhanced T1-weighted images were obtained in at least two orthogonal planes.
Image analysis
The images were evaluated independently by two musculoskeletal radiologists, who were blinded to the histological diagnosis of the tumours. One of the readers was an attending radiologist with 7 years of experience in musculoskeletal radiology, and the other was a dedicated musculoskeletal MRI fellow. The T2WI and Gd-T1WI with FS in any plane were used to assess the location, size, configuration, and pattern of the adjacent fascial enhancements of the tumours. The location was recorded as subcutaneous, deep seated, or both involvements. The size was measured as the largest diameter of the mass in any imaging plane. The configuration of the tumour was classified as mass forming or superficial spreading, which is defined as localised fascial thickening without discrete mass formation or tiny nodules within the thickened fascia (Fig. 1). The pattern of the adjacent fascial enhancement was classified as absent, thin, or tail-like thick enhancement. Tail-like thick enhancement was defined as tapered fascial enhancement extending from the tumour margin with more than 2 mm thickness and recognised as a tail sign (Figs. 2 and 3). The adjacent fascia could be either investing fascia or intermuscular deep fascia. The thickness of the enhancing fascia was also measured. In the superficial spreading tumour, the “tail” of the tumour could not be differentiated from the tumour itself, but localised fascial thickening in the superficial spreading tumour was considered as a similar MR feature to the “tail” extending from the tumour. Therefore, all superficial spreading lesions were thought to be having a tail sign. After independent image analyses had been completed, the observers reread MR images in consensus and determined the pattern of the adjacent fascial enhancements of the tumour.
A 77-year-old man with multiple recurrences of undifferentiated sarcoma in his right forearm. a Axial T2-weighted image demonstrates localised fascial thickening over the extensor carpi ulnaris (arrow). b Axial T1-weighted image with fat suppression after gadolinium administration shows localised thick fascial enhancement (arrow). c Preoperative ultrasound also demonstrates the localized fascial thickening corresponding to MR images. d, e Photomicrographs (H&E staining, ×1 in d and × 25 in e) of the histopathological specimen demonstrate tumour nodules (asterisk) less than 1 cm in size in the deep fascia with tumour cell infiltration along the deep fascial plane and fibrous septa of subcutaneous fat tissue (arrows)
A 69-year-old woman with undifferentiated sarcoma in her left back. a Axial T2-weighted image demonstrates a soft tissue mass located in the subcutaneous fat layer and a tapered tail-like signal alteration in the deep fascia and subcutaneous fat layer adjacent to the main mass (arrow). b Axial T1-weighted image with fat suppression after gadolinium administration shows a tail-like contrast enhancement spreading from the main mass, indicating a tail sign
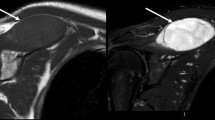
A 79-year-old woman with myxofibrosarcoma in the medial aspect of her left thigh. a Axial T2-weighted image demonstrates soft tissue sarcoma with T2 high-signal intensity, in keeping with a myxoid-rich soft tissue mass. b Axial T1-weighted image with fat suppression after gadolinium administration identified thick fascial enhancement extending from the main mass indicating a tail sign
Radiological-pathological correlation
Pathological reports describing the pathological features were carefully reviewed and the final histological diagnosis was confirmed. Then, a comparison between MR images and histopathological findings was performed on a lesion-by-lesion basis. If necessary, pathological slides were reviewed by an experienced musculoskeletal pathologist and a radiologist.
Statistical analysis
First, tumours were classified as primary or recurrent tumour at presentation. Second, tumours were subdivided into the following two groups: MFS and US comprised group 1, and the remaining tumours were assigned to group 2. The independent t-test was used to evaluate the differences in tumour sizes and enhancing fascial thicknesses between groups. The other nonparametric variables were processed by chi-square analysis or Fisher’s exact test. For all statistical analyses, a two-tailed P < 0.05 was considered statistically significant. The kappa value was used to assess inter-observer variability in the evaluation of adjacent fascial enhancements. Local recurrence-free survival (LRFS) was calculated from the date of surgery to the date of first local recurrence. Patients who had not experienced a local recurrence as of the date of last contact were censored at this date. If a patient died (non-recurring), the date of death was the end date. LRFS was calculated using the Kaplan-Meier survival estimates and analysed by log-rank test. All time intervals were calculated from the date of surgery. Degrees of agreement were categorised as follows: k values of 0.00 to 0.20 were considered to indicate poor agreement; k values of 0.21 to 0.40, fair agreement; k values of 0.41 to 0.60, moderate agreement; k values of 0.61 to 0.80, good agreement; and k values of 0.81 to 1.00, excellent agreement. Statistical analyses were performed using MedCalc version 12.4.2 (MedCalc Software, Mariakerke, Belgium) and SPSS version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
There were 63 tumours in group 1 and 115 tumours in group 2. They were also divided into two categories: 116 primary tumours and 62 recurrent tumours. The mean age, sex, tumour size, and imaging features are summarised in Table 1. Although there was no statistically significant difference in sex between the groups, the mean age of the patients was significantly different between the groups.
Tumour location and configuration
In primary tumours, group 1 tumours were more frequently found in the subcutaneous fat layer, whereas group 2 tumours were more common in deep soft tissue (p = 0.001). Recurrent tumours were distributed nearly equally between deep and superficial locations. The configuration of most tumours was mass forming; however, 13 tumours (4 primary and 9 recurrent) presented as superficial spreading, which is characterised only by localised fascial thickening, without discrete mass formation. All 13 tumours except one were group 1 tumours. Among them, two patients with US experienced multiple recurrences with a superficial spreading pattern during the 5 years of follow-up (Fig. 1).These patients underwent repeated wide excisions of localised thickened fasciae. After careful retrospective review of the images, we found four more missed tumours in four patients because they presented only as a localised fascial thickening with enhancement on preoperative MRI. They became nodular masses on the follow-up MRI and were then treated with wide excisions (Fig. 4).
A 71-year-old man with myxofibrosarcoma in the right lower leg (not shown). a Axial T2-weighted image below the main mass shows mildly thickened crural fascia (arrow). b Axial T1-weighted image with fat suppression after gadolinium administration also shows subtle localised fascial thickening (arrow). This finding was missed at the time of initial diagnosis. After wide excision of the main mass, 1-year follow-up MR was taken. c Axial T2-weighted image identifies that a large recurrent tumour from the site of localized thick fascial enhancement on previous MRI. d Axial T1-weighted image with fat suppression after gadolinium administration demonstrates peripheral mild enhancement without a tail sign
Adjacent thick fascial enhancement (tail sign)
Tail-like thick enhancement along the adjacent fascial plane of the tumour (tail sign) was more common in group 1 in both primary and recurrent tumours (p < 0.001 and p = 0.001, respectively). The tail sign was found in 18 (53 %) of group 1 and 8 (10 %) of group 2 in primary tumours, and in 16 (55 %) of group 1 and 6 (18 %) of group 2 in recurrent tumours. Moreover, group 1 tumours had significantly thicker enhancing fascia adjacent to the tumour (thickness of the tail) in both primary and recurrent tumours (p < 0.001 in both). The mean thickness of the enhancing fascia in group 1 was 2.8 ± 2.4 mm (mean ± SD) in primary tumours and 2.4 ± 1.9 mm (mean ± SD) in recurrent tumours (Table 1). In the subgroup analysis based on tumour location, a significant difference in the presence of the tail sign between groups was observed only in tumours within the subcutaneous layer and not in deep-seated tumours. In subcutaneous tumours, group 1 tumours had a higher proportion of tumours with the tail sign than group 2 tumours [14 of 19 (74 %) vs. 1 of 15 (7 %), p < 0.001 in primary tumours; 11 of 17 (65 %) vs. 3 of 18 (17 %), p = 0.009 in recurrent tumours]. In deep-seated tumours, there was no significant difference in the presence of the tail sign between groups [4 of 14 (29 %) vs. 4 of 57 (7 %), p = 0.059 in primary tumours; 5 of 12 (42 %) vs. 3 of 14 (21 %), p = 0.071 in recurrent tumours] (Table 2). There was good interobserver agreement between the two readers in the classification of the adjacent fascial enhancement pattern (k = 0.672; p < 0.001).
Histopathological examination of the tail sign
Tail-like thick enhancement along the fascial plane extending from the main tumour on MRI was compared with histopathological findings in 20 patients with a tail sign. Although there was no discernible satellite tumour nodule within the thick enhancing fascia on MRI, histological examination revealed extensive tumour cell infiltrations along the deep fascia and fibrous septa of subcutaneous fat tissue, resulting in tumour clusters away from the main mass (Fig. 5d). In tumours with a superficial spreading pattern (n = 4), tiny tumour nodules less than 1 cm in size were found along the thickened fascial plane on microscopic examination (Fig. 1d).
A 44-year-old woman with undifferentiated sarcoma in the right lower leg. a Axial T2-weighted image demonstrates a soft tissue mass located in the anteromedial subcutaneous fat layer of the lower leg and localised deep fascial thickening in the pretibial region adjacent to the main mass (arrow). b Axial T1-weighted image with fat suppression after gadolinium administration shows a tail-like contrast enhancement spreading from the main mass, indicating a tail sign. c Preoperative ultrasound also demonstrates the localised fascial thickening corresponding to the MR images. d, e Photomicrographs (H&E staining, ×1in d and × 25 in e) of histopathological specimen show main mass (M) and satellite small tumour nodules along the fascia (asterisk) and irregular tumour infiltrative projections into the subcutaneous fat tissue and fascia (arrows)
Myxofibrosarcoma vs. undifferentiated sarcoma
Subgroup analysis was performed between MFS and US within group 1 (Table 3). There was no significant difference in the age and sex of patients or in the size, location, tumour configuration, and presence of a tail sign. Approximately half of the tumours were located in the subcutaneous fat layer and exhibited a 2 − 3-mm-thick tail sign.
Tumour recurrence
Kaplan-Meier estimates for LRFS are shown in Fig. 6. Local recurrence occurred in 19 of the group 1 tumours (n = 58) at a mean of 62.7 months (range 1.1-70.2 months) and 7 of the group 2 tumours (n = 100) at a mean of 92.0 months (range 1.5-108.3 months) after diagnosis. Two-year LRFS was 78.2 % in group 1 and 94.6 % in group 2. Patients with a tail sign had a worse LRFS than patients without it (2-year LRFS: 75.1 % vs. 92.8 %, p < 0.01). There was a significantly higher rate of tumour recurrence in the group 1 tumours with a tail sign than in those without it (2-year LRFS: 64.8 % vs. 87.7 %, p = 0.019), whereas the presence of a tail sign did not influence the LRFS in group 2 (2-year LRFS: 91.7 % vs. 95.1 %, p = 0.142).
Kaplan-Meier curve for local recurrence-free survival (LRFS) based on the presence of a tail sign. a Patients with a tail sign have a worse LRFS than patients without a tail-sign (2-year LRFS: 70.2 % vs. 92.2 %, p < 0.01) in all tumour groups. b In group 1, patients with a tail sign also have a worse LRFS than patients without a tail sign (2-year LRFS: 62.2 % vs. 84.0 %, p = 0.007)
Discussion
Although MFS and US are pathologically distinct, the infiltrative growth pattern of the tumour has been well described for both tumours in the literature. Mentzel et al. [11] classified MFS into superficial and deep groups. They reported that the superficial tumours tend to infiltrate and often spread extensively in a longitudinal manner. Sometimes these tumours extend to the deep fascia, losing their nodular appearance in the process and demonstrating a more infiltrative growth pattern [11]. Kaya et al. showed that MFS often showed abnormal signal infiltration along the fascial plane on MRI, which corresponds to an infiltrative growth pattern histologically [9]. Waters et al. also demonstrated that a tapering tail of enhancing tumour extending from a nodular recurrence, which is consistent with spread along fascial planes, was seen in as many as 60 % of recurred low-grade MFS [14]. According to Enzinger and Weiss, MFH also often spreads for a considerable distance microscopically along fascial planes or between muscle fibres instead of having a gross circumscribed appearance [13]. As this study was performed before the reclassification of MFH (2002 WHO classification system), both MFS and US may be included in the study. These previous studies suggested that both MFS and US often have an infiltrative border that extends to the adjacent deep fascia and subcutaneous tissue, which could be represented as a tapered thick fascial enhancement spread from the main mass on MRI.
Of these, only three studies noted and reported the presence of a tail sign on MRI. Kaya et al. assessed the growth pattern of 21 MFSs on MRI and pathological findings [9]. Whereas 17 of 21 tumours showed a pattern of tail-like multidirectional extensive spread along the fascial plane on MRI, the remaining four tumours demonstrated a focal growth pattern. However, most MFSs showed an infiltrative growth pattern histologically regardless of the MR characteristics. This discrepancy may be due to the use of T2-weighted images instead of gadolinium-enhanced images that depict tumour infiltration more accurately. Actually, we could easily find tumours showing a focal growth pattern on T2-weighted image but also demonstrating a tail sign on post-contrast images in our study. Water et al. [14] analysed 13 patients with locally recurrent low-grade MFS and identified a tapering tail of enhancing tumour extending from a nodular recurrence in 60 % of cases. However, the study had limitations because only a small number of locally recurrent MFSs were included, and only eight patients were imaged with MRI. Recently, Lefkowitz et al. [10] reported a tail sign of MFS on MRI, which was described as a well-defined, sharp or tapering, pointed curvilinear projection that enhanced, as a moderately specific (79-90 %) and sensitive (64-77 %) sign for diagnosis of MFS relative to other myxoid-containing tumours. Although this is the first study assessing the frequency and diagnostic value of the tail sign in a large number of patients with MFS, they only focused on myxoid-predominant tumours; thus, they did not assess how many non-myxoid tumours had tails. Six patients with US were comprised of their 52 control patients showing a tail sign in 50-66.7 % of cases, but it is difficult to draw any conclusions about US from this paper based on such a small sample. However, in our experience, the tail sign had been frequently seen in a definite US regardless of a myxoid component. Therefore, MRI findings of MFS and US were analysed together in this study to evaluate infiltrative tumour growth patterns. We divided tumours into the following two groups: group 1, including US and MFS, and group 2, including all other soft tissue sarcomas.
In our study, the tail sign was more frequently observed in group 1 than group 2, especially in patients with subcutaneous tumours. Superficial spreading, which was defined as a localised thick fascial enhancement without discrete mass formation, was also more common in recurrent tumours of group 1. Our results are similar to those of previous studies [9–11, 14]. On histological examination, extensive tumour infiltration along the deep fascia and fibrous septa separating the lobules of the subcutaneous fat tissue and skeletal muscles was observed, which was extended for several centimetres away from the edge of the main mass. Even in the cases without histological confirmation, we considered localised thick fascial enhancement to be a reflection of microscopic tumour infiltration when a discrete mass had developed from the site on follow-up MRIs. The specificity (85-90 %) of the tail sign for the diagnosis of group 1 tumours was relatively high, but the sensitivity (49-53 %) was marginal, as reported by Lefkowitz et al. [10].
Although the diagnostic value of the tail sign was marginal, its recognition may be critical in preoperative planning to reduce the risk of local recurrence and distant metastasis. Several studies have assessed the risk of local recurrence and distant metastasis in tumours known as MFH before MFS and US were recognised as a distinct entity. It has been reported that local recurrence occurs in up to 50-60 % of tumours known as MFH, unrelated to histological grade [8, 11, 13, 15, 16]. According to a comparative study of infiltrative subcutaneous and deep-seated MFH, the local recurrence rate was very high in the infiltrative type of MFH [7, 17]. The high rate of local recurrence of infiltrative-type tumours may be attributed to both their higher incidence of having infiltrative margins and an insufficient surgical margin. Tumours confined to the subcutaneous layer, while capable of local recurrence, rarely metastasise, but once a tumour involves the fascia or deeper structures, the risk of metastasis is substantial [7, 17]. In addition, the MFH is known to become progressively higher in grade at the time of recurrence, which places the patient at risk for metastases and death [8, 14, 16]. As described in the literature, our results showed that patients with a tail sign had a worse LRFS than patients without it (p < 0.001, Fig. 6). The tail-like fascial enhancement may represent infiltrative tumour growth and poorly defined tumour margins, which likely contribute to local recurrence. Therefore, it is important to alert the surgeon that a very wide primary resection margin may be necessary for complete surgical excision. Because diffuse thick fascial enhancement without discrete mass formation was not uncommon in recurrences in group 1 tumours, radiologists evaluating patients with previously resected group 1 tumours need to be aware of this pattern of tumour recurrence on follow-up MRI.
In the subgroup analysis, there was no significant difference in the prevalence of tail-like fascial enhancement between MFS and US. Therefore, a tail sign extending from the main mass could be a common finding of both tumours. Although the two tumours share a tail sign as a common imaging finding, the myxoid nature of MFS usually results in distinct imaging findings on MRI from tumours that are predominantly cellular, fibrous, or lipomatous, allowing differential diagnosis.
This retrospective study has several limitations. First, due to its retrospective analytic nature, selection bias was inherent. Second, radiological-pathological correlation could not be performed in all cases. Although an effort was made to match the MR images with the pathological reports and excised gross specimen, precise lesion-by-lesion correlation was difficult. However, reconfirmation was achieved by experienced musculoskeletal pathologists in some selected cases, which allowed lesion-by-lesion correlation. Third, the assessment of fascial enhancement was confounded by the superimposed radiation and postsurgical changes in patients with recurrent tumours. It was often difficult to determine whether perilesional oedema was due to treatment effects or the recurrent tumour itself. Measurement of the enhanced fascial thickness was also extremely difficult owing to its complex appearance. Other limitations include referral bias. Skewing may be possible in patient inclusion, imaging, and statistics based on data generated from our referral and tertiary care centre, which may be representative of more complicated or atypical presentations.
In conclusion, both MFS and US frequently showed a tail-like enhancement extending from the main mass or localised thick fascial enhancement on preoperative MRI. These MRI appearances, the tail sign, may indicate tumour infiltration along the fascial plane and also be associated with worse local recurrence-free survival. Radiologists should be aware of these MRI findings and inform the surgeon preoperatively so that they can obtain a sufficient surgical margin to minimise the risk of local tumour recurrence.
Abbreviations
- MFH:
-
Malignant fibrous histiocytoma
- MFS:
-
Myxofibrosarcoma
- US:
-
Undifferentiated sarcoma
- MRI:
-
Magnetic resonance imaging
- LRFS:
-
Local recurrence-free survival
References
Enjoji M, Hashimoto H, Tsuneyoshi M, Iwasaki H (1980) Malignant fibrous histiocytoma. A clinicopathologic study of 130 cases. Acta Pathol Jpn 30:727–741
Fletcher CD, Gustafson P, Rydholm A, Willen H, Akerman M (2001) Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol 19:3045–3050
Gibbs JF, Huang PP, Lee RJ et al (2001) Malignant fibrous histiocytoma: an institutional review. Cancer Investig 19:23–27
Fletcher CD (2006) The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology 48:3–12
Rosenberg AE (2013) WHO classification of soft tissue and bone, fourth edition: summary and commentary. Curr Opin Oncol 25:571–573
Petersen I (2013) The new WHO classification and recent results in soft tissue tumor pathology. Pathologe 34:436–448
Fanburg-Smith JC, Spiro IJ, Katapuram SV, Mankin HJ, Rosenberg AE (1999) Infiltrative subcutaneous malignant fibrous histiocytoma: a comparative study with deep malignant fibrous histiocytoma and an observation of biologic behavior. Ann Diagn Pathol 3:1–10
Huang HY, Lal P, Qin J, Brennan MF, Antonescu CR (2004) Low-grade myxofibrosarcoma: a clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of the efficacy of 3-tier and 4-tier grading systems. Hum Pathol 35:612–621
Kaya M, Wada T, Nagoya S et al (2008) MRI and histological evaluation of the infiltrative growth pattern of myxofibrosarcoma. Skeletal Radiol 37:1085–1090
Lefkowitz RA, Landa J, Hwang S et al (2013) Myxofibrosarcoma: prevalence and diagnostic value of the "tail sign" on magnetic resonance imaging. Skeletal Radiol 42:809–818
Mentzel T, Calonje E, Wadden C et al (1996) Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol 20:391–405
Wada T, Hasegawa T, Nagoya S, Kawaguchi S, Kaya M, Ishii S (2000) Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol 30:458–462
Weiss SW, Enzinger FM (1978) Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer 41:2250–2266
Waters B, Panicek DM, Lefkowitz RA et al (2007) Low-grade myxofibrosarcoma: CT and MRI patterns in recurrent disease. AJR Am J Roentgenol 188:W193–W198
Weiss SW, Enzinger FM (1977) Myxoid variant of malignant fibrous histiocytoma. Cancer 39:1672–1685
Merck C, Angervall L, Kindblom LG, Oden A (1983) Myxofibrosarcoma. A malignant soft tissue tumor of fibroblastic-histiocytic origin. A clinicopathologic and prognostic study of 110 cases using multivariate analysis. Acta Pathol Microbiol Immunol Scand Suppl 282:1–40
Matsumoto S, Ahmed AR, Kawaguchi N, Manabe J, Matsushita Y (2003) Results of surgery for malignant fibrous histiocytomas of soft tissue. Int J Clin Oncol 8:104–109
Acknowledgements
The scientific guarantor of this publication is Sung Hwan Hong,1M.D., Ph.D. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this article. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, H.J., Hong, S.H., Kang, Y. et al. MR imaging of myxofibrosarcoma and undifferentiated sarcoma with emphasis on tail sign; diagnostic and prognostic value. Eur Radiol 24, 1749–1757 (2014). https://doi.org/10.1007/s00330-014-3181-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3181-2