Abstract
The surgical management of extremity bone and soft tissue sarcomas has evolved significantly over the last 50 years. The introduction and refinement of high-resolution cross-sectional imaging has allowed accurate assessment of anatomy and tumor extent, and in the current era more than 90% of patients can successfully undergo limb-salvage surgery. Advances in imaging have also revolutionized the clinician’s ability to assess treatment response, detect metastatic disease, and perform intraoperative surgical navigation. This review summarizes the broad and essential role radiology plays in caring for sarcoma patients from diagnosis to post-treatment surveillance. Present evidence-based imaging paradigms are highlighted along with key future directions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcomas constitute a heterogeneous group of neoplasms of mesenchymal origin. While they may arise in any area of the body with connective tissue, the majority of bone and soft tissue sarcomas arise in the extremities. The management of sarcoma patients is truly a multidisciplinary effort. While surgeons, radiation oncologists, and medical oncologists compose the core of the team, radiologists are essential partners in achieving successful clinical outcomes.
The surgical management of extremity bone and soft tissue sarcomas has evolved significantly over the last 50 years. Prior to 1980, amputation was the primary means of local disease control owing to the lack of advanced imaging and adjuvant therapies. The advent of high-resolution cross-sectional imaging allowed for detailed assessment of anatomic compartments, thereby improving the visualization of nearby neurovascular structures and satellite lesions, making limb-salvage resections feasible. As image resolution improved, the surgeon’s ability to preserve tissue and improve function followed. In conjunction with these imaging advances, the introduction of chemotherapy improved disease-free survival, and advances in radiation oncology further improved local control of soft tissue sarcomas. Currently, over 90% of patients with extremity sarcomas can be treated with limb-salvage surgery. This review summarizes the broad and essential role radiology plays in caring for sarcoma patients. Present evidence-based imaging paradigms are highlighted, along with key future directions.
Diagnosis
Radiographs
Radiographs are part of the initial evaluation of bone and soft tissue lesions [1]. While advanced imaging modalities, like MRI, continue to improve and are more accessible than ever, radiographs still provide valuable diagnostic information and should not be overlooked. For bone lesions, standard practice involves obtaining radiographs of the entire bone in two orthogonal projections with the adjacent joint. Key imaging features—including bone destruction pattern, zone of transition, cortical destruction, type of periosteal reaction, matrix and tumor mineralization, and soft tissue involvement—help differentiate indolent from aggressive bone tumors and identify a histologic character (Fig. 1) [2]. While radiographs are seldom diagnostic for soft-tissue lesions, they remain a key component of evaluating soft tissue tumors, many of which have distinctive patterns of mineralization. For example, hemangiomas may contain phleboliths, liposarcomas can contain large coarse calcifications, and synovial sarcomas tend to have coarse calcifications that are amorphous or spiculated [3]. Radiographs may also assist in identifying bony erosion from soft tissue sarcomas adjacent to the bone.
A 24-year-old man with a small painless mass on his left anterior tibia just distal to the knee. Lateral (A) and AP (B) radiographs of the left tibia and fibula show an aggressive skeletal lesion (arrow) with osteoid matrix (asterisk) in the left proximal tibial diametaphysis. Close up of the lateral view (C) shows aggressive periosteal reaction (dashed arrow)
Magnetic resonance imaging
The American College of Radiology considers MRI to be the gold standard for the evaluation of soft tissue sarcomas of the extremity and for identifying bone marrow infiltration and tumor margins in osseous lesions [4]. MRI provides excellent tissue contrast resolution and anatomic information detailing the tumor’s relationship to nearby critical structures, especially with the advent of fast, high-resolution 3D volumetric sequences [1, 5, 6]. During imaging of suspected sarcomas, it is important for the protocol to include the entire anatomic compartment containing the tumor, to evaluate for skip lesions in the bone, multifocal disease, or tumor spread along fascial and vascular planes (Fig. 2) [7].
A 24-year-old man with a small painless mass on his left proximal tibia with aggressive radiographic features underwent MRI for assessment of anatomic extent (same patient as in Fig. 1). Coronal 2D T1-weighted images (A) optimally delineate tumor extent (by differentiating marrow replacement from normal marrow elements on T1-weighted images). A large-field-of-view T1-weighted image from proximal to distal joint was obtained to detect skip lesions. Isotropic volumetric 3D T1 (B) and T2 fat-suppressed (C) imaging was also performed. The 3D acquisition enables reformation of images into any desired plane, as shown here. At the level of the tibial tuberosity, note a T2-hyperintense proximal tibial lesion with slightly higher T2 content (dashed arrow)
For the assessment of a bone tumor, an MRI protocol should include T1-weighted and fluid-sensitive sequences (typically with fat-suppressed T2-weighted or short tau inversion recovery imaging) to assess for tumor extent (by differentiating marrow replacement from normal marrow elements on T1-weighted images) and lesional character (by identifying aggressive features, such as perilesional edema, periosteal reaction, and soft tissue extension). The administration of gadolinium-based contrast agents further aids in the identification of solid tumor areas (differentiating them from cystic areas or areas of necrosis) (Fig. 3), and is a useful adjunct to identify hemorrhage and joint involvement [8]. When standard fast-spin echo T1-weighted imaging is inconclusive, a useful non-contrast fast technique is chemical shift imaging with in-phase and opposed-phase gradient echo imaging (the Dixon technique), which helps distinguish areas of marrow replacement from normal marrow elements or bone marrow edema (Fig. 4); this is an important technique for evaluating tumor extent and characterizing marrow signal abnormalities [9, 10]. In addition, functional imaging sequences with diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI are techniques that do not add significant time to the MRI protocol (Fig. 5), and provide information on tumor character, treatment response, and postsurgical distinction of recurrence [11,12,13].
A 24-year-old man with a small painless mass on his left anterior proximal tibia (same patient as in Fig. 1). Sequential dynamic-contrast–enhanced sequences (A, B) show early arterial and progressive enhancement of the proximal tibial lesion, a functional MRI feature of aggressivity or malignancy. Isotropic volumetric 3D T1 fat-suppressed (B) images with multiplanar reformations show heterogeneous enhancement and extraosseous extension of the aggressive lesion in the proximal diametaphysis and peripheral enhancement of the proximal lesion at the level of the tibial tuberosity
A 24-year-old man with a small painless mass on his left anterior tibia (same patient as in Fig. 1). Axial in-phase (A), opposed-phase (B), and apparent diffusion coefficient (ADC) map images (C) through the proximal tibial at the level of the tibial tuberosity show lack of signal drop-out on chemical shift imaging (solid arrow on A and B) compatible with bone marrow replacement and elevated signal on the ADC map (dashed arrow) compatible with free diffusion and benign disease
A 24-year-old man with a small painless mass on his left anterior tibia (same patient as in Fig. 1). Axial in-phase (A), opposed-phase (B), and ADC map (C) images through the anterior tibial symptomatic lesion in the proximal diametaphysis show lack of signal drop-out on chemical shift imaging (arrow in A and B) compatible with bone marrow replacement, and hypointense signal on the ADC map (dashed arrow in C) compatible with restricted diffusion and malignancy. The two lesions ultimately were histologically confirmed as grade 3 of 3 osteosarcoma in the diametaphysis and simple bone cyst at the level of the tibial tuberosity
For the assessment of a soft tissue tumor, T1-weighted sequences, fluid-sensitive sequences, and contrast-enhanced sequences are important to identify the extent of a mass. The use of contrast is particularly important when trying to differentiate between tumor extension and reactive inflammation, as edema does not typically enhance on postcontrast sequences [14]; this distinction can be further confirmed with DWI [15]. Similar to bone tumor assessment, tumor character and histology can be assessed with conventional anatomic sequences (T1-weighted, T2-weighted, and postcontrast T1-weighted features); as a general rule, malignant lesions are larger and more heterogeneous. The histology of certain tumors can also be suggested sometimes by some conventional features, such as lipid content in lipomatous masses; flow voids and time-sensitive contrast enhancement in vascular malformations; the target sign in peripheral nerve sheath tumors (Fig. 6); the triple sign in synovial sarcoma (T2-weighted images showing three different signal patterns—areas that are hypointense, isointense, and hyperintense within the lesion—related to necrosis, cystic degeneration, dystrophic calcifications, and fibrotic regions); the low-signal internal bands of desmoid tumor; and the tail sign that can be characteristic of a desmoid tumor, nodular fasciitis, and such malignancies as myxofibrosarcoma (Fig. 7) and undifferentiated pleomorphic sarcoma [16]. When tumors lack convincing classic signal characteristics on conventional anatomic imaging, functional techniques can be helpful for further characterization as malignancies typically exhibit restrictive diffusion by quantitative DWI and early enhancement by DCE MRI (Fig. 8) [17, 18].
A 31-year-old woman with neurofibromatosis type 1. Coronal maximum intensity projection of WB-MRI (A) performed using isotropic volumetric 3D STIR sequence shows internal nodular plexiform tumor burden along the brachial plexus and lumbosacral plexus. Reticular elevated T2 signal in the skin and subcutaneous tissues with redundancy, hypertrophy, and flow voids in the right neck and face (solid arrows in A and B) is compatible with a diffuse infiltrative plexiform neurofibroma. Focal right abdominal wall lesion (dashed arrows in A and D) appears solitary with target sign (central hypointense and peripheral hyperintense signal) on axial DWI using low b-values (C) and ADC map (D)
A 73-year-old woman with intermediate-grade myxofibrosarcoma arising in the setting of a precursor hemosiderotic fibrolipomatous tumor. Sagittal T2 fat-suppressed (A) and T1 postcontrast fat-suppressed (B) imaging through the left hand show an indeterminate solid soft tissue mass in the dorsal soft tissues with heterogeneity, a feature associated with malignancy. Note the “tail” of elevated T2 signal and enhancement extending proximally (arrow in A and B). This “tail” is a nonspecific feature of both benign entities (e.g., desmoid tumors) and malignancies (e.g., myxofibrosarcoma). MRI provides key information regarding the anatomic extent of soft tissue sarcoma lesions, particularly for malignancies such as myxofibrosarcoma that can exhibit an infiltrative growth pattern along fascial planes visible as a “tail” of enhancement
A 58-year-old man with neurofibromatosis type 1 and enlarging soft tissue mass in the forearm. Axial T2 fat-suppressed (A), ADC map (B), and T1 fat-suppressed postcontrast images through the forearm show a large heterogeneous soft tissue mass (arrows) with perilesional edema, restricted diffusion, and central necrosis suspicious for malignancy. Note the absence of a target sign on T2 fat-suppressed and ADC map images. Dynamic contrast–enhanced sequences (D) show early arterial enhancement of the forearm lesion, a functional MRI feature of aggressivity or malignancy. This was histologically confirmed to be a malignant peripheral nerve sheath tumor
Ultrasound
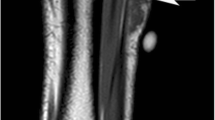
Ultrasonography is less commonly used in the initial assessment of a soft tissue mass, largely due to surgeons’ lack of familiarity in interpreting the images. It can be helpful to confirm the presence of tumor, determine the tumor’s relationships to surrounding structures, estimate dimensions, characterize internal contents of the lesion, and describe margins [19]. Surgeons also find it helpful in differentiating superficial cystic from solid masses (Fig. 9). Additionally, ultrasound is useful in evaluating soft tissue masses in young children who would otherwise require anesthesia for MRI. Positive findings on ultrasound should be correlated with additional advanced imaging as ultrasound is rarely sufficient on its own.
Computed tomography
Due to the superior contrast resolution of MRI, CT is not routinely used in evaluating suspected sarcomas [20]. However, CT can be helpful in certain clinical scenarios. For osseous lesions, it can provide details on tumor matrix, occult matrix mineralization, intra-articular extension, cortical involvement, and degree/depth of endosteal scalloping (Fig. 10) [21]. Surgeons often prefer CT for the evaluation of pelvic tumors because of their familiarity with interpreting anatomic relationships using this modality and because CT provides greater clarity than MRI owing to its higher spatial resolution and lower susceptibility to motion artifacts.
A 24-year-old man with a left proximal tibial osteosarcoma (same patient as in Fig. 1). Axial noncontrast CT (A) and volume rendered 3D CT (B) images of the left tibia provide details regarding osteoid tumor matrix and cortical destruction (arrows)
Biopsy
To establish a definitive diagnosis, a biopsy of the lesion typically must be obtained in order to perform tissue analysis. The diagnostic imaging work-up should be completed prior to biopsy, as it will not only guide the location of the biopsy to the most active portion of the lesion but also can provide additional context to the reviewing pathologist. Additionally, the biopsy tract may make it difficult to evaluate the extent of the lesion due to edema and reactive changes from the biopsy itself. While open biopsy is considered the gold standard, advances in pathologists’ ability to perform elaborate analysis on small amounts of tissue have shifted the biopsy paradigm to percutaneous needle biopsy. In the current era, image-guided percutaneous fine needle aspiration and core needle biopsy are most commonly utilized. Core needle biopsy is the preferred technique for image-guided biopsies of suspected sarcomas (Fig. 9). Many studies have reported on the diagnostic accuracy of core needle biopsy, highlighted by a recent meta-analysis showing that core needle biopsy is similarly accurate, while resulting in fewer complications, than incisional biopsy [22].
The primary modes of image guidance are CT and ultrasound. Ultrasound is ideally used to assess superficial soft tissue lesions, although lesions deep to the fascia can also be accurately sampled using ultrasound guidance [23]. Doppler ultrasound can improve sampling by identifying vascular regions in the tumor, which are likely to contain active cells, and avoid areas of necrosis [24]. Newer techniques, such as contrast-enhanced ultrasound [25] or CT/MRI-fusion ultrasound [26], also can improve visualization of the tumor and minimize sampling error by identifying viable portions of the tumor for biopsy. For deep or intraosseous lesions, CT guidance provides superior resolution [27].
Regardless of the image guidance used, there are multiple considerations in choosing the path for biopsying a lesion, and the shortest route to the tumor is not necessarily the best option [28]. Ideally, the biopsy should be oriented along the limb salvage incision. Errors in biopsy placement lead to more invasive procedures and worse patient outcomes at the time of final surgical resection [29]. Historically, biopsy tracts were resected as part of the definitive resection, although the recent literature has questioned the necessity of this approach [30]. The importance of communication between the surgical and radiology teams regarding the biopsy trajectory cannot be overemphasized.
Staging
Once a sarcoma diagnosis has been established, the next step is disease staging. There are a variety of different staging systems for sarcomas, all of which incorporate some combination of tumor size, tumor grade, local/nodal spread, and distant metastasis. Tumor size is evaluated on the initial diagnostic images, and grade is typically determined by biopsy, although studies have shown that both PET-CT [31] and MRI [32] may provide metrics to help predict the grade of a sarcoma. While staging studies can be completed before biopsy, having a working diagnosis from the biopsy may help guide the choice of staging studies and prevent the patient from undergoing unnecessary or incomplete initial studies. Additionally, some studies (particularly PET-CT) may not be approved by insurance prior to a formal cancer diagnosis.
Evaluations of the extent of disease focus on the most common sites of metastatic spread. For bone and soft tissue tumors, the lung is the most common site of metastatic disease. CT of the chest without contrast is the study of choice, as the addition of contrast does not improve diagnostic accuracy [33]. Multiple studies have shown that while PET-CT can identify larger pulmonary metastatic lesions, it performs worse than CT in evaluating pulmonary disease, particularly for nodules less than 1 cm (Fig. 11) [34]. While CT of the chest is the most sensitive modality for evaluating patients for pulmonary metastasis, rates of false-positive findings remain high due to the prevalence of benign and transient lung nodules, and in indeterminate cases expert consultation and serial exams are often necessary to make a final diagnosis of pulmonary metastasis [35].
Bone sarcomas also have a predilection for metastasizing to other osseous sites. Historically, such metastases have been evaluated using bone scintigraphy, in which Tc-99 methylene diphosphonate is used to identify sites of osteoblastic activity (a surrogate for metastatic lesions). This modality lacks specificity, as other bone-altering pathologies, such as osteoarthritis, fracture, and osteomyelitis, also are identified on bone scan. This led to interest in using more specific modalities to assess patients for distant metastases. Multiple investigations have evaluated PET-CT and found it to be superior to bone scintigraphy for identifying bony metastasis [36, 37]. When available, whole-body MRI, especially in combination with whole-body DWI, has the benefit of detecting both bone and soft tissue metastatic disease without exposing patients to radiation [38, 39].
Certain soft tissue sarcomas—specifically, angiosarcoma, epithelioid sarcoma, clear cell sarcoma, and rhabdomyosarcoma—have a propensity to metastasize to the lymph nodes, and thus require nodal assessment [40]. PET-CT has been used to assess for nodal involvement but does not have a high positive predictive value for nodal metastasis [41] and did not perform as well as sentinel lymph node biopsy in a study of adolescents and young adults with high-grade soft tissue sarcomas [42]. While PET-CT is currently the best imaging modality we have to evaluate for lymph node metastasis, this remains an area that requires further study. Newer imaging methodologies, like whole-body MRI, DWI MRI, and PET/MRI [43], increasingly have a role in better identifying pathologic nodal involvement and in the staging of sarcoma patients in general, when available.
CT of the chest, abdomen, and pelvis (CT CAP) is not routinely used in the staging of sarcomas but may be of use with certain histologies. The most common soft tissue sarcomas of the extremities that metastasize to the abdomen and retroperitoneum are myxoid liposarcoma, leiomyosarcoma, epithelioid sarcoma, myxofibrosarcoma, angiosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumor, and CT CAP may be considered for these histologies [44]. The use of CT CAP in the staging of sarcoma is an evolving field, and further data are required to identify the proper indications for its use in sarcoma staging.
Preoperative considerations
Treatment response
Once a patient has undergone appropriate diagnostic imaging, treatment commences, which typically involves some combination of surgery, chemotherapy, and radiation. Decisions regarding the types of treatment and their order are typically made in the setting of multidisciplinary tumor boards. For patients undergoing preoperative chemotherapy and/or radiation, repeat local imaging close to the date of surgery is critical, as response to treatment impacts the surgical plan. The imaging modalities most commonly used to assess treatment response are MRI and PET-CT, although the exact parameters to accurately assess treatment response are poorly defined. Moreover, not all changes seen on imaging correlate with a good histologic response; at least a 90% (bone tumors) or 95% (soft tissue tumors) histologic response is necessary for a good outcome [45,46,47]. Although the RECIST criteria are commonly used to measure progression/response to treatment in other solid tumors, they do not correlate well with histologic treatment response in sarcoma patients [48]. This is due primarily to the RECIST criteria’s reliance on tumor size to assess treatment response; even when a sarcoma has a strong histologic response to treatment (i.e., a high rate of tumor necrosis), the lesion does not always shrink due to the underlying collagenous or bony matrix, and in some cases may even increase in volume due to hemorrhage, necrosis, and edema [49]. In light of this, other modalities for assessing therapeutic response have been investigated, particularly functional MRI sequences and PET-CT. In a comparison of two methods for assessing radiologic response, Stacchiotti et al. found that the modified Choi criteria (which incorporate both size and internal enhancement patterns/characteristics on post-treatment MRI) were superior to the RECIST criteria for predicting survival [48]. Functional MRI sequences, such as DCE MRI and DWI MRI, have also been shown to accurately predict histological response [50], and to do better than RECIST criteria [51]; in particular, sarcomas that respond with tumor sclerosis rather than necrosis are best assessed with functional MRI sequences [45]. PET-CT studies have consistently shown that changes in standard uptake values on post-treatment PET-CT scans correlate with histologic response in both soft tissue sarcoma [52] and bone sarcoma [53]. New response criteria (named PERCIST) that incorporate PET-CT data are superior to the RECIST criteria in predicting local control and distant progression in soft tissue sarcoma.
In addition to MRI and PET-CT, newer imaging techniques and technologies for assessing response to treatment are being investigated. Techniques such as MR elastography [54] and combined PET/MRI [55] have shown promise in evaluating treatment response. The rapidly evolving field of radiomics (in which artificial intelligence, neural networks, and machine learning are used to analyze raw imaging data to produce clinically relevant information) has also shown promise in assessing treatment response [56, 57].
Radiation effects
The purpose of radiation is to reduce local recurrence of the tumor by treating satellite lesions in and beyond the pseudocapsule of the tumor that the imaging and surgeon cannot see. Decisions regarding the timing of radiation (preoperative, intraoperative, or postoperative) are complex and beyond the scope of this review. Some surgeons prefer preoperative radiation to facilitate a margin-negative surgery. However, the changes that occur in the tumor and surrounding tissues following preoperative radiation can make interpreting post-treatment MRI challenging. Specifically, post-radiation scarring and fibrosis can be difficult to differentiate from tumor or edema. DCE MRI and other functional MRI sequences can aid in distinguishing between the two [16]. Additional considerations when imaging a patient after radiation include allowing at least 3–4 weeks for the effects of treatment to be apparent and recognizing that new enhancement on postradiation images may indicate vascular disruption or hemorrhage rather than disease progression [58]. With conventional 2D MRI sequences, it is important to acquire images in the same plane and with the same imaging parameters as the pre-radiation study of the tumor to facilitate comparison; however, with the advent of 3D volumetric sequences, this issue is mitigated, and the original acquisition plane can be reconstructed into any plane of choice for optimal comparison of the pre- and postradiation studies.
Preoperative planning
Post-treatment imaging not only provides insight on the tumor’s response to the neoadjuvant treatment, but also serves a critical role in preoperative planning for the surgical team. The goal of surgery is to perform a wide excision, meaning that the tumor is taken out en bloc with a cuff of normal tissue and a negative microscopic margin. MRI is commonly used to assess involvement of key anatomical structures, determine the surgical approach, and form a reconstruction plan. When planning level of resection in bone sarcomas, surgeons should keep in mind that in a comparison of MRI pulse sequences (T1- vs. T2-weighted) and timing (before or after neoadjuvant chemotherapy), post-treatment T1 sequences had the strongest correlation with the resected specimen on final pathology review [59].
For soft tissue sarcomas, MRI also provides key information for planning margins. One particularly challenging scenario is the resection of myxofibrosarcoma and undifferentiated pleomorphic sarcoma, which may demonstrate an infiltrative growth pattern (Fig. 7), frequently identified as “tails” extending along fascial planes on MRI [60]. If this is not identified preoperatively, surgery may leave positive margins, which are associated with high rates of local recurrence [61]. DWI MRI [15] and contrast-enhanced, fat-suppressed images [62] aid in identifying the extent of these tails and may provide useful information in the planning of these cases. Even when resecting all concerning tissue identified on MRI, surgeons may have difficulty obtaining a negative margin on the first attempt, and some consider approaching these cases in a staged fashion, waiting for final pathology margin results prior to reconstruction and closure.
Intraoperative imaging
Imaging plays a key role in the operating room during resection of sarcomas. Intraoperative fluoroscopy is routinely used during the resection of bone sarcomas to help identify levels of resection determined during preoperative planning, and to aid in performing the reconstructive part of the procedure. Some institutions also have begun using intraoperative ultrasound to aid in resecting ill-defined or infiltrative soft tissue lesions [63].
One rapidly developing area in sarcoma surgery is the use of image-guided surgical navigation. Its use is particularly beneficial in procedures involving the pelvis and sacrum, which present the surgeon with anatomic challenges related to spatial orientation and the proximity of critical structures. Historically, rates of positive-margin resection and local recurrence have been higher for sarcomas in these locations than for extremity sarcomas, but intraoperative navigation is a promising method of reducing rates of these events [64]. Navigation may also assist in treating periarticular sarcomas in young patients by facilitating the performance of physeal- or articular-sparing wide resection, thus avoiding the need for arthroplasty [65].
Specific workflow may vary based on surgeon preference and institutional resources. Often, patients will undergo a CT scan in the operating room after placement of a fixed reference array. A computer will register these images with spatial orientation information provided by the array. The surgeon may then use a calibrated probe or other instrument with accompanying real-time visualization of orientation in axial, coronal, sagittal, and in-line-with-the-instrument planes. For bone sarcomas with large soft-tissue components, preoperative MRI may be reliably fused to the CT, allowing resections of lesions that are otherwise occult on CT (Fig. 12). Future developments in image-guided navigation may be characterized by increasing use of MRI-based referencing, as well as the introduction of augmented and mixed reality [66].
Surveillance
Patients with bone and soft tissue sarcomas are at risk for both local recurrence and distant metastases, which negatively impact overall survival [67]. The frequency of disease recurrence is risk-stratified by stage. Strong evidence is lacking regarding the method and time-frame for surveillance imaging of sarcoma patients. The National Comprehensive Cancer Network provides general guidelines for surveillance of high-grade soft tissue sarcoma and recommends imaging of the primary site and chest every 2–6 months for 2–3 years, every 6 months for the next 2 years, and annually thereafter [68]. There is no consensus for how long surveillance should continue, though metastasis and local recurrence beyond 10 years is uncommon for most sarcoma subtypes [69].
Local recurrence
Many factors contribute to local recurrence, including the size, location/depth, and histology of the tumor and whether surgery produced negative margins. Long-term prognosis after local recurrence is poor but repeat resection prior to the development of metastases may improve patient survival [70], highlighting the importance of detecting these events.
MRI with and without contrast is commonly performed for surveillance imaging of the primary disease site [4]. Advanced MRI sequences, such as DCE and DWI MRI, can improve detection rate and can help differentiate tumor from postoperative scarring and hematoma [16, 71]. Certain treatment factors may increase the difficulty of identifying local recurrence on MRI imaging. The use of metal implants can create signal artifact in the surgical field, an issue that may be circumvented by utilization of metal artifact–reducing MRI sequences (Fig. 13) (especially in combination with DCE MRI [72]) or PET-CT [73]. Additionally, certain motorized or expandable implants are not MRI-compatible and require evaluation with other imaging modalities. Soft-tissue defects may be reconstructed with myocutaneous flaps, which both alter the local anatomy and may have mass-like signal alterations; identifying this from the patient records can be helpful in distinguishing recurrence from normal postoperative anatomy (Figs. 14 and 15) [74]. Additionally, post-radiation changes such as radiation osteitis can be challenging to differentiate from malignancy in the bone marrow [75].
An 18-year-old woman with a history of osteosarcoma with periprosthetic recurrence in the anterior thigh detected on MRI using metal artifact–reduction sequences. Axial STIR (A), subtracted T1 fat-suppressed postcontrast (B), coronal intermediate weighted sequence (C), and coronal T1-weighted postcontrast (D) images through the left leg show a hyperintense enhancing nodule (dashed arrow) with associated faint early arterial enhancement (E) on dynamic contrast–enhanced (DCE) sequence compatible with subsequent histologically proven recurrence. Evaluation of sagittal subtraction (F) DCE raw images enables localization of the faint early arterial enhancement detected on DCE maximum intensity projections to soft tissue nodule (dashed arrow) located anterior to the femoral prosthesis
A 24-year-old man with left proximal tibial osteosarcoma status after resection and allograft/autograft reconstruction (same patient as in Fig. 1). Although radiographs (A) and multiplanar reformation dual-energy CT optimized for metal reduction (B, C) are optimal for assessment of graft- and instrumentation-related complications, MRI with intravenous contrast is most commonly obtained for surveillance imaging of the primary site of disease
A 24-year-old man with left proximal tibial osteosarcoma status after resection and allograft reconstruction extent (same patient as in Fig. 1). Coronal 2D T1-weighted images (A), in addition to 3D T2 fat-suppressed (B) and 3D T1 (C) images with multiplanar reconstructions, show early postoperative changes from resection of proximal tibial osteosarcoma and reconstruction using allograft (solid arrow) and free fibula strut graft (dashed arrow) and from medial gastrocnemius rotation flap reconstruction (asterisk). MRI, in the post-treatment setting, should be carefully assessed in the context of prior therapeutic approaches
Other imaging modalities are also routinely used to assess for local recurrence. In some types of surgery, radiography is used to evaluate hardware (Fig. 14) and may be helpful in identifying new bony lesions as well. For soft tissue tumors, ultrasound may be effective as an initial screening test due to its high accuracy and negative predictive value; one study even found ultrasound to be equivalent to MRI for detecting local recurrence [76]. Ultrasound may also be used when visualization is limited by metal artifact. While imaging plays a key role in identifying local recurrence, a large portion of local recurrences are identified on physical exam or by the patients themselves [77], so patients should be counseled to notify the clinician of any changes they notice between surveillance visits.
Distant metastasis
As is the case for local recurrence, there is a paucity of high-powered studies to provide strong recommendations regarding the type and interval of chest screening. The two main modalities for such screening are chest radiography and CT. While CT is the most sensitive modality to assess lung nodules, this added sensitivity has not consistently correlated with better patient survival when compared to chest radiography, and additionally it increases the cost and radiation exposure to the patient [35]. Other modalities, such as PET-CT, have also been investigated, but PET-CT alone may not be able to detect small (< 1 cm) malignant nodules and therefore is not the current standard of care [34]. Surveillance of non-pulmonary sites at risk for metastases is driven by the histologic diagnosis (Fig. 16).
A 24-year-old man with left proximal tibial osteosarcoma after resection and allograft reconstruction (same patient as in Fig. 1). Coronal 2D T1-weighted images (A) and sagittal CT images (B) through the left lower extremity show improved incorporation of the fibula and distal femoral osteonecrosis (solid arrow in A, not visualized on the chosen cut in B) and a new lytic lesion in the posterior acetabulum (dashed arrow in B) that was subsequently shown to be a distant metastasis
The TOSS randomized controlled trial attempted to compare survival outcomes between chest radiography and chest CT, and between 3-month versus 6-month follow-up, in patients who had undergone surgery for sarcoma of an extremity. The researchers found the less intensive follow-up method (chest radiography every 6 months) to be non-inferior to CT or to more frequent follow-up [77]. In contrast, another study found that rates of second complete remission and overall survival were higher for CT than for chest radiography among patients with osteosarcoma [78]. Since there is no consensus regarding the optimal modality and timing of chest screening, future studies should consider the role of patient-specific factors, such as histology type and grade, tumor size and location, and whether the patient would be a candidate for metastasectomy. Stratifying patients’ risk on the basis of these and other factors will likely prove more fruitful than attempting to prove the superiority of a single overarching surveillance protocol.
Conclusions
Over the past 5 decades, surgical care for sarcoma has benefited from the tremendous advances in radiology capabilities and techniques. Even in the past decade, many of the classic imaging paradigms in the management of bone and soft tissue sarcoma have begun to shift. Particularly, PET-CT and functional MRI scans have become more readily available and are being used to provide additional insights into a patient’s diagnosis, prognosis, and treatment response. As these technologies, and newer fields such as radiomics, continue to mature, this new data hopefully will provide us with more accurate diagnoses, a better ability to assess response to therapies, and nimbleness to alter treatment plans to better serve the individual patient’s needs. Furthermore, soft tissue sarcomas are commonly treated and studied as a single disease, while in reality the term “soft tissue sarcoma” encompasses over 100 different tumor histologies. As more data is collected on each individual sarcoma diagnosis, we will be able to move from generalized imaging protocols to specific protocols based on diagnostic risk stratification. More than ever, collaborative multi-institutional and registry-based efforts to reach the data thresholds required to make such improvements are needed.
References
Sharon CE, Straker RJ 3rd, Karakousis GC. The role of imaging in soft tissue sarcoma diagnosis and management. Surg Clin North Am. 2022;102(4):539–50.
de Sá Neto JL, Simão MN, Crema MD, Engel EE, Nogueira-Barbosa MH. Diagnostic performance of magnetic resonance imaging in the assessment of periosteal reactions in bone sarcomas using conventional radiography as the reference. Radiol Bras. 2017;50(3):176–81.
Banks KP, Bui-Mansfield LT, Chew FS, Collinson F. A compartmental approach to the radiographic evaluation of soft-tissue calcifications. Semin Roentgenol. 2005;40(4):391–407.
Roberts CC, Kransdorf MJ, Beaman FD, Adler RS, Amini B, Appel M, et al. ACR appropriateness criteria follow-up of malignant or aggressive musculoskeletal tumors. J Am Coll Radiol. 2016;13(4):389–400.
Ahlawat S, Morris C, Fayad LM. Three-dimensional volumetric MRI with isotropic resolution: improved speed of acquisition, spatial resolution and assessment of lesion conspicuity in patients with recurrent soft tissue sarcoma. Skeletal Radiol. 2016;45(5):645–52.
Luna R, Fritz J, Del Grande F, Ahlawat S, Fayad LM. Determination of skeletal tumor extent: is an isotropic T1-weighted 3D sequence adequate? Eur Radiol. 2021;31(5):3138–46.
Sajadi KR, Heck RK, Neel MD, Rao BN, Daw N, Rodriguez-Galindo C, et al. The incidence and prognosis of osteosarcoma skip metastases. Clin Orthop Relat Res. 2004;426:92–6.
Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv51–67.
Tang H, Ahlawat S, Fayad LM. Multiparametric MR imaging of benign and malignant bone lesions. Magn Reson Imaging Clin N Am. 2018;26(4):559–69.
Del Grande F, Tatizawa-Shiga N, Jalali Farahani S, Chalian M, Fayad LM. Chemical shift imaging: preliminary experience as an alternative sequence for defining the extent of a bone tumor. Quant Imaging Med Surg. 2014;4(3):173–80.
Ahlawat S, Khandheria P, Subhawong TK, Fayad LM. Differentiation of benign and malignant skeletal lesions with quantitative diffusion weighted MRI at 3T. Eur J Radiol. 2015;84(6):1091–7.
Yin P, Xu J, Sun X, Liu T, Chen L, Hong N. Intravoxel incoherent motion and dynamic contrast-enhanced magnetic resonance imaging for neoadjuvant chemotherapy response evaluation in patients with osteosarcoma. Eur J Radiol. 2023;162:110790.
Saleh MM, Abdelrahman TM, Madney Y, Mohamed G, Shokry AM, Moustafa AF. Multiparametric MRI with diffusion-weighted imaging in predicting response to chemotherapy in cases of osteosarcoma and Ewing’s sarcoma. Br J Radiol. 2020;93(1115):20200257.
Lefkowitz RA, Landa J, Hwang S, Zabor EC, Moskowitz CS, Agaram NP, et al. Myxofibrosarcoma: prevalence and diagnostic value of the “tail sign” on magnetic resonance imaging. Skeletal Radiol. 2013;42(6):809–18.
Hong JH, Jee WH, Jung CK, Jung JY, Shin SH, Chung YG. Soft tissue sarcoma: adding diffusion-weighted imaging improves MR imaging evaluation of tumor margin infiltration. Eur Radiol. 2019;29(5):2589–97.
Fayad LM, Jacobs MA, Wang X, Carrino JA, Bluemke DA. Musculoskeletal tumors: how to use anatomic, functional, and metabolic MR techniques. Radiology. 2012;265(2):340–56.
Ahlawat S, Fritz J, Morris CD, Fayad LM. Magnetic resonance imaging biomarkers in musculoskeletal soft tissue tumors: review of conventional features and focus on nonmorphologic imaging. J Magn Reson Imaging. 2019;50(1):11–27.
Del Grande F, Ahlawat S, Subhawong T, Fayad LM. Characterization of indeterminate soft tissue masses referred for biopsy: what is the added value of contrast imaging at 3.0 tesla? J Magn Reson Imaging. 2017;45(2):390–400.
Noebauer-Huhmann IM, Weber MA, Lalam RK, Trattnig S, Bohndorf K, Vanhoenacker F, et al. Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging. Semin Musculoskelet Radiol. 2015;19(5):475–82.
Kransdorf MJ, Murphey MD, Wessell DE, Cassidy RC, Czuczman GJ, Demertzis JL, et al. ACR Appropriateness Criteria(®) Soft-Tissue Masses. J Am Coll Radiol. 2018;15(5s):S189-s197.
Igrec J, Fuchsjager MH. Imaging of bone sarcomas and soft-tissue sarcomas. Rofo. 2021;193(10):1171–82.
Birgin E, Yang C, Hetjens S, Reissfelder C, Hohenberger P, Rahbari NN. Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: a systematic review and meta-analysis. Cancer. 2020;126(9):1917–28.
Yoon MA, Chung HW, Chee CG, Lee MH, Lee SH, Shin MJ. Risk factors for diagnostic failure of ultrasound-guided core needle biopsy of soft-tissue tumors based on World Health Organization classification category and biologic potential. AJR Am J Roentgenol. 2020;214(2):413–21.
Peer S, Freuis T, Loizides A, Gruber H. Ultrasound guided core needle biopsy of soft tissue tumors; a fool proof technique? Med Ultrason. 2011;13(3):187–94.
Coran A, Di Maggio A, Rastrelli M, Alberioli E, Attar S, Ortolan P, et al. Core needle biopsy of soft tissue tumors, CEUS vs US guided: a pilot study. J Ultrasound. 2015;18(4):335–42.
Khalil JG, Mott MP, Parsons TW 3rd, Banka TR, van Holsbeeck M. 2011 Mid-America Orthopaedic Association Dallas B Phemister Physician in Training Award: can musculoskeletal tumors be diagnosed with ultrasound fusion-guided biopsy? Clin Orthop Relat Res. 2012;470(8):2280–7.
Rimondi E, Staals EL, Errani C, Bianchi G, Casadei R, Alberghini M, et al. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008;17(7):975–81.
Liu PT, Valadez SD, Chivers FS, Roberts CC, Beauchamp CP. Anatomically based guidelines for core needle biopsy of bone tumors: implications for limb-sparing surgery. Radiographics. 2007;27(1):189–205; discussion 206.
Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78(5):656–63.
Binitie O, Tejiram S, Conway S, Cheong D, Temple HT, Letson GD. Adult soft tissue sarcoma local recurrence after adjuvant treatment without resection of core needle biopsy tract. Clin Orthop Relat Res. 2013;471(3):891–8.
Fendler WP, Chalkidis RP, Ilhan H, Knosel T, Herrmann K, Issels RD, et al. Evaluation of several FDG PET parameters for prediction of soft tissue tumour grade at primary diagnosis and recurrence. Eur Radiol. 2015;25(8):2214–21.
Manikis GC, Nikiforaki K, Lagoudaki E, de Bree E, Maris TG, Marias K, et al. Differentiating low from high-grade soft tissue sarcomas using post-processed imaging parameters derived from multiple DWI models. Eur J Radiol. 2021;138:109660.
Iemsawatdikul K, Wonglaksanapimon S, Mingkwansook V, Lornimitdee W. Evaluation of pulmonary metastases in children by non-contrast chest computed tomography. J Med Assoc Thai. 2013;96(3):334–9.
O JH, Yoo IR, Kim SH, Sohn HS, Chung SK. Clinical significance of small pulmonary nodules with little or no 18F-FDG uptake on PET/CT images of patients with nonthoracic malignancies. J Nucl Med. 2007;48(1):15–21.
Chiesa AM, Spinnato P, Miceli M, Facchini G. Radiologic assessment of osteosarcoma lung metastases: state of the art and recent advances. Cells. 2021;10(3):553. https://doi.org/10.3390/cells10030553.
Byun BH, Kong CB, Lim I, Kim BI, Choi CW, Song WS, et al. Comparison of (18)F-FDG PET/CT and (99 m)Tc-MDP bone scintigraphy for detection of bone metastasis in osteosarcoma. Skeletal Radiol. 2013;42(12):1673–81.
Treglia G, Salsano M, Stefanelli A, Mattoli MV, Giordano A, Bonomo L. Diagnostic accuracy of (1)(8)F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: a systematic review and a meta-analysis. Skeletal Radiol. 2012;41(3):249–56.
Wu Q, Yang R, Zhou F, Hu Y. Comparison of whole-body MRI and skeletal scintigraphy for detection of bone metastatic tumors: a meta-analysis. Surg Oncol. 2013;22(4):261–6.
Cruz IAN, Fayad LM, Ahlawat S, Lederman HM, Nico MAC, Ormond Filho AG, et al. Whole-body MRI in musculoskeletal oncology: a comprehensive review with recommendations. Radiol Imaging Cancer. 2023;5(3):e220107.
Ecker BL, Peters MG, McMillan MT, Sinnamon AJ, Zhang PJ, Kelz RR, et al. Implications of lymph node evaluation in the management of resectable soft tissue sarcoma. Ann Surg Oncol. 2017;24(2):425–33.
Fuglo HM, Jorgensen SM, Loft A, Hovgaard D, Petersen MM. The diagnostic and prognostic value of (1)(8)F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur J Nucl Med Mol Imaging. 2012;39(9):1416–24.
Wagner LM, Kremer N, Gelfand MJ, Sharp SE, Turpin BK, Nagarajan R, et al. Detection of lymph node metastases in pediatric and adolescent/young adult sarcoma: sentinel lymph node biopsy versus fludeoxyglucose positron emission tomography imaging-A prospective trial. Cancer. 2017;123(1):155–60.
Orsatti G, Zucchetta P, Varotto A, Crimi F, Weber M, Cecchin D, et al. Volumetric histograms-based analysis of apparent diffusion coefficients and standard uptake values for the assessment of pediatric sarcoma at staging: preliminary results of a PET/MRI study. Radiol Med. 2021;126(6):878–85.
Smolle MA, Leithner A, Bernhardt GA. Abdominal metastases of primary extremity soft tissue sarcoma: a systematic review. World J Clin Oncol. 2020;11(2):74–82.
Soldatos T, Ahlawat S, Montgomery E, Chalian M, Jacobs MA, Fayad LM. Multiparametric assessment of treatment response in high-grade soft-tissue sarcomas with anatomic and functional MR imaging sequences. Radiology. 2016;278(3):831–40.
Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–9.
Bacci G, Mercuri M, Longhi A, Ferrari S, Bertoni F, Versari M, et al. Grade of chemotherapy-induced necrosis as a predictor of local and systemic control in 881 patients with non-metastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy in a single institution. Eur J Cancer. 2005;41(14):2079–85.
Stacchiotti S, Collini P, Messina A, Morosi C, Barisella M, Bertulli R, et al. High-grade soft-tissue sarcomas: tumor response assessment–pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251(2):447–56.
Gui C, Morris CD, Meyer CF, Levin AS, Frassica DA, Deville C, et al. Characterization and predictive value of volume changes of extremity and pelvis soft tissue sarcomas during radiation therapy prior to definitive wide excision. Radiat Oncol J. 2019;37(2):117–26.
Oka K, Yakushiji T, Sato H, Hirai T, Yamashita Y, Mizuta H. The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol. 2010;39(2):141–6.
Cousin S, Crombe A, Stoeckle E, Brouste V, Loarer FL, Lucchesi C, et al. Clinical, radiological and genetic features, associated with the histopathologic response to neoadjuvant chemotherapy (NAC) and outcomes in locally advanced soft tissue sarcoma (STS) patients (pts). J Clin Oncol. 2017;35(15_suppl):11014–11014.
Lim HJ, Johnny Ong CA, Tan JW, Ching Teo MC. Utility of positron emission tomography/computed tomography (PET/CT) imaging in the evaluation of sarcomas: a systematic review. Crit Rev Oncol Hematol. 2019;143:1–13.
Hongtao L, Hui Z, Bingshun W, Xiaojin W, Zhiyu W, Shuier Z, et al. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: a meta-analysis. Surg Oncol. 2012;21(4):e165-170.
Pepin K, Grimm R, Kargar S, Howe BM, Fritchie K, Frick M, Wenger D, Okuno S, Ehman R, McGee K, James S, Laack N, Herman M, Pafundi D. Soft tissue sarcoma stiffness and perfusion evaluation by MRE and DCE-MRI for radiation therapy response Aassessment: a technical feasibility study. Biomed Phys Eng Express. 2019;5(4). https://doi.org/10.1088/2057-1976/ab2175.
Pourmehdi Lahiji A, Jackson T, Nejadnik H, von Eyben R, Rubin D, Spunt SL, et al. Association of tumor [(18)F]FDG activity and diffusion restriction with clinical outcomes of rhabdomyosarcomas. Mol Imaging Biol. 2019;21(3):591–8.
Gao Y, Ghodrati V, Kalbasi A, Fu J, Ruan D, Cao M, et al. Prediction of soft tissue sarcoma response to radiotherapy using longitudinal diffusion MRI and a deep neural network with generative adversarial network-based data augmentation. Med Phys. 2021;48(6):3262–372.
Fields BKK, Demirjian NL, Cen SY, Varghese BA, Hwang DH, Lei X, et al. Predicting soft tissue sarcoma response to neoadjuvant chemotherapy using an MRI-based delta-radiomics approach. Mol Imaging Biol. 2023;25(4):776–87.
Messiou C, Bonvalot S, Gronchi A, Vanel D, Meyer M, Robinson P, et al. Evaluation of response after pre-operative radiotherapy in soft tissue sarcomas; the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) and Imaging Group recommendations for radiological examination and reporting with an emphasis on magnetic resonance imaging. Eur J Cancer. 2016;56:37–44.
Thompson MJ, Shapton JC, Punt SE, Johnson CN, Conrad EU 3rd. MRI identification of the osseous extent of pediatric bone sarcomas. Clin Orthop Relat Res. 2018;476(3):559–64.
Waters B, Panicek DM, Lefkowitz RA, Antonescu CR, Healey JH, Athanasian EA, et al. Low-grade myxofibrosarcoma: CT and MRI patterns in recurrent disease. AJR Am J Roentgenol. 2007;188(2):W193-198.
Jang WY, Kim HS, Han I. Impact of surgical margin on survival in extremity soft tissue sarcoma: a systematic review and meta-analysis. Medicine. 2021;100(3):e24124 (Baltimore).
Iwata S, Araki A, Funatsu H, Yonemoto T, Kamoda H, Itami M, et al. Optimal surgical margin for infiltrative soft tissue sarcomas: assessing the efficacy of excising beyond the infiltration. J Surg Oncol. 2018;118(3):525–31.
Takeuchi A, Yamamoto N, Hayashi K, Miwa S, Igarashi K, Yonezawa H, et al. Intraoperative ultrasonography-guided surgery for malignant soft tissue tumor. J Surg Oncol. 2020;122(8):1791–801.
Bosma SE, Cleven AHG, Dijkstra PDS. Can navigation improve the ability to achieve tumor-free margins in pelvic and sacral primary bone sarcoma resections? A Historically Controlled Study. Clin Orthop Relat Res. 2019;477(7):1548–59.
Li J, Shi L, Chen GJ. Image navigation assisted joint-saving surgery for treatment of bone sarcoma around knee in skeletally immature patients. Surg Oncol. 2014;23(3):132–9.
Wong KC, Sun EY, Wong IOL, Kumta SM. Mixed reality improves 3D visualization and spatial awareness of bone tumors for surgical planning in orthopaedic oncology: a proof of concept study. Orthop Res Rev. 2023;15:139–49.
Gronchi A, Lo Vullo S, Colombo C, Collini P, Stacchiotti S, Mariani L, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251(3):506–11.
von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J Natl Compr Canc Netw. 2020;18(12):1604–12.
Sawamura C, Matsumoto S, Shimoji T, Okawa A, Ae K. How long should we follow patients with soft tissue sarcomas? Clin Orthop Relat Res. 2014;472(3):842–8.
Blackmon SH, Shah N, Roth JA, Correa AM, Vaporciyan AA, Rice DC, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg. 2009;88(3):877–884; discussion 884–875.
Del Grande F, Subhawong T, Weber K, Aro M, Mugera C, Fayad LM. Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology. 2014;271(2):499–511.
Fritz J, Levin A, Morris C, Fayad LM. Surveillance imaging in patient with tumor prostheses using anatomic and functional metal reduction MRI sequences. Proceedings International skeletal society annual meeting scientific session. New York, NY; 2017. https://internationalskeletalsociety.com/sites/default/files/mtgbooks/2017%20ISS%20Program%20Book_NewYork.pdf.
Park SY, Chung HW, Chae SY, Lee JS. Comparison of MRI and PET-CT in detecting the loco-regional recurrence of soft tissue sarcomas during surveillance. Skeletal Radiol. 2016;45(10):1375–84.
Fox MG, Bancroft LW, Peterson JJ, Kransdorf MJ, Terkonda SP, O’Connor MI. MRI appearance of myocutaneous flaps commonly used in orthopedic reconstructive surgery. AJR Am J Roentgenol. 2006;187(3):800–6.
Vijayakumar G, Jones CM, Supple S, Meyer J, Blank AT. Radiation osteitis: incidence and clinical impact in the setting of radiation treatment for soft tissue sarcoma. Skeletal Radiol. 2023;52(9):1747–54.
Choi H, Varma DG, Fornage BD, Kim EE, Johnston DA. Soft-tissue sarcoma: MR imaging vs sonography for detection of local recurrence after surgery. AJR Am J Roentgenol. 1991;157(2):353–8.
Puri A, Ranganathan P, Gulia A, Crasto S, Hawaldar R, Badwe RA. Does a less intensive surveillance protocol affect the survival of patients after treatment of a sarcoma of the limb? updated results of the randomized TOSS study. Bone Joint J. 2018;100-B(2):262–8.
Paioli A, Rocca M, Cevolani L, Rimondi E, Vanel D, Palmerini E, et al. Osteosarcoma follow-up: chest X-ray or computed tomography? Clin Sarcoma Res. 2017;7:3.
Funding
This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• The initial imaging work-up of a suspected sarcoma includes extremity radiographs, MRI of the entire compartment, and chest CT to evaluate for pulmonary metastases. For bone sarcomas, the work-up also incorporates PET-CT or bone scintigraphy to evaluate other bones.
• Post-treatment surveillance evaluates the primary site, lung, and disease-specific areas at risk for metastases at regularly scheduled intervals.
• Advances in such imaging technologies as PET-CT, functional MRI, and radiomics are improving our ability to diagnose, stage, treat, and surveil patients with sarcoma.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kantzos, A.J., Fayad, L.M., Abiad, J.E. et al. The role of imaging in extremity sarcoma surgery. Skeletal Radiol 53, 1937–1953 (2024). https://doi.org/10.1007/s00256-024-04586-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-024-04586-7