Abstract
Actinomycetes are the most important producers of secondary metabolites for medical, agricultural and industrial applications. Efficient engineering of bacterial genomes to improve their biosynthetic capabilities largely depends on the available arsenal of tools and vectors. One of the most widely used vector systems for actinomycetes is derived from the Streptomyces ghanaensis DSM2932 plasmid pSG5. pSG5 is a broad host range multicopy plasmid replicating via a rolling circle mechanism. The unique feature of pSG5, which distinguishes it from other Streptomyces plasmids, is its naturally thermosensitive mode of replication. This allows the efficient elimination of the plasmid from its host by simply shifting the incubation temperature to non-permissive 37–39 °C. This property makes pSG5 derivatives ideal facultative suicide vectors required for selection of gene disruption/gene replacement, transposon delivery or CRISPR/Cas9-mediated genome editing. Whereas these techniques depend on the fast elimination of the vector, stably replicating expression vectors for the production of recombinant proteins have been constructed more recently. This mini-review describes the generation and application of the pSG5 vector family, highlighting the specific features of the distinct vector plasmids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In times of ‘synthetic biology’, plasmid replicons are often regarded as biobricks for vector constructions that can be randomly combined with appropriate selection markers and other cassettes (http://parts.igem.org/Plasmid_backbones) (Shetty et al. 2008). Thus, many available cloning vectors were constructed by such approaches. Although such ‘biobrick-vectors’ are suitable at least for standard applications, they have severe drawbacks since initiation of plasmid replication is not sufficient to ensure efficient establishing of the plasmid in the host cell. Natural plasmids evolved into complex selfish DNA molecules, which developed elaborate functions and machineries to ensure persistence in their host and strategies to spread in the environment by ‘infecting’ other bacteria (Hulter et al. 2017; Million-Weaver and Camps 2014). Persistence in the host is achieved by reducing the metabolic burden of the plasmid by complex regulatory networks controlling replication (and copy number) and conjugative transfer (Silva et al. 2012). The need to co-exist with other plasmids was the driving force for evolving various mechanisms of autonomous replication and accordingly manifold ways to direct initiation of plasmid replication (Espinosa et al. 2000). Acquirement of beneficial traits, like antibiotic resistance, metabolic properties or virulence functions provides a selective advantage to their hosts (Top et al. 2000). Moreover, plasmids developed various stability functions that complement one another to avoid elimination from the host cell. These include dimer resolution systems, active partitioning systems and toxin-antitoxin systems for the post-segregational killing of host cells that succeeded to get rid of the plasmid (Gerdes et al. 2000).
Maybe the most intriguing acquisition of plasmids was the ability to make their host cells contact other bacteria allowing transfer of the plasmid to a new host cell. Transfer of most plasmids depends on the coupling of a rolling circle type replication system and a specialized type IV protein secretion system (Zechner et al. 2000). Plasmids of the Gram-positive mycelium forming streptomycetes, however, adapted the FtsK/SpoIIIE chromosome segregation system for plasmid transfer (Sepulveda et al. 2011; Vogelmann et al. 2011a).
Many mobile genetic elements have been discovered in Streptomyces and related mycelial actinomycetes (Kataoka et al. 1994; Kieser et al. 1982; Pernodet et al. 1984; Servín-González et al. 1995; Vogelmann et al. 2011b). In general, naturally occurring Streptomyces plasmids are cryptic, meaning that they only encode functions for stable replication and conjugative transfer and do not encode other traits, e.g. resistance genes. Some of these plasmids were used to construct simple cloning vectors (Katz et al. 1983; Kieser et al. 1982; Muth et al. 1989; Wehmeier 1995). Most of these vectors lack stability functions, e.g. a single-stranded origin sso (Suzuki et al. 2004) or a SpdA-like DNA-binding protein that binds to a palindromic sequence (Thoma et al. 2014) and are readily lost, when omitting antibiotic selection. Only few of these vectors had found a broader distribution and are still in frequent use. And even fewer are available from public deposit sites, e.g. Addgene (www.addgene.org/).
The most widely applied Streptomyces replicon for vector constructions is pSG5, a multicopy plasmid with a broad host range (Maas et al. 1998). During the last 35 years, pSG5 was used in many labs to construct different vectors with manifold applications. The scope of this mini-review is to give detailed insights into the pSG5 sequences affecting replication and stability and to provide for the first time a comprehensive compilation of the available vectors, describing their construction, their properties, and their applications. Therefore, this review will not only be helpful in the identification and selection of appropriate vectors but will also assist those intending to construct new pSG5 derivatives.
The conjugative pSG5 plasmid
pSG5 (Fig. 1) is a 12.207-bp plasmid isolated from S. ghanaensis DSM2932 (Muth et al. 1988; Maas et al. 1998). It has an estimated copy number of ~ 50 (Labes et al. 1990) and is self-transmissible. Its DNA transfer region is similar to that of plasmid pSVH1 (Reuther et al. 2006b), the best characterized Streptomyces plasmid (Thoma et al. 2015). Unlike other conjugative Streptomyces plasmids, pSG5 does not cause formation of pock structures. This was attributed to the missing promoter activity upstream of the putative pSG5 spd-operon (Maas et al. 1998).
Map of the S. ghanaensis DSM2932 plasmid pSG5. The pSG5 minimal replicon (thick line, a) contains the double-stranded origin dso (grey box), the rep promoter (thin arrow), and the rep gene (grey arrow), encoding the rolling circle replication initiator protein Rep. Positions of three strong hairpin structures (a 11397/11453, b 11776/11829, c 12077/12118), probably terminating rep transcription are shown. The single-stranded origin sso (grey box) for lagging strand synthesis is located outside the minimal replicon (b). Relevant restriction sites and nucleotide sequences are shown. Brackets indicate that additional sites are present on pSG5. Arrows on the map show the replication gene (grey), regulatory genes (black), genes involved in conjugative plasmid transfer and spreading (light grey), and genes of unknown function (white)
The intriguing feature of pSG5 is its thermosensitive mode of replication. The plasmid is stably inherited only at temperatures below 34 °C, but becomes efficiently eliminated from the host cell at incubation temperatures above 37 °C (Muth et al. 1989). This characteristic seems to be independent of the host strain and has been demonstrated in many Streptomyces species (Arrowsmith et al. 1992; Blanco et al. 1992), but also in other actinomycetes, e.g. Micromonospora (Rose and Steinbuchel 2002) and Actinoplanes (Ostash et al. 2015; Wolf et al. 2016).
Identification of the pSG5 minimal replicon
The minimal replicon of pSG5 (NC_008792.1) was located by transposon mutagenesis of the bifunctional Escherichia coli–Streptomyces shuttle vector pSW344E and deletion/subcloning analyses to nt positions 9433-11623 (Muth et al. 1988, 1989). This fragment (Fig. 1a) contains the rep gene, encoding the initiator protein for rolling circle replication (RCR).
Downstream of rep, there are three dyad symmetry structures (nt 11193-11145, 11776-11829, 12077-12118) with 26, 24 and 19 nucleotides, respectively, in the stem probably forming strong transcriptional terminators with negative delta-G values (Clone Manager 9 professional) of − 51.0, − 45.4 and − 39.1 kcal.
The transcriptional start site and the promoter region of rep have not been mapped so far. The double-stranded origin dso lies immediately upstream of rep and includes the SphI site (nt 9507). In contrast to dso sequences of other Streptomyces RCR plasmids, the dso of pSG5 does not contain the conserved nicking site sequence CCTTGG, which is processed by a Rep-dimer to initiate plasmid replication via the rolling circle mode (Muth et al. 1995; Vogelmann et al. 2011b). Replication of pSG5 might occur at the membrane, since Rep was identified as a membrane associated protein by immunoblotting. After separating subcellular fractions of S. ghanaensis DSM2932, anti-Rep-specific antibodies detected Rep in the membrane fraction, from which it could be eluted with 1% NaCl (Maas and Muth, unpublished).
However, the minimal replicon does not include the single-stranded origin sso, where host factors initiate synthesis of the second strand. Sequence comparison of putative sso regions of various Streptomyces plasmids (Vogelmann et al. 2011b) localized a sso consensus sequence (nt 2125-2299) to the intergenic region between the putative regulatory gene prg and the traB repressor gene traR (Fig. 1b). This fragment prevented accumulation of single-stranded plasmid DNA and enhanced stable maintenance of a pSG5 minimal replicon-based bifunctional plasmid considerably (unpublished data, see below).
Streptomyces plasmid vectors
Based on the pSG5 minimal replicon, a series of Streptomyces cloning vectors were constructed quite a long time ago. For details on their constructions see (Muth et al. 1989). All these vectors lack the sso and accumulate single-stranded DNA. Nevertheless, they proved to be useful and even allowed the shotgun cloning of chromosomal genes (Behrmann et al. 1990).
pGM4
Plasmid pGM4 has a molecular size of 4809 bp and contains the 2687 bp PvuII–SacII fragment (nt 8934-11621) of pSG5. It carries the aminoglycoside phosphotransferase gene aph from Streptomyces fradiae, conferring low-level resistance to neomycin and the rRNA methyltransferase gene tsr from Streptomyces azureus, conferring thiostrepton resistance.
pGM7
Plasmid pGM7 has a molecular size of 5052 bp and contains the 2188-bp Sau3A–SacII fragment (nt 9433-11621) of pSG5. It carries the tsr gene from S. azureus and the tyrosinase gene mel from Streptomyces antibioticus, which confers melanin production. Thus, colonies carrying an intact mel gene are surrounded by the brownish melanin. Insertions into the singular BglII site abolish melanin production.
pGM8
Plasmid pGM8 has a molecular size of 6726 bp and contains the 3122 bp PvuII–XhoI fragment (nt 8934-12056) of pSG5. It carries a fragment of the Tn21 integron (Wohlleben et al. 1989). The Tn21 part consists of the integrase gene, the aminoglycoside acetyltransferase gene aac(3)-I and a aadA fragment encoding the N-terminal 58 aa. The aac(3)-I gene is expressed from the native integron promoter in Streptomyces and confers low level resistance to gentamicin (8 μg/ml) and mediates cross resistance to apramycin. As a second selection marker, pGM8 carries the tsr gene.
pGM9
Plasmid pGM9 has a molecular size of 6265 bp and contains the 3122-bp PvuII–XhoI fragment (nt 8934-12056) of pSG5. It carries the tsr gene and the co-transcribed aphII-ble genes from Tn5. Both aphII and ble are expressed in Streptomyces from the native Tn5 promoter. aphII encodes a phosphotransferase conferring high-level resistance to kanamycin and neomycin, while ble encodes the bleomycin binding protein BLMT (Kumagai et al. 1999). pGM9 was used in fusions with T7-vectors (e.g. pRSETB) to express genes in Streptomyces lividans T7 (J. Altenbuchner, Stuttgart, see below), which contains the T7-polymerase gene (Michta et al. 2012; Pfeifer et al. 2001).
pGM11
Plasmid pGM11 has a molecular size of 5501 bp and contains the 2437-bp PvuII–SalI fragment (nt 8934-11371) of pSG5. It carries the aphII gene from Tn5 for selection and a promoter probe cassette, consisting of the transcriptional terminator of the phage fd (Gentz et al. 1981) and the promoterless gentamicin acetyltransferase gene aac(3)-I from Tn21 (Wohlleben et al. 1989). It was constructed by replacing the 2451-bp HindIII–XhoI fragment of pGM103 (Muth et al. 1988) by the aac(3)-I cassette of pGL500 (Labes et al. 1997), resulting in pGM10. Subsequently, an 850-bp fragment upstream of dso was deleted by replacing the HindIII–PvuII fragment of pGM10 by the HindIII–SmaI aphII fragment of Tn5. As a consequence of the construction of pGM103 involving a partial SalI digest, the stop codon of rep was removed and the very last four amino acids of Rep were replaced by 15 amino acids originating from vector sequences. However, this change did not affect replication of pGM11.
‘Unstable’ bifunctional plasmids for gene disruption and gene replacement studies
Usually, Streptomyces plasmids are introduced by PEG-mediated protoplast transformation, a time-consuming process with often not reproducible efficiency. This hampers more complex plasmid constructions. Therefore, bifunctional vectors, able to replicate in E. coli and Streptomyces have been constructed. Unfortunately, bifunctional Streptomyces plasmids are well known for their instability (Pigac et al. 1988), often making it difficult to isolate such plasmids from Streptomyces cultures. The exact reason for the negative effect of E. coli replicons on Streptomyces plasmids is not known. Nevertheless, many bifunctional pSG5 derivatives were generated. The main advantage of the pSG5-derived plasmids is that they can easily be cleared from the cell by increasing the temperature to non-permissive > 37 °C (Muth et al. 1989). This feature make these plasmids particularly useful to select recombination events via single and double crossover, for transposon delivery and, more recently, for genome editing by CRISPR/Cas9 (see below).
pGM160
Plasmid pGM160 (Muth et al. 1989) has a molecular size of 7792 bp and contains the 2188 bp Sau3A–SacII fragment (nt 9433-11621) of pSG5. It contains the pMB1 ori for replication in E. coli and can be selected in E. coli with ampicillin (bla) and gentamicin (aac(3)-I). pGM160 found broad application as thermosensitive suicide vector for gene disruption and gene replacement experiments (Arrowsmith et al. 1992; Blanco et al. 1992; Muth et al. 1989).
pKC1139
The 3122-bp PvuII–XhoI fragment (nt 8934-12056) of pSG5 containing the thermosensitive replicon was inserted between the Kpnl and Spel sites in pOJ260 after treatment with T4 DNA polymerase. pOJ260 is a pUC-derivative containing the oriT region (including traJ) of plasmid RK2 (Bierman et al. 1992). The resulting plasmid pKC1139 carries the apramycin resistance gene aac(3)IV for selection in E. coli and Streptomyces.
pKG1139
Myronovskyi et al. constructed a derivative of pKC1139, which carried the glucuronidase gene gusA, allowing detection of plasmid carrying strains by the X-Gluc colour reaction. A synthetic DNA fragment containing the gusA gene under the control of the PtipA promoter was cloned as a BamHI fragment into the BglII sites of pKC1139, yielding pKG1139 (Myronovskyi et al. 2011). Induction by thiostrepton requires the thiostrepton responsive activator TipA, encoded by most streptomycetes (Murakami et al. 1989).
pGM446
Plasmid pGM446 was constructed by replacing the 3.4-kb EcoRI–BamHI fragment, containing the SCP2 ori, from the vector pOJ446 by the 3515-bp EcoRI–BamHI fragment of pGM160, which includes the tsr gene, the promoter of the aphI gene and the dso and rep gene of pSG5 (Rose and Steinbuchel 2002). pGM446 contains the thermosensitive pSG5 replicon, the apramycin resistance gene aac(3)IV and the thiostrepton resistance gene tsr for selection, the RK2 oriT for conjugation and cos sites to support DNA packaging. pGM446 was introduced into Micromonospora aurantiaca W2b by intergeneric conjugation. M. aurantiaca exconjugants harbouring plasmid pGM446 grew well at 30 °C but not at 40 °C (Rose and Steinbuchel 2002), demonstrating that the thermosensitive replication of the pSG5 replicon is not restricted to Streptomyces strains.
pGMGus
The β-glucuronidase gene gusA, able to mediate conversion of X-Gluc into coloured 5,5′-dibromo-4,4′-dichloro-indigo, was cut out from pKCLP2 (Myronovskyi et al. 2011) with SpeI/AvrII and cloned into NheI/SpeI digested pRM4.3 (Chevillotte et al. 2008) to place gusA under control of the constitutive PermE* promoter. Subsequently, the 5286-bp SphI–XbaI fragment of the resulting plasmid was replaced by the 82-bp SphI–XbaI polylinker from pUC21, yielding pGus21. To insert the replication region of plasmid pSG5, the dso-rep cassette of pSG5 (nt 9368-11536) was amplified with primers adding SpeI and SnaBI/MunI sites at the ends. The 2002-bp SpeI–MunI fragment was cloned into XbaI/EcoRI-digested pGus21, generating the 7552-bp pGMGus (Addgene: #115678, Muth, unpublished). pGMGus (Fig. 2) carries the aac(3)IV gene allowing selection in E. coli and Streptomyces with apramycin. Presence of the gusA gene makes pGMGus in particular suitable for gene knockout experiments, since double crossover events resulting in the deletion of the vector can be easily distinguished from single crossovers, still carrying the vector integrated, by applying X-Gluc onto the agar plate.
Restriction map of the thermosensitive suicide plasmid pGMGUS. The bifunctional pGMGUS carries the pMB1 origin for replication in E. coli, the oriT and traJ from RK2 for efficient mobilization, dso and rep of pSG5, conferring thermosensitive replication in Streptomyces, a PermE*-gusA cassette and the aac(3)IV gene for apramycin selection. Only unique restriction sites are shown
pSG5 derivatives with counter selectable markers
Efficiency of mutant selection in targeted gene deletion and replacement experiments can be considerably increased by applying a counter selectable marker, which inhibits or eliminates growth of the non-mutated host organism upon selection. Two distinct systems have been incorporated in pSG5 derived plasmids.
pSrpsl14
Based on pGM160, a gene replacement vector containing the rpsL gene as a counter selectable marker for Streptomyces was built. Streptomycin-resistant alleles of the rpsL gene, encoding the 30S ribosomal S12 protein, arise spontaneously by acquiring specific point mutations and can be easily selected in most bacteria. The wild type rpsL gene is dominant over the chromosomal mutant allele, conferring resistance against streptomycin. Thus, presence of the plasmid encoded wild type rpsL gene makes a streptomycin-resistant cell sensitive to streptomycin allowing positive selection of rare genetic events that lead to loss of plasmid sequences. The positive selection plasmid pSrpsl14 was constructed by cloning an EcoRI fragment containing the wild type rpsL (Sms) gene of Streptomyces coelicolor A3(2) into pGM160 (Martinez et al. 2004).
pIJ12739 and pIJ12742
The yeast mitochondrial I-sceI gene encodes the monomeric homing endonuclease I-SceI, which generates double-strand breaks at the 18-base pair recognition sequence TAGGGATAACAGGGTAAT. Fernández-Martínez and Bibb synthesized a codon-optimised I-sceI gene and inserted it as a NdeI–EcoRI fragment into pGM1190 (see below). The resulting plasmid pIJ12739 carried I-sceI under control of the thiostrepton inducible PtipA promoter. In the similar plasmid pIJ12742, I-sceI is expressed from the strong constitutive PermE* promoter.
To demonstrate efficacy and efficiency of this approach, the non-replicative plasmid pIJ12740, containing sequences flanking the biosynthesis genes redD and redX for production of the red-pigmented undecylprodigiosine and the adjacent I-SceI recognition site, was integrated into the S. coelicolor M1141 genome via a single crossover. Subsequently, pIJ2739 (or pIJ12742) was introduced and expression of I-SceI was induced by thiostrepton. Between 29 and 52% of the colonies displayed a mutant phenotype (cream). Arbitrarily, ten cream and ten red colonies were chosen and analysed by PCR. In all colonies, the absence of pIJ12740 was demonstrated. Deletion of redDX was confirmed in all cream isolates, while reversion to the wild type genotype was shown in the red colonies (Fernandez-Martinez and Bibb 2014).
pSG5 derivatives encoding site-specific recombinases
Site-specific recombinases, catalysing recombination between two short non-identical target sequences are widespread in bacteria and phages. They are important tools for in vivo genetic engineering and are used for gene cloning, inversion of DNA fragments, deletion of large chromosomal fragments, integration of heterologous DNA into the chromosome and marker excision (Olorunniji et al. 2016). Besides the integrases of actinophages (ΦC31, ΦBT1, VWB) and integrative conjugative elements (pSAM2), several tyrosine recombinase genes from other organisms were used in various actinomycetes (Herrmann et al. 2012). These included Cre from the P1 phage, recognizing loxP, the yeast Flp recombinase, recognizing FRT and Dre of a Salmonella P1-like phage, which recognizes the rox site (Fedoryshyn et al. 2008a, b). After codon optimization, the recombinase genes were cloned on unstable pUWL-based plasmids (Wehmeier 1995) or expressed from the pSG5 derivatives pNL1 or pAL1 (Fedoryshyn et al. 2008b). Both plasmids are derivatives of pKC1139 (see above). They carry the thermosensitive pSG5 replicon (nt 8934-12056) and contain the PtipA promoter, controlling expression of the respective recombinase gene. They are distinguished by the selection marker (pNL1: aac(3)IV, pAL1: hyg). Application of these plasmids for excision of antibiotic resistance genes yielded marker-free mutants with efficiencies of up to 100% (Herrmann et al. 2012). Availability of this arsenal of site-specific recombinases encoded on different plasmids enables even complex engineering approaches of actinomycetes genomes.
pSG5-based transposon delivery vectors
The thermosensitive mode of replication makes pSG5-based vectors efficient suicide vectors for transposon delivery (Baltz 2016; Bilyk et al. 2013; Petzke and Luzhetskyy 2009; Volff and Altenbuchner 1997). However, presence of the transposase for an extended period can cause problems (multiple insertions, reduced diversity of transposon insertions). Therefore, more recent reports favor the use of non-replicative delivery vectors (Xu et al. 2017).
The first transposon mutagenesis in a streptomycete was reported for IS493-derived transposons (Solenberg and Baltz 1991). Later on, a derivative (Tn5099-IO) was isolated that transposed at elevated frequencies and also a hypertransposable cassette, Tn5100-4, lacking transposase function was constructed (Solenberg and Baltz 1994). The IS493-derived transposons were inserted in a derivative of pGM160 and a colony sectoring procedure was applied to isolate transposition mutants. Such mutants were recovered as colony sectors that continued to grow on a medium selective for the transposon marker after shifting the temperature up to cure the thermosensitive transposon delivery plasmid (Solenberg and Baltz 1991).
Volff and Altenbuchner constructed the mini Tn5 transposon Tn5493, which consists of the thiostrepton resistance gene tsr and the inverted repeats of Tn5. The delivery vector pJOE2577 (Volff and Altenbuchner 1997) is an E. coli-Streptomyces shuttle plasmid based on pGM11 (see above) and provides a mutant tnpA gene leading to higher transposition frequencies. The tnpA gene is under control of the mercury resistance promoter merp and equipped with the ribosomal binding site of the Streptomyces griseofuscus xylA gene. Since pJOE2577 lacks an oriT, it was introduced into S. lividans by PEG-mediated protoplast transformation. After shifting the incubation temperature and eliminating of the autonomously replicating pJOE2577, ~ 3% of the colonies growing at the non-permissive temperature contained a chromosomal Tn5493 insertion (Volff and Altenbuchner 1997).
More recently, Petzke and Luzhetskyy synthesized a codon-optimized Tn5 hyperactive transposase gene tpn(α) and placed it under control of the thiostrepton inducible PtipA promoter (Petzke and Luzhetskyy 2009). The delivery vector pTNM carries besides tpn(α) the hygromycin resistance gene hph, the oriT, the pSG5 replicon and a mini transposon. The mini transposon is flanked by the ME mosaic end recognition sequences of the Tn5 transposase and contains the apramycin resistance gene aac(3)IV and the R6Kγ origin, which supports replication in E. coli pir+ cells. Therefore, the transposon can be excised together with flanking streptomycete DNA and rescued in E. coli to determine the insertion site.
Bilyk et al., used a similar delivery system for the mariner-based synthetic Himar1 transposon (Bilyk et al. 2013). The codon-optimized transposase is expressed from the PtipA promoter and directs transposition of a mini transposon containing a resistance gene (either aac(3)IV, aadA(1) or hph) and the the R6Kγ origin of replication. Moreover, the mini transposon is equipped with loxP and rox sites allowing excision of transposon resistance markers by Cre and/or Dre recombinases. The oriT allows mobilization of the delivery vector from E. coli and the thermosensitive pSG5 replicon enables elimination of the replicative plasmid after transposon mutagenesis.
pSG5-based vectors for CRISPR/Cas9 applications
Genome editing using the CRISPR/Cas9 system depends on the strict control of Cas9 activity to avoid unwanted side effects, like secondary mutations or genome rearrangements. The thermosensitive pSG5 replicon turned out to be an ideal Cas9 vector for Streptomyces, since it can be easily eliminated from the host by increasing the incubation temperature. At least three distinct Cas9 vectors for genome editing of Streptomyces and related actinomycetes have been reported.
pCRISPomyces
pCRISPomyces plasmids encode an engineered CRISPR/Cas system for rapid multiplex genome editing of Streptomyces strains (Wang et al. 2016; Zhang et al. 2017). pCRISPomyces-1 (Addgene: #61736) includes both tracrRNA and CRISPR array expression cassettes along with cas9, while pCRISPomyces-2 (Addgene: #61737) contains a single-guide RNA (sgRNA) expression cassette and cas9.
A precursor of plasmid pCRISPomyces-1 was assembled from its building blocks via homologous recombination in yeast. Subsequently the yeast helper fragment containing URA3 and CEN6/ARS4 was deleted by restriction digest, releasing pCRISPomyces-1. In pCRISPomyces-1, the strong rpsLXC promoter from Xylanimonas cellulosilytica drives expression of the codon-optimized cas9 gene. The tracrRNA is a product of the Cellulomonas flavigena promoter rpsLCF. To facilitate seamless insertion of custom-designed spacers into the CRISPR array by Golden Gate assembly, a lacZ cassette flanked by unique BbsI restriction sites was incorporated between two direct repeat sequences of the empty CRISPR array. Expression of the CRISPR array is directed from the constitutive gapdhEL promoter of the Eggerthella lenta glyceraldehyde-3-phosphate dehydrogenase gene. Thermosensitive replication in Streptomyces is mediated by the pSG5 replicon (nt 8934-12056; an endogenous BbsI site within rep was removed). The apramycin resistance gene aac(3)IV serves as resistance marker for Streptomyces and E. coli. For replication in E. coli, pCRISPomyces-1 carries the ColE1 origin and the oriT of RK2 allows mobilization from appropriate E. coli strains.
Plasmid pCRISPomyces-2 (Addgene: #61737) was constructed via isothermal assembly of the EcoRI/XbaI-digested pCRISPomyces-1 backbone with a synthetic guide RNA expression cassette, containing a BbsI-flanked lacZ cassette in place of the spacer sequence. Both pCRISPomyces plasmids contain a unique XbaI site for insertion of editing template sequences for recombination driven repair of Cas9-induced gaps and found broad application in Streptomyces (Qin et al. 2017) and other actinomycetes (Wolf et al. 2016).
pKCcas9dO
The 13,382-bp plasmid pKCcas9dO (Addgene: # 62552) is a derivative of pKC1139 (see above). It contains a codon-optimized cas9 gene (scocas9) under control of the thiostrepton inducible PtipA promoter (Huang et al. 2015). Since PtipA induction depends on the thiostrepton responsive activator TipA, use of pKCcas9dO is restricted to species encoding a TipA homologue (Murakami et al. 1989). Transcription of the sgRNA is driven by the synthetic j23119 promoter. pKCcas9dO contains the thermosensitive replicon of pSG5 (nt 8934-12056), the pMB1 ori and the apramycin resistance gene aac(3)IV.
pCRISPR-Cas9
A complete toolkit for engineering actinomycetal genomes with CRISPR-Cas9 was developed by Tong et al. (2018). This CRISPR-Cas9 system consists of a variety of suitable vector plasmids and a website for the selection of 20 nt target sequences to be incorporated into sgRNA. After loading a genomic sequence, the program searches selected genes for the presence of the Cas9 PAM motif (NGG) and lists the number of potential secondary binding sites in the genome to avoid unwanted side effects due to the possible generation of off target lesions in the genome.
To construct pCRISPR-Cas9, a single-guide RNA (sgRNA) scaffold, under control of the strong constitutive PermE* promoter, where the 20 nt target sequence is flanked by NcoI and SnaBI restriction sites, respectively, was inserted into the SnaBI site upstream of the to terminator in pGM1190 (see below). Subsequently, a codon-optimized cas9 gene replaced the 29-bp NdeI–XbaI fragment, placing cas9 under control of the thiostrepton inducible PtipA promoter, which requires a functional tipAL gene (Murakami et al. 1989). Single-restriction sites allow the insertion of template sequences for repair of the generated double-strand break in the chromosome by homologous recombination.
The efficiency of pCRISPR-Cas9 in engineering Streptomyces genomes has been widely demonstrated (Low et al. 2018; Tong et al. 2015). Suitability of pCRISPR-Cas9 was extended by replacing cas9 by the dcas9 variant, where the RuvC1 and the HNH nuclease domain encoding sequences were mutated to generate a catalytically inactive dCas9 protein. pCRISPR-dCas9 can be used for sgRNA directed gene silencing (CRISPRi), since binding of dCas9 to the target gene impairs its transcription (Tong et al. 2015).
Following the sgRNA/Cas9 directed DNA cleavage, mutants arise by repairing the chromosomal lesion by the error prone non-homologous end joining (NHEJ) pathway. Since most actinomycetes seem to lack the LigD DNA ligase, a core component of the NHEJ pathway, a modified Streptomyces carnosus ligD gene (scaligD) was cloned to stimulate NHEJ in actinomycetes. Insertion of the scaligD into the StuI site of pCRISPR-Cas9 generated pCRISPR-Cas9-ScaligD (Tong et al. 2015).
To simplify CRISPR vector constructions, pCRISPR-Cas9 was recently modified by adaptation to the uracil-specific excision reagent (USER) cloning technology (Tong et al. 2018).
Stable replicating plasmids for gene expression in Streptomyces
Whereas the above-described vector plasmids were designed for efficient elimination from the host cell, successful application of a vector for gene expression depends on high stability, to avoid loss of the expression plasmid. Although multicopy plasmids are not thought to require specific stability functions, certain parameters affect stability of RCR plasmids. A characteristic of many natural occurring RCR plasmids is that all genes are transcribed in the same direction as the rep gene, encoding the replication initiator protein. The only exceptions in native Streptomyces RCR plasmids are the regulatory genes traR (korA), which control expression of the divergently transcribed traB and spd genes, involved in conjugative transfer. Although it is possible to insert genes in the ‘wrong’ orientation without a dramatic effect on replication, it seems to have consequences for the stability of the respective plasmid. The pIJ101-derived shuttle plasmid pWHM3 (Vara et al. 1989) carries the tsr and the bla gene in the opposite orientation from rep. This plasmid is so unstable that it becomes nearly completely lost after one sporulation cycle under non-selective conditions, allowing its successful use as a ‘suicide’ vector for gene knock out experiments (Fink et al. 1999).
pGM190
In constructing a stable replicating bifunctional expression plasmid it was emphasized to clone all genes in the direction of rep. To allow inducible gene expression, the PtipA expression cassette for inducing transcription upon addition of the thiopeptide antibiotic thiostrepton was amplified from plasmid pIJ6021 (Takano et al. 1995). The PtipA cassette consisted of (i) the transcriptional terminator to from phage lambda (Zukowski and Miller 1986) to prevent transcriptional read-through from the vector, (ii) the PtipA promoter, (iii) the native tipAL ribosome binding site followed by (iv) a polylinker including a NdeI site overlapping with the translational start codon (Murakami et al. 1989), and (v) the transcriptional terminator tfd from the E. coli phage fd (Gentz et al. 1981), located downstream from the polylinker to prevent potentially deleterious transcription of vector sequences. During amplification, the 794-bp PtipA cassette was equipped with MunI/SnaBI and StuI/NheI sites, respectively, and cloned into EcoRI/NheI digested pK18 (Pridmore 1987), yielding pK18-tipA (Muth and Franco, unpublished). To introduce the pSG5 minimal replicon, the rep-tsr cassette was excised from pGM160 as a 3494-bp EcoICR1 fragment and inserted into the StuI site of pK18-tipA, generating plasmids pGM180A and pGM180B. In pGM180B, all genes were in the same orientation as rep. Both plasmids were introduced into S. lividans TK64 by PEG-mediated protoplast transformation. After a single round of sporulation on antibiotic free medium, only about 12.5% of the colonies contained plasmid pGM180A. In contrast, pGM180B, was maintained in 51.5% (Franco and Muth, unpublished), indicating a stabilizing effect, if all genes were transcribed in the same direction as rep.
To further increase stability of pGM180B, the sso of pSG5 was introduced to convert the accumulating single-stranded plasmid molecules more efficiently into double-stranded ones. The sso containing sequence of pSG5 (nt 2103-2442) was amplified with primers adding restriction sites and inserted as a MluI–NheI fragment into MluI/NheI digested pGM180B. As a side effect of the ligation, the aph sequence remains, which originated from the parental plasmid pGM160 were eliminated in the resulting plasmid pGM180sso (Muth and Franco, unpublished). To eliminate the second NdeI site upstream of the tsr gene, which would interfere with subsequent cloning experiments, the tsr gene was amplified with a primer replacing the NdeI site upstream of tsr by XbaI/StuI sites. Subsequently, the 514-bp XbaI–EcoRV (internal within tsr) fragment, lacking the NdeI site was used to replace the 543-bp SpeI–EcoRV fragment of pGM180sso, yielding pGM190 (Fig. 3, Addgene: #69614).
Stability of pGM190 was compared to that of pGM180B for three successive cycles of sporulation on antibiotic free medium. Whereas pGM180B was almost completely lost and retained in only 1.2% of the colonies, pGM190 was more stable and maintained in > 88% of the colonies (Muth and Franco, unpublished). Evidence for the increased stability of pGM190 was also obtained by alkaline lysis of S. lividans, resulting in considerably increased plasmid yields, compared to pGM180B. pGM190 was successfully used for overexpression studies and purification of various proteins (Eys et al. 2008; Reuther et al. 2006a; Thoma and Muth 2015; Vogelmann et al. 2011a).
Restriction maps of the expression vectors pGM190 und pGM202T7 (a). To increase stability, all genes were cloned in the same orientation as rep and the sso for lagging strand synthesis was inserted. The PtipA promoter of pGM190 allows induction of gene expression by thiostrepton. The aphII gene from Tn5 confers kanamycin resistance in E. coli and Streptomyces. pGM202T7 carries the PT7 promoter depending on the RNA polymerase of phage T7. Therefore, use of this plasmid is restricted to E. coli (e.g. BL21) and Streptomyces strains (e.g. S. lividans T7) providing the T7 RNA polymerase. Only unique restriction sites are given. The polylinker sequences of some pGM190 derivatives are shown (b). Unique sites are written in bold, the start codons are underlined. Affinity tags and an enterokinase cleavage site (grey) for protein purification are shown as translation
pGM191.1 and pGM202
The egfp gene encoding the ‘enhanced’ green fluorescent protein (Cormack et al. 1996) was amplified from pTST101 (J. Altenbuchner, Stuttgart) with primers containing a Strep-tagII encoding sequence and subcloned into Litmus 28 (New England Biolabs). Subsequently, the strep-tagII-egfp cassette was cut out and used to replace the 56-bp NdeI–EcoRI fragment of pGM190, generating pGM191.1 (Fig. 3). Due to the presence of the egfp gene under control of the PtipA promoter, Streptomyces mycelium shows green fluorescence, when grown on thiostrepton containing media. By cloning a gene of interest (GOI) as a BamHI–HindIII fragment, it can be fused to an N-terminal Strep-tagII encoding sequence. Alternatively, a C-terminal egfp fusion gene can be expressed by the insertion of an NdeI–BamHI fragment.
Plasmid pGM202 (Fig 2) was constructed by replacing the 35-bp NdeI–BamHI fragment of pGM190 by a NdeI/BglII digested PCR fragment encoding a His-tag encoding sequence. By cloning a NdeI–BamHI fragment, a GOI can be fused to the C-terminal his-tag encoding sequence.
pGM202T7 and pGM202T7-mCherry
The 382-bp PciI–BglII fragment of pRSETB was ligated into PciI/BamHI cut pGM202, generating pGM202T7 (Fig. 3, Addgene: #69993). pGM202T7 carries the PT7 promoter for inducible gene expression in certain E. coli hosts (e.g. BL21) or, respectively, engineered Streptomyces strains, providing the T7 RNA polymerase. Such strains have been generated by J. Altenbuchner, Stuttgart, who integrated a streptomycin/spectinomycin/thiostrepton resistance-mediating pSAM2 derivative, carrying the T7 polymerase gene under control of the PtipA promoter, into the genome of S. lividans (J. Altenbuchner, pers. communication). More recently, F.X. Lussier reported construction of the S. lividans 10 T7 strain, which carried a pSET152 derivative containing a codon-modified T7 polymerase gene under control of the PtipA promoter (Lussier et al. 2010). Depending on the used enzyme, pGM202T7 allows generation of N-terminal or C-terminal His-tag fusions. The N-terminal His-tag can be removed from the affinity purified protein by enterokinase cleavage.
The 729-bp NdeI–HindIII fragment, encoding mCherry, was cut out from pRM43-mCherry (Thoma et al. 2016) and ligated into pGM202T7, yielding pGM202T7-mCherry (Fig. 3). BL21, carrying pGM202T7-mCherry, produces reddish colonies upon induction and S. lividans T7 mycelium, carrying pGM202T7-mCherry, displays bright red fluorescence when irradiated with long-wave UV light. The NdeI and BamHI sites can be used to generate C-terminal mCherry fusion proteins.
Mobilizable expression plasmids
Since introduction of plasmid DNA into Streptomyces by PEG-mediated protoplast transformation (Bibb et al. 1978) is often inefficient and tedious compared to the mobilization of oriT containing plasmids during intergeneric conjugation from E. coli (Flett et al. 1997), mobilizable derivatives of pGM190 were generated.
pGM190-oriT
The mob site of RK2, consisting of the relaxosome component traJ and the nicking site was amplified from pSET152 (Bierman et al. 1992) and inserted via in-fusion cloning (http://www.takarabio.com) into MluI linearized pGM190. Thus, pGM190-oriT (Addgene: #115679) carries the oriT fragment between rep and the sso.
pGM1190 and pGM1202
The oriT-aac cassette of pIJ773 (Gust et al. 2003) was amplified using primers adding XbaI and AsuII sites, respectively. The 1268 bp XbaI–AsuII fragment was used to replace the 1168-bp NheI–AsuII fragment of pGM190, yielding plasmid pGM1190 (Fig. 4, Addgene: #69994).
Restriction map of the mobilizable plasmids pGM1190 and pGM1192 (a). Presence of the RK2 oriT allows mobilization of pGM1190 from suitable E. coli strains providing the conjugative transfer functions of RK2 (e.g. S17-1 (Simon et al. 1983) or ET12562 (pUZ8002) (Kieser et al. 2000). pGM1192 carries a codon-optimized mCherry gene (red arrow) under control of the strong PSF14* promoter of the actinophage I19. Only unique sites are given. Polylinker sequences of pGM1190, pGM1202 and pGM1192 are shown (b); − 35 and − 10 regions and transcriptional start sites of the tandem PSF14 promoter are indicated according to (Labes et al. 1997). PSF14* is distinguished from PSF14 by a missing G residue (grey letter above the sequence). Translation above the nucleotide sequence shows the affinity tags. Unique sites are written in bold, the start codons are underlined. Colonies of E. coli (left) or S. lividans (right), carrying pGM1192 appear pink/red on LBApra plates, with and without blue light (470 nm) irradiation (c)
In an identical approach, pGM1202 (Fig. 4, Addgene: #69615) was generated by replacing the aphII gene from pGM202 against the oriT-aac cassette.
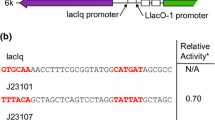
pGM1192
To construct pGM1192 (Fig. 4, Addgene: #115683), the 387-bp SnaBI–BamHI fragment of pGM1190, including the thiostrepton inducible PtipA promoter, was replaced by a synthetic mCherry cassette. This cassette is under transcriptional control of the strong constitutive PSF14* promoter from the actinophage I19 (Labes et al. 1997). PSF14 contains two tandemly arranged promoters, p14-1 and p14-ll, with overlapping and adjacent − 10 and − 35 regions, respectively. Both promoters are recognized by the major RNA polymerase holoenzyme (hrdB) of S. coelicolor resulting in two transcriptional start sites with 4-nt spacing. Both promoters are also functional in E. coli (Labes et al. 1997). Compared to the native PSF14, PSF14* misses a G residue (Fig. 4b) between − 10 and − 35 regions. The codon-optimized mCherry gene is equipped with a synthetic ribosome binding site and an N-terminal Strep-tagIIsc encoding sequence. E. coli or S. lividans transformants carrying pGM1192 form pinkish-red colonies (Fig. 4c), even without UV irradiation.
By replacing the BamH–HindIII mCherry fragment, a GOI can be fused to the Step-tagIIsc sequence. Expression and Streptactin-affinity chromatography purification of the respective protein can be achieved either in E. coli or in Streptomyces. By replacing the NdeI–BamHI fragment, encoding the Step-tagIIsc sequence, the GOI can be fused to the codon-optimized mCherry gene, generating a C-terminal mCherry fusion for protein localization studies in Streptomyces.
Concluding remarks
The most intriguing property of pSG5 is its thermosensitive mode of replication, which makes it in particular appropriate for use in poorly transformable bacteria. If transformation frequency is low, non-replicating suicide vectors cannot be introduced with sufficient efficiency to allow selection of rare events, e.g. gene replacement by double crossovers or random transposition of IS elements. Even if the initial transformation frequency was non-satisfying, a thermosensitive replicon can be propagated at multiple copies in a large population, once introduced. Hence, many plasmid copies can recombine with the chromosome and all recombination/transposition events occurring during the whole period of growth at permissive temperature can be selected after raising the temperature to the non-permissive level.
Thermosensitive replication of plasmids can be achieved by mutagenesis (Birch and Cullum 1985; Eichenlaub 1979; Maguin et al. 1992). For the cryptic Corynebacterium glutamicum plasmid pBL1, it was shown that a single-point mutation resulting in a Pro to Ser substitution of the replication initiator protein made replication of pBL1 thermosensitive (Nakamura et al. 2006). Also, host factors were reported to cause thermosensitive replication of plasmids (Feirtag et al. 1991). However, pSG5 is naturally thermosensitive. Most probably, it had adapted to replication at a low temperature, since its original host S. ghanaensis DSM2932 does not grow above 39 °C. Thermosensitive replication of pSG5 seems to be independent of the host strain and was reported even in a non-Streptomyces host (Wolf et al. 2016).
The pSG5-derived vector family allows broad applications, including use of pure Streptomyces plasmids, shuttle vectors and unstable suicide plasmids for genome editing. Beyond that, the construction of stable expression vectors has to be emphasized, since these vectors represent the only multicopy Streptomyces vectors that have been engineered for stable replication, a prerequisite for reliable gene expression studies.
Besides pSG5, only a few other autonomously replicating Streptomyces plasmids, e.g. pIJ101, pJV1, pSN22, SCP2 and pSVH1 were used to build cloning vectors, often with limited functionality. Of course, it might be possible to develop similar vector families also from other Streptomyces plasmids. But this requires detailed understanding of the biology of the respective plasmid, beyond mere sequence analysis. Whereas in former times, many groups started to isolate and characterize new Streptomyces plasmids (Kataoka et al. 1994; Kieser et al. 1982; Pernodet and Guerineau 1981; Reuther et al. 2006b; Servín-González et al. 1995), plasmid biology is currently not of common interest. Thus, the lack in fully understanding basic plasmid functions will hamper the development of novel vector families based on other Streptomyces replicons.
References
Arrowsmith TJ, Malpartida F, Sherman DH, Birch A, Hopwood DA, Robinson JA (1992) Characterisation of actI-homologous DNA encoding polyketide synthase genes from the monensin producer Streptomyces cinnamonensis. Mol Gen Genet 234:254–264
Baltz RH (2016) Genetic manipulation of secondary metabolite biosynthesis for improved production in Streptomyces and other actinomycetes. J Ind Microbiol Biotechnol 43:343–370
Behrmann I, Hillemann D, Pühler A, Strauch E, Wohlleben W (1990) Overexpression of a Streptomyces viridochromogenes gene (glnII) encoding a glutamine synthetase similar to those of eucaryotes confers resistance against the antibiotic phosphinothricyl-alanyl- ala. J Bacteriol 1729:5326–5334
Bibb MJ, Ward JM, Hopwood DA (1978) Transformation of plasmid DNA into Streptomyces at high frequency. Nature 274:398–400
Bierman M, Logan R, O’Brien K, Seno ET, Nagaraja-Rao R, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Bilyk B, Weber S, Myronovskyi M, Bilyk O, Petzke L, Luzhetskyy A (2013) In vivo random mutagenesis of streptomycetes using mariner-based transposon Himar1. Appl Microbiol Biotechnol 97:351–359
Birch AW, Cullum J (1985) Temperature-sensitive mutants of the Streptomyces plasmid pIJ702. J Gen Microbiol 131:1299–1303
Blanco G, Pereda A, Mendez C, Salas JA (1992) Cloning and disruption of a fragment of Streptomyces halstedii DNA involved in the biosynthesis of a spore pigment. Gene 112:59–65
Chevillotte M, Menges R, Muth G, Wohlleben W, Stegmann E (2008) A quick and reliable method for monitoring gene expression in actinomycetes. J Biotechnol 135:262–265
Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38
Eichenlaub R (1979) Mutants of the mini-F plasmid pML31 thermosensitive in replication. J Bacteriol 138:559–566
Espinosa M, Cohen S, Couturier M, Del Solar G, Diaz Orejas R, Giraldo R, Janniere L, Miller C, Osborn M, Thomas CM (2000) Plasmid replication and copy number control. In: Thomas CM (ed) The horizontal gene pool. Harwood, Amsterdam, pp 1–48
Eys S, Schwartz D, Wohlleben W, Schinko E (2008) Three thioesterases are involved in the biosynthesis of phosphinothricin tripeptide in Streptomyces viridochromogenes Tu494. Antimicrob Agents Chemother 52:1686–1696
Fedoryshyn M, Petzke L, Welle E, Bechthold A, Luzhetskyy A (2008a) Marker removal from actinomycetes genome using Flp recombinase. Gene 419:43–47
Fedoryshyn M, Welle E, Bechthold A, Luzhetskyy A (2008b) Functional expression of the Cre recombinase in actinomycetes. Appl Microbiol Biotechnol 78:1065–1070
Feirtag JM, Petzel JP, Pasalodos E, Baldwin KA, McKay LL (1991) Thermosensitive plasmid replication, temperature-sensitive host growth, and chromosomal plasmid integration conferred by Lactococcus lactis subsp. cremoris lactose plasmids in Lactococcus lactis subsp. lactis. Appl Environ Microbiol 57:539–548
Fernandez-Martinez LT, Bibb MJ (2014) Use of the meganuclease I-SceI of Saccharomyces cerevisiae to select for gene deletions in actinomycetes. Sci Rep 4:7100. https://doi.org/10.1038/srep07100
Fink D, Falke D, Wohlleben W, Engels A (1999) Nitrogen metabolism in Streptomyces coelicolor A3(2): modification of glutamine synthetase I by an adenylyltransferase. Microbiology 145:2313–2322
Flett F, Mersinias V, Smith CP (1997) High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol Lett 155:223–229
Gentz R, Langner A, Chang AC, Cohen SN, Bujard H (1981) Cloning and analysis of strong promoters is made possible by the downstream placement of a RNA termination signal. Proc Natl Acad Sci U S A 78:4936–4940
Gerdes K, Ayora S, Canosa I, Ceglowski P, Diaz Orejas R, Franch T, Gultyaev AP, Bugge Jensen R, Kobayashi I, Macpherson C, Summers D, Thomas CM, Zielenkiewicz U (2000) Plasmid maintenance systems. In: Thomas CM (ed) The horizontal gene pool. Harwood, pp 49–86
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A 100:1541–1546
Herrmann S, Siegl T, Luzhetska M, Petzke L, Jilg C, Welle E, Erb A, Leadlay PF, Bechthold A, Luzhetskyy A (2012) Site-specific recombination strategies for engineering actinomycete genomes. Appl Environ Microbiol 78:1804–1812
Huang H, Zheng G, Jiang W, Hu H, Lu Y (2015) One-step high-efficiency CRISPR/Cas9-mediated genome editing in Streptomyces. Acta Biochim Biophys Sin 47:231–243
Hulter N, Ilhan J, Wein T, Kadibalban AS, Hammerschmidt K, Dagan T (2017) An evolutionary perspective on plasmid lifestyle modes. Curr Opin Microbiol 38:74–80
Kataoka M, Kiyose YM, Michisuji Y, Horiguchi T, Seki T, Yoshida T (1994) Complete nucleotide sequence of the Streptomyces nigrifaciens plasmid, pSN22: genetic organization and correlation with genetic properties. Plasmid 32:55–69
Katz E, Thompson CJ, Hopwood DA (1983) Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol 129:2703–2714
Kieser T, Hopwood DA, Wright HM, Thompson CJ (1982) pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet 185:223–238
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces Genetics. The John Innes Foundation, Norwich
Kumagai T, Nakano T, Maruyama M, Mochizuki H, Sugiyama M (1999) Characterization of the bleomycin resistance determinant encoded on the transposon Tn5. FEBS Lett 442:34–38
Labes G, Simon R, Wohlleben W (1990) A rapid method for the analysis of plasmid content and copy number in various Streptomycetes grown on agar plates. Nucleic Acids Res 18:2197–2197
Labes G, Bibb M, Wohlleben W (1997) Isolation and characterization of a strong promoter element from the Streptomyces ghanaensis phage I19 using the gentamicin resistance gene (aacC1) of Tn1696 as reporter. Microbiol 143:1503–1512
Low ZJ, Pang LM, Ding Y, Cheang QW, Le Mai Hoang K, Thi Tran H, Li J, Liu XW, Kanagasundaram Y, Yang L, Liang ZX (2018) Identification of a biosynthetic gene cluster for the polyene macrolactam sceliphrolactam in a Streptomyces strain isolated from mangrove sediment. Sci Rep 8:1594. https://doi.org/10.1038/s41598-018-20018-8
Lussier FX, Denis F, Shareck F (2010) Adaptation of the highly productive T7 expression system to Streptomyces lividans. Appl Environ Microbiol 76:967–970
Maas RM, Gotz J, Wohlleben W, Muth G (1998) The conjugative plasmid pSG5 from Streptomyces ghanaensis DSM 2932 differs in its transfer functions from other Streptomyces rolling-circle-type plasmids. Microbiology 144:2809–2817
Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A (1992) New thermosensitive plasmid for gram-positive bacteria. J Bacteriol 174:5633–5638
Martinez A, Kolvek SJ, Yip CL, Hopke J, Brown KA, MacNeil IA, Osburne MS (2004) Genetically modified bacterial strains and novel bacterial artificial chromosome shuttle vectors for constructing environmental libraries and detecting heterologous natural products in multiple expression hosts. Appl Environ Microbiol 70:2452–2463
Michta E, Schad K, Blin K, Ort-Winklbauer R, Rottig M, Kohlbacher O, Wohlleben W, Schinko E, Mast Y (2012) The bifunctional role of aconitase in Streptomyces viridochromogenes Tu494. Environ Microbiol 14:3203–3219
Million-Weaver S, Camps M (2014) Mechanisms of plasmid segregation: have multicopy plasmids been overlooked? Plasmid 75:27–36
Murakami T, Holt TG, Thompson CJ (1989) Thiostrepton-induced gene expression in Streptomyces lividans. J Bacteriol 171:1459–1466
Muth G, Wohlleben W, Puhler A (1988) The minimal replicon of the Streptomyces ghanaensis plasmid pSG5 identified by subcloning and Tn5 mutagenesis. Mol Gen Genet 211:424–429
Muth G, Nußbaumer B, Wohlleben W, Pühler A (1989) A vector system with temperature-sensitive replication for gene disruption and mutational cloning in streptomycetes. Mol Gen Genet 219:341–348
Muth G, Farr M, Hartmann V, Wohlleben W (1995) Streptomyces ghanaensis plasmid pSG5: nucleotide sequence analysis of the self-transmissible minimal replicon and characterization of the replication mode. Plasmid 33:113–126
Myronovskyi M, Welle E, Fedorenko V, Luzhetskyy A (2011) Beta-glucuronidase as a sensitive and versatile reporter in actinomycetes. Appl Environ Microbiol 77:5370–5383
Nakamura J, Kanno S, Kimura E, Matsui K, Nakamatsu T, Wachi M (2006) Temperature-sensitive cloning vector for Corynebacterium glutamicum. Plasmid 56(3):179–186
Olorunniji FJ, Rosser SJ, Stark WM (2016) Site-specific recombinases: molecular machines for the genetic revolution. Biochem J 473:673–684
Ostash B, Yushchuk O, Tistechok S, Mutenko H, Horbal L, Muryn A, Dacyuk Y, Kalinowski J, Luzhetskyy A, Fedorenko V (2015) The adpA-like regulatory gene from Actinoplanes teichomyceticus: in silico analysis and heterologous expression. World J Microbiol Biotechnol 31:1297–1301
Pernodet JL, Guerineau M (1981) Isolation and physical characterization of Streptomycete plasmids. Mol Gen Genet 182:53–59
Pernodet JL, Simonet JM, Guerineau M (1984) Plasmids in different strains of Streptomyces ambofaciens: free and integrated form of plasmid pSAM2. Mol Gen Genet 198:35–41
Petzke L, Luzhetskyy A (2009) In vivo Tn5-based transposon mutagenesis of Streptomycetes. Appl Microbiol Biotechnol 83:979–986
Pfeifer V, Nicholson GJ, Ries J, Recktenwald J, Schefer AB, Shawky RM, Schroder J, Wohlleben W, Pelzer S (2001) A polyketide synthase in glycopeptide biosynthesis: the biosynthesis of the non-proteinogenic amino acid (S)-3,5-dihydroxyphenylglycine. J Biol Chem 276:38370–38377
Pigac J, Vujaklija D, Toman Z, Gamulin V, Schrempf H (1988) Structural instability of a bifunctional plasmid pZG1 and single-stranded DNA formation in Streptomyces. Plasmid 19:222–230
Pridmore RD (1987) New and versatile cloning vectors with kanamycin-resistance marker. Gene 56:309–312
Qin Z, Munnoch JT, Devine R, Holmes NA, Seipke RF, Wilkinson KA, Wilkinson B, Hutchings MI (2017) Formicamycins, antibacterial polyketides produced by Streptomyces formicae isolated from African Tetraponera plant-ants. Chem Sci 8:3218–3227
Reuther J, Gekeler C, Tiffert Y, Wohlleben W, Muth G (2006a) Unique conjugation mechanism in mycelial streptomycetes: a DNA-binding ATPase translocates unprocessed plasmid DNA at the hyphal tip. Mol Microbiol 61:436–446
Reuther J, Wohlleben W, Muth G (2006b) Modular architecture of the conjugative plasmid pSVH1 from Streptomyces venezuelae. Plasmid 55:201–209
Rose K, Steinbuchel A (2002) Construction and intergeneric conjugative transfer of a pSG5-based cosmid vector from Escherichia coli to the polyisoprene rubber degrading strain Micromonospora aurantiaca W2b. FEMS Microbiol Lett 211:129–132
Sepulveda E, Vogelmann J, Muth G (2011) A septal chromosome segregator protein evolved into a conjugative DNA-translocator protein. Mob Genet Elements 1:225–229
Servín-González L, Sampieri A, Cabello J, Galván L, Juárez V, Castro C (1995) Sequence and functional analysis of the Streptomyces phaeochromogenes plasmid pJV1 reveals a modular organization of Streptomyces plasmids that replicate by rolling circle. Microbiology 141:2499–2510
Shetty RP, Endy D, Knight TF Jr (2008) Engineering BioBrick vectors from BioBrick parts. J Biol Eng 2:5. https://doi.org/10.1186/1754-1611-2-5
Silva F, Queiroz JA, Domingues FC (2012) Evaluating metabolic stress and plasmid stability in plasmid DNA production by Escherichia coli. Biotechnol Adv 30:691–708
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnol 1:784–791
Solenberg PJ, Baltz RH (1991) Transposition of Tn5096 and other IS493 derivatives in Streptomyces griseofuscus. J Bacteriol 173:1096–1104
Solenberg PJ, Baltz RH (1994) Hypertransposing derivatives of the streptomycete insertion sequence IS493. Gene 147(1):47–54
Suzuki I, Kataoka M, Yoshida T, Seki T (2004) Lagging strand replication of rolling-circle plasmids in Streptomyces lividans: an RNA polymerase-independent primer synthesis. Arch Microbiol 181:305–313
Takano E, White J, Thompson CJ, Bibb MJ (1995) Construction of thiostrepton-inducible, high-copy-number expression vectors for use in Streptomyces spp. Gene 166:133–137
Thoma L, Muth G (2015) The conjugative DNA-transfer apparatus of Streptomyces. Int J Med Microbiol 305:224–229
Thoma L, Sepulveda E, Latus A, Muth G (2014) The stability region of the Streptomyces lividans plasmid pIJ101 encodes a DNA-binding protein recognizing a highly conserved short palindromic sequence motif. Front Microbiol 5:499. https://doi.org/10.3389/fmicb.2014.00499
Thoma L, Dobrowinski H, Finger C, Guezguez J, Linke D, Sepulveda E, Muth G (2015) A multiprotein DNA translocation complex directs intramycelial plasmid spreading during Streptomyces conjugation. MBio 6:e02559–e02514. https://doi.org/10.1128/mBio.02559-14
Thoma L, Vollmer B, Muth G (2016) Fluorescence microscopy of Streptomyces conjugation suggests DNA-transfer at the lateral walls and reveals the spreading of the plasmid in the recipient mycelium. Environ Microbiol 18:598–608
Tong Y, Charusanti P, Zhang L, Weber T, Lee SY (2015) CRISPR-Cas9 based engineering of Actinomycetal genomes. ACS Synth Biol 4:1020–1029
Tong Y, Robertsen HL, Blin K, Weber T, Lee SY (2018) CRISPR-Cas9 toolkit for Actinomycete genome editing. Methods Mol Biol 1671:163–184
Top EM, Moenne-Loccoz Y, Pembroke T, Thomas CM (2000) Phenotypic traits conferred by plasmids. In: Thomas CM (ed) The horizontal gene pool. Harwood Academic Publishers, pp 249–286
Vara J, Lewandowska-Skarbek M, Wang YG, Donadio S, Hutchinson CR (1989) Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J Bacteriol 171:5872–5881
Vogelmann J, Ammelburg M, Finger C, Guezguez J, Linke D, Flotenmeyer M, Stierhof YD, Wohlleben W, Muth G (2011a) Conjugal plasmid transfer in Streptomyces resembles bacterial chromosome segregation by FtsK/SpoIIIE. EMBO J 30:2246–2254
Vogelmann J, Wohlleben W, Muth G (2011b) Streptomyces conjugative elements. In: Dyson P (ed) Streptomyces—molecular biology and biotechnology. Caister Academic Press, Norfolk, pp 27–42
Volff JN, Altenbuchner J (1997) High frequency transposition of the Tn5 derivative Tn5493 in Streptomyces lividans. Gene 194:81–86
Wang Y, Cobb RE, Zhao H (2016) High-efficiency genome editing of Streptomyces species by an engineered CRISPR/Cas system. Methods Enzymol 575:271–284
Wehmeier UF (1995) New multifunctional Escherichia coli-Streptomyces shuttle vectors allowing blue-white screening on XGal plates. Gene 165:149–150
Wohlleben W, Arnold W, Bissonnette L, Pelletier A, Tanguay A, Roy PH, Gamboa GC, Barry GF, Aubert E, Davies J, Kagan SA (1989) On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I(AAC(3)-I), another member of the Tn21-based expression cassette. Mol Gen Genet 217:202–208
Wolf T, Gren T, Thieme E, Wibberg D, Zemke T, Puhler A, Kalinowski J (2016) Targeted genome editing in the rare actinomycete Actinoplanes sp. SE50/110 by using the CRISPR/Cas9 system. J Biotechnol 231:122–128
Xu Z, Wang Y, Chater KF, Ou HY, Xu HH, Deng Z, Tao M (2017) Large-scale transposition mutagenesis of Streptomyces coelicolor identifies hundreds of genes influencing antibiotic biosynthesis. Appl Environ Microbiol 83. https://doi.org/10.1128/AEM.02889-16
Zechner EL, de la Cruz F, Eisenbrandt R, Grahn AM, Koraimann G, Lanka E, Muth G, Pansegrau W, Thomas CM, Wilks M, Zatyka M (2000) Conjugative-DNA transfer processes. In: Thomas CM (ed) The horizontal gene pool. Harwood Academic Publishers, pp 87–175
Zhang MM, Wong FT, Wang Y, Luo S, Lim YH, Heng E, Yeo WL, Cobb RE, Enghiad B, Ang EL, Zhao H (2017) CRISPR-Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nat Chem Biol 13:607–609. https://doi.org/10.1038/nchembio.2341
Zukowski MM, Miller L (1986) Hyperproduction of an intracellular heterologous protein in a sacUh mutant of Bacillus subtilis. Gene 46:247–255
Acknowledgements
The author thanks J. Authenrieth, A. Franco, A. Latus and E. Sulz for assistance in the vector constructions.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB766).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals.
Rights and permissions
About this article
Cite this article
Muth, G. The pSG5-based thermosensitive vector family for genome editing and gene expression in actinomycetes. Appl Microbiol Biotechnol 102, 9067–9080 (2018). https://doi.org/10.1007/s00253-018-9334-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9334-5