Abstract
Biotechnological production of vanillin is gaining momentum as the natural synthesis of vanillin that is very expensive. Ferulic acid (FA), a costly compound, is used as the substrate to produce vanillin biotechnologically and the making process is still expensive. Therefore, this study investigated the practical use of an agrobiomass waste, rice bran, and provides the first evidence of a cost-effective production of vanillin within 24 h of incubation using recombinant Pediococcus acidilactici BD16 (fcs +/ech +). Introduction of two genes encoding feruloyl CoA synthetase and enoyl CoA hydratase into the native strain increased vanillin yield to 4.01 g L−1. Bioconversion was monitored through the transformation of phenolic compounds. A hypothetical metabolic pathway of rice bran during the vanillin bioconversion was proposed with the inserted pathway from ferulic acid to vanillin and compared with that of other metabolic engineered strains. These results could be a gateway of using recombinant lactic acid bacteria for industrial production of vanillin from agricultural waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acceptance and quality of food and beverages are influenced by their flavors. In food industry, vanillin is used as the most popular flavoring agent in terms of consumption level (Kaur and Chakraborty 2012). Vanilla pod is very expensive to grow and takes a long time for its maturation (Odoux and Grisoni 2010). According to European regulation on flavors (EC no. 1334/2008), biotechnological approaches can be labeled as “natural” for product formation and thus can be followed as an interesting alternative for the production of vanillin. Various substrates have been employed to fulfill the increasing demand for natural production of vanillin. For example, ferulic acid (FA) using Streptomyces setonii ATCC 39116 (Achterholt et al. 2000), Pycnoporus cinnabarinus MUCL39533 (Lesage-Meessen et al. 2002), Pseudomonas putida (Plaggenborg et al. 2003), and Amycolatopsis sp. HR167 (Overhage et al. 2006); eugenol using Pseudomonas sp. HR199 (Rabenhorst et al. 2000) and Rhodococcus opacus PD630 (Plaggenborg et al. 2006); isoeugenol using Pseudomonas chlororaphis CDAE5 (Kasana et al. 2007); and vanillic acid using P. cinnabarinus MUCL39533 (Lesage-meessen et al. 2002). FA precursors from common agricultural waste residues such as cereal bran, sugar beet pulp, rice bran oil, and maize bran were used as the sources for FA to synthesize vanillin using fungi (Aspergillus niger I-1472, P. cinnabarinus, A. niger I-1472, P. cinnabarinus MUCL 39533, A. niger CGMCC0774, P. cinnabarinus CGMCC1115). However, this process has been accomplished in two steps; in the first step, FA was released from waste residue using ferulic acid esterase (FAE) producing strains (A. niger I-1472 and A. niger CGMCC0774) but in the second step, FA was converted into vanillin using Pycnoporus sp. (Kaur and Chakraborty 2013). Besides the expensive substrates, two-step processes which are consuming long biotransformation time (e.g., more than 72 h) also make the process very expensive in the industrial settings. Therefore, with the aim for the production of inexpensive and natural food grade vanillin with less biotransformation time, the application of lactic acid bacteria (LAB) has recently been proposed recently due to its generally recognized as safe (GRAS) status.
The LAB isolate (strain was isolated from milk product, e.g., Paneer) Pediococcus acidilactici BD16 previously designated as MTCC 10973 was used to release the phytosterols of rice to be used as the FA precursor and eventually to produce vanillin (Cicero and Derosa 2005; Kaur et al. 2013a, b). However, low vanillin productivity (1.06 g L−1) due to vanillin degradation was observed when the native BD16 strain was used (Kaur and Chakraborty 2013) as also observed in other studies (Overhage et al. 1999; Yoon et al. 2005a, b). Therefore, to enhance the vanillin yield, P. acidilactici BD16 was genetically engineered by subcloning vanillin biosynthetic cassette having feruloyl CoA synthetase (fcs) and enoyl CoA synthetase (ech) genes into pLES003 shuttle vector (accession no. AB370338) from pCC1 (T7/fcs +/ech +) vector (accession no KJ543568) of Escherichia coli top 10 (fcs +/ech +). Expression of fcs and ech genes and FA to vanillin biotransformation through coenzyme A-dependent pathway in the recombinant P. acidilactici BD16 (fcs +/ech +) was reported recently (Kaur et al. 2014). For vanillin production, FA was used as substrate for many other engineered strains like E. coli DH5α, E. coli M109, E. coli DH5α (pTAHEF-gltA+/icd), Pseudomonas fluorescens (vdh −), E. Coli BL21 and E. coli top 10 (fcs +/ech +) where ech and fcs genes originated from various organisms like Amycolatopsis sp. HR167, Amycolatopsis sp. HR104, Delftia acidovorans, Pseudomonas sp. HR199, P. fluorescens BF13, and the genes were expressed using E. coli-inducible expression systems (Overhage et al. 1999; Achterholt et al. 2000; Overhage et al. 2003; Yoon et al. 2005a, b; Barghini et al. 2007; Yoon et al. 2007; Ruzzi et al. 2008; Lee et al. 2009; Song et al. 2009; Di Gioia et al. 2011; Yang et al. 2013; Chakraborty et al. 2016) (Table 1). High price of FA and its low solubility in water, low vanillin productivity, complex downstream processing costs, and genetic instability were major drawbacks of using FA as a substrate for vanillin synthesis. Therefore, in this study, we focused on utilizing rice bran as sole substrate for vanillin production using recombinant strain P. acidilactici BD16 (fcs +/ech +). Statistically optimized conditions of rice bran medium (RBM) as cultivation medium for the recombinant strain for vanillin production were investigated and vanillin degradation of recombinant strain was evaluated using vanillin dehydrogenase (Vdh) activity assay. Furthermore, the process efficiency of P. acidilactici BD16 (fcs +/ech +) was evaluated in terms of FA consumption to vanillin production and compared with another metabolic engineered strain E. coli top 10 (fcs +/ech +). In addition, a metabolic pathway of biotransformation of the rice bran phenolics to vanillin was predicted through chromatographic metabolite analysis.
Materials and methods
Chemicals
Coenzyme A (99% pure, MP chemicals), adenosine tri phosphate (99%, HiMedia Laboratories), vanillin (99%) and hydrochloric acid (35–38%) (s.d. Fine-Chem. Ltd), ammonium nitrate (98%, Nice Chemicals Pvt. Ltd.), maltose (98%, Sisco Research Laboratories Pvt. Ltd.), thiobarbituric acid (99%, BDH), adenosine tri phosphate (99%), dextrose (99%), tri-ammonium citrate (98.5%), di-potassium hydrogen phosphate (99%), magnesium sulfate (99.5–100%), manganous sulfate (98%), magnesium chloride (99%), sodium acetate (82.03%), sodium di-hydrogen orthophosphate (99%), and di-sodium hydrogen orthophosphate (99%) (HiMedia Laboratories Pvt. Ltd.) were obtained and used for biotransformation assay without any processing.
Microorganisms and culture condition
P. acidilactici BD16 (fcs +/ech +) (Kaur et al. 2014) was used as host for the expression of FA catabolic genes fcs-encoding feruloyl CoA synthetase and ech-encoding enoyl CoA hydratase. P. acidilactici BD16 (fcs +/ech +) was revived in de Man, Rogosa, and Sharpe (MRS) broth (containing 20 g L−1 dextrose, 10 g L−1 beef extract, 10 g L−1 peptone, 5 g L−1 sodium acetate, 5 g L−1 yeast extract, 2 g L−1 tri ammonium citrate, 2 g L−1 di-potassium hydrogen phosphate, 0.1 g L−1 magnesium sulfate, 0.05 g L−1 manganese sulfate, and 1 mL tween-80, pH 5.6) under stationary conditions at 37 °C for 24 h. Erythromycin was added at a final concentration of 25 μg mL−1 for selection of recombinant strains. After two subculturings, 1% (v/v) inoculum of culture was transferred into 250-mL flasks containing 50 mL of MRS medium. After overnight cultivation, each culture was used as final inoculum for all biotransformation reactions. Recombinant E. coli top 10 (fcs +/ech +) (Chakraborty et al. 2016) was grown in Luria broth (LB) (containing 10 g L−1 tryptone, 5 g L−1 yeast extract, 1 g L−1 sodium chloride, pH 7) under shaking conditions at 30 °C for 24 h. Chloramphenicol and l-arabinose were added at a final concentration of 25 μg mL−1 and 0.1% for selection of strain and as inducer, respectively. After two subculturings, 1% (v/v) inoculum of each culture was transferred into 250-mL flasks containing 50 mL of MRS and LB medium, respectively. After overnight cultivation, each inoculum was used as final inoculum for all biotransformation reactions.
Vdh activity assay by HPLC
Vdh activity was determined in synthetic medium consisting of 1 g L−1 vanillin, 1 mL tween-80, 2 g L−1 triammonium citrate, 5 g L−1 sodium acetate, 0.1 g L−1 magnesium sulfate, 0.05 g L−1 manganous sulfate, and 2 g L−1 di-potassium hydrogen phosphate. The Vdh assay was carried out at pH 5.6, and vanillin was used as sole carbon source. Recombinant strain P. acidilactici BD16 (fcs +/ech +) was cultured in 500-mL flasks containing 100 mL vanillin synthetic medium at 37 °C for 24 h. One milliliter of culture aliquot from the synthetic medium was withdrawn after 24 h and centrifuged. Vanillin and other phenolic metabolites produced on synthetic medium by selected isolates were partitioned with equal volume of ethyl acetate. HPLC analysis was performed of ethyl acetate part according to the protocol described previously Landete et al. (2010). FA, vanillin, vanillic acid, 4-ethylphenol, and vanillyl alcohol (VAL) were used as standards.
Comparative study of FA to vanillin biotransformation in recombinant strains using synthetic medium
E. coli top 10 (fcs +/ech +) and P. acidilactici BD16 (fcs +/ech +) were transferred to minimal media containing 5 g L−1 sodium acetate, 2 g L−1 di-potassium hydrogen phosphate, 2 g L−1 tri ammonium citrate, 0.1 g L−1 magnesium sulfate, 0.05 g L−1 manganous sulfate, and 1 mL L−1 tween-80 (pH 5.6), supplemented with 0.2 g L−1 (1030 nM) FA. FA to vanillin biotransformation was assayed at 37 °C for 24 h, using 1% v/v inoculum of recombinant P. acidilactici BD16 (fcs +/ech +) and E. coli top 10 (fcs +/ech +). The rate of FA consumption was measured using spectroscopic method (A 310) (Kaur et al. 2013b), and the rate of vanillin production in synthetic medium was estimated using thiobarbutyric acid (TBA) reagent (Converti et al. 2010) at 6 h intervals for 24 h.
RBM
Critically composite design (CCD)-optimized rice bran media containing 150 g L−1 rice bran, 0.05 g L−1 FA (257.5 nM) (as inducer), 5 g L−1 peptone, 2 g L−1 ammonium nitrate, and 1 mL L−1 tween-80, pH 5.6, developed by Kaur and Chakraborty (2013), was used in this study, and FA release in the fermenting medium from rice bran and FA to vanillin transformation was estimated according to Kaur et al. (2013b) and Converti et al. (2010) after inoculating with 1% v/v culture of recombinant P. acidilactici BD16 (fcs +/ech +). Fermented media was centrifuged to remove rice bran partials, and the supernatant was acidified to pH 2.0 with 6 N HCl. Finally, phenolics were extracted using equal volume of ethyl acetate (1:1 v/v). Release of FA in RBM and the molar conversion rate of FA to vanillin were estimated at 6 h intervals for 24 h using the standard method of Narbad and Gasson (1998).
Recovery of vanillin from fermentation medium
Aliquots of fermented media were centrifuged at 8 h interval up to 24 h of incubation to remove rice bran partials. Solvent extraction was carried out to separate vanillin from fermentation medium. Supernatant was acidified to pH 2.0 with 6 N HCl; extracted three times with equal volume of ethyl acetate; and concentrated using rotary vacuum evaporator at 50 °C and recovered in 5 mL of 80% of methanol (Narbad and Gasson 1998; Kaur et al. 2013b). Vanillin from phenolic fraction was initially recovered in water, followed by refrigeration at 4 °C to crystallize vanillin (Eugene 1958). Yellow needle-like crystal vanillin was separated and characterized.
Metabolome profiling by LCMS-ESI
Biotransformation of rice bran to vanillin was evaluated in CCD optimized RBM at 37 °C and 180 rpm for 24 h using 1% v/v inoculum of recombinant P. acidilactici BD16 (fcs +/ech +). Rice bran fermented media was sampled for every 8 h and centrifuged to remove bran partials; supernatant was acidified to pH 2 with 6 N HCl followed by extraction with equal volume of ethyl acetate (Narbad and Gasson 2000). The extracts were concentrated using rotary vacuum evaporator and residue was used for LCMS-MS analysis. Release of FA from rice bran and its further bioconversion to other phenolics including vanillin was estimated using LCMS-electron spray ionization (ESI) method using LCMSn (n = 9) (Thermo, Model-LTQ-XL) with ESI probe at 8 h interval for 24 h. Metabolites were analyzed in a positive mode over a m/z range of 100 to 300 using a C18 column (length—25 cm, and ID—4.6 mm). Metabolomic profiling data was processed as described previously by Liu and Rochfort (2013) to identity each metabolite using Mass Data Bank.
Statistical analysis of data
SPSS 20.0 statistical software was used to analyze correlation coefficient (two-tailed) and regression analysis. Metabolites were detected using LCMS-MS spectral analysis method based upon their relative abundance during biotransformation and fragmentation patterns represented in MS. The significant correlations and regression analysis among FA, caffeic acid, vanillin, catechol, homovanillic acid, trans-cinnamic acid, and meta-methoxy cinnamic acid were analyzed.
Results
Vdh activity assay by HPLC
Vanillin was determined in synthetic medium fermenting P. acidilactici BD16 (fcs +/ech +) after 0 h (early of the biotransformation) and 24 h to detect whether vanillin was degraded into VAL by Vdh activity of the strain or not. But the peaks obtained during HPLC at 0 h and after 24 h confirmed that more than 90% of the vanillin levels remained the same as before (Fig. 1 ). This is due to absence of Vdh activity in the medium or due to microorganism’s lack of interest to uptake vanillin as a carbon source.
FA to vanillin biotransformation
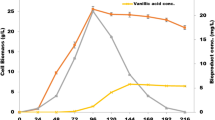
FA to vanillin biotransformation by recombinant P. acidilactici BD16 (fcs +/ech +) and E. coli top 10 (fcs +/ech +) was comparatively studied in different media. Within 6 h, 20 nM of FA was transformed into 7 nM of vanillin with P. acidilactici BD16 (fcs +/ech +) in (minimal media + dextrose + FA inoculated with P. acidilactici BD16 (fcs +/ech +) (Fig. 2a; Table 2) medium whereas 9 nM of FA to 41.2 nM of vanillin biotransformation was observed in E. coli top 10 (fcs +/ech +) (minimal media + dextrose + FA inoculated with E. coli top 10 (fcs +/ech +) (Fig. 2c; Table 2) when synthetic medium was used. However, in CCD optimized RBM (Fig. 2b; Table 2), 29.86 nM FA was released into the RBM medium and simultaneously 101.1 nM of vanillin production was also observed with P. acidilactici BD16 (fcs +/ech +). Release of FA is early hours due to FAE activity of the strain (Fig. 2b; Table 2). With increasing time from 6 to 12 h, FA level was increased where 594.5 nM of FA production was observed in CCD optimized RBM by P. acidilactici BD16 (fcs +/ech +), but no vanillin production was detected. In synthetic medium (Fig. 2a, c; Table 2), vanillin production was also not observed during this time with P. acidilactici BD16 (fcs +/ech +) and E. coli top 10 (fcs +/ech +), respectively. With further increase in time (12–18 h), FA conversion rate was increased in A medium where only 3 nM of FA retained but 155 nM of vanillin was produced with P. acidilactici BD16 (fcs +/ech +) while 1 nM of FA retention and 12.64 nM vanillin production by E. coli top 10 (fcs +/ech +) were observed in synthetic medium (Fig. 1c). The rate at which FA is liberated from rice bran and the rate of production of vanillin were found to be directly correlated in CCD optimized RBM (Fig. 1b) during this time. In recombinant P. acidilactici BD16 (fcs +/ech +), 703.2 nM of FA to 41,097.6 nM vanillin production was observed which is the highest yield reported among all the reported values available in the literature. At last stage, within (18–24 h) of fermentation, 15.88 nM of FA to 6.31 nM of vanillin conversion was observed in recombinant E. coli top 10 (fcs +/ech +) in case of synthetic medium (Fig. 1c), which was a little less as compared to recombinant P. acidilactici BD16 (fcs +/ech +), in which 10 nM of FA to 26.82 nM of vanillin conversion was observed. But, in RBM medium, 55.62 nM of FA production was observed during this time (18–24 h). In RBM (Fig. 1b), in total, conversion of 1383.2 nM of FA to 41,198.7 nM of vanillin was observed after 24 h of fermentation with P. acidilactici BD16 (fcs +/ech +), whereas 54.84 nM of FA to 188.82 nM vanillin and 29.88 nM of FA to 60.15 nM vanillin productions were observed in synthetic media using P. acidilactici BD16 (fcs +/ech +) and E. coli top 10 (fcs +/ech +), respectively, after 24 h of fermentation (Table 2; Fig. 2a–c). Overall study revealed that the rate of FA production, consumption, and its transformation to vanillin was more in recombinant P. acidilactici BD16 (fcs +/ech +) than E. coli top 10 (fcs +/ech +) in RBM than synthetic medium. It may be due to the fact that extracellular enzyme expression was more in case of P. acidilactici BD16 (fcs +/ech +) than E. coli top 10 (fcs +/ech +) as described earlier during kinetic analysis of cloned genes (Kaur et al. 2014).
Metabolic profiling to identify vanillin and metabolic intermediates using LCMS-ESI
Within 1 h of fermentation FA, cinnamic acid fragmentation products and FA decarboxylation products such as caffeic acid, homovanillic acid, and vanillin were confirmed in the biotransforming medium. LCMS study clearly indicates that the release of cell wall-bound FA from rice bran and other FA degradation products confirms FAE activity of the recombinant P. acidilactici BD16 (fcs +/ech +) strain which helped to release FA from highly cross-linked cell wall matrix of rice bran. Intensity of cinnamic acid fragmentation product was highest than all other metabolites in the biotransforming medium. Same compounds with different amplitudes including dihydroferulic acid appeared after 8 h. It was observed that FA fragmentation product increases but meta-methoxy cinnamic acid and cinnamic acid fragmentation product decreases due to continuous biotransformation flow into benzyl ion. Vanillin production in early hour was probably due to FA into vanillin biotransformation through homovanillic acid. De-esterifying activity of the recombinant strain released FA in monomer and dimer from the cell wall. Overall results indicate that with increasing time, FA bioconversion to metabolites like meta-methoxy cinnamic acid, caffeic acid, and vanillic acid was observed during FA to vanillin biotransformation. After 16 h of biotransformation, increased amounts of FA fragmentation product were detected than other metabolites. trans-Cinnamic acid and meta-methoxy cinnamic acid were also detected but not in much abundance as reported in early hours. On the other hand, concentration of benzyl ion increased, with the detection of catechol and vanillin fragmentation product in the RBM medium. Overall analysis confirms that a consistent FAE activity of the strain and detection of 4-vinyl guaiacol in the medium also confirms vanillin formation through decarboxylation pathway which was indicated in our previous report on native strain M16 of recombinant P. acidilactici (fcs +/ech +) (Kaur et al. 2013a). On the same time, vanillin fragmentation via catechol was also observed in the RBM medium (Table 3; Figs. S1–S3).
After 18 h of biotransformation, cleavage of benzene ring with their fragmentation products and FA fragmentation product along with catechol in low abundance was detected. Very interesting data was obtained from peak with RT of 9.93 min, which reveals complete biotransformation of caffeic acid into cinnamic acid as no caffeic acid was detected. Similarly, RTs 9.93 and 12.8 min (Table 3; Fig. S4) are very important as vanillin was detected in the RBM medium in maximum abundance for the first time than early hours which confirms FA to vanillin biotransformation as a function of FA concentration in the biotransforming medium. Enzymatic activity of Fcs and Ech results in higher vanillin production during fermentation. Probability of vanillin production through vanillic acid and homovanillic acid from FA through reductive pathway cannot be neglected here as it is already confirmed in the native strain P. acidilactici BD16. Sharp peak of vanillin after 24 h of biotransformation confirmed successful expression of cloned Fcs and Ech enzymes by the recombinant P. acidilactici BD16 (fcs +/ech +) which was detected from LCMS peak of RT at 12.98 min (Fig. 3). RT at 3.07 min revealed the presence of FA fragmentation product, catechol, and meta-methoxy cinnamic acid in the fermentation medium. Existence of FAE activity in recombinant strain releases FA simultaneously in the medium with FA fragmentation to cinnamic acid (Fig. 3). Presence of vanillin degradation product like vanillyl alcohol was not detected in the fermentation medium which is one of the major advantages of using recombinant P. acidilactici BD16 (fcs +/ech +) for vanillin biosynthesis gene expression.
A significant correlation was found among FA, vanillin, caffeic acid, meta-methoxy cinnamic acid, trans-cinnamic acid, and homovanillic acid. There is a negative correlation between abundance of FA, vanillin, catechol, and trans-cinnamic acid, which means that the concentrations of vanillin, catechol, and trans-cinnamic acid increases as the amount of FA falls in the RBM. Whereas correlations of FA with caffeic acid, homovanillic acid, and methoxy cinnamic acid were positive (Table 4), correlations between homovanillic acid with vanillin and vanillin with catechol were negative, which emphasizes that homovanillic acid to vanillin and vanillin to catechol bioconversion in rice bran containing biotransformation medium are reversely linked.
Regression analysis of FA with vanillin was found significant at r = 0.727, P = 0.043, and r 2 = 0.529 (Fig. S5a). The regression equation representing FA and vanillin correlation is as follows: y 1 = −1.323x 1 + 12.89. Regression analysis between FA with caffeic acid, trans-cinnamic acid, and meta-methoxy cinnamic acid is represented with y 2 = −1.323x 2 + 12.89 (r = 0.684, r 2 = 0.468), as observed from Fig. S5b; y 3 = −0.455x 3 + 12,724,425 (r = 0.477, r 2 = 0.227), as observed in Fig. S5c; and y 4 = −0.112x 4 + 5,250,881.81 (r = 0.360, r 2 = 0.130), as indicated in Fig. S5d. Homovanillic acid to vanillin bioconversion and vanillin to catechol were confirmed and can be explained using the following equation: y 5 = −0.045x 5 + 721,468.10 (r = 0.349, r 2 = 0.122) and y 6 = −0.064x 6 + 4,779,782.83 (r = 0.262, r 2 = 0.069), respectively, as represented in Fig. S5e, f (shown in Fig. S5).
Recovery of vanillin from fermentation media
The amounts of vanillin crystal separated from the fermented broth at 0 and 8 h were very low (0.17 ± 0.012 and 0.67 ± 0.032 g L−1, respectively), but the amounts increased as the fermentation proceeded, and finally, 4.01 g of crystal vanillin was recovered from 1 L of media after 24 h of incubation (Fig. S6). Till date, such a high vanillin yield using either native or recombinant LAB strain was not reported. Molecular and biotechnological approaches of vanillin production by Kaur and Chakraborty (2012) discussed for the first time about benefits and probability of using LAB strain for vanillin production.
Discussion
Till date, only few LAB strains were reported to synthesize trace amounts of vanillin along with side products during phenolic biotransformations (Bloem et al. 2007). Earlier, various approaches were followed including metabolic engineering for production of vanillin from FA using E. coli and Pseudomonas sp. (Table 1). With the aim of vanillin degradation, vdh knockout mutants of Pseudomonas sp. HR199 strain were designed which accumulated only 2.9 mM of vanillin from FA (Overhage et al. 1999) and same strategy was used for Amycolatopsis sp. HR 167 (Overhage et al. 2003). In another strategy, the vanillin dehydrogenase (vdh)-encoding gene was inactivated via targeted mutagenesis and fcs and ech genes were expressed in P. fluorescens BF13 (fcs +/ech +). Resulted strain accumulated 8.41 mM of vanillin from FA using wheat bran as FA precursor and longer incubation leads to degrade vanillin (Di Gioia et al. 2011). With the aim to increase genetic stability of the recombinant strain E. coli FR13, Ruzzi et al. (2008) cloned genes encoding Fcs and Ech of P. fluorescens BF13 into E. coli JM109 using low copy plasmid vector pFR12. Using solid-state fermentation, green coconut husk as substrate led to a final production of 6.6 kg of vanillin per kilogram wet of biomass, which is the highest yield found in the literature reporting recombinant E. coli. But it makes the process more complex, and pathogenic strain genetic instability (prone to mutation and unable to express recombinant genes) was the other major concern for vanillin production. Metabolic engineering approaches were implemented for yeast strain (yeast; Schizosaccharomyces pombe and Saccharomyces cerevisiae) by introducing shikimic acid pathway to confer their ability to convert glucose to vanillin and gene knockout approach of the S. cerevisiae for alcohol dehydrogenase ADH6 to prevent its further conversion into vanillyl alcohol (Hansen et al. 2009; Brochado et al. 2010). Glucose was converted into vanillic acid which was further reduced to vanillin with 92% molar conversion within 7 h of incubation in both the recombinants. The major drawback of this strategy was the glycosylation step which implies reduction in the maximum theoretical yield and downstream processing cost of vanillin was too high. This strain was reported to produce up to 0.5 g L−1 of vanillin-d-glucoside. But, toxic product accumulation poses severe limitations on the use of engineered strains S. cerevisiae at industrial scale (Brochado et al. 2010).
Ours was the first report on utilization of a LAB strain P. acidilactici BD16, which is also a natural vanillin producer (Kaur and Chakraborty 2013; Kaur et al. 2013b). HPLC of Vdh activity of the recombinant strain confirms retention of vanillin in the medium. Both LCMS analysis and spectrophotometric analysis revealed that first 12 h biotransformation process was dominated with FA production in the RBM with the help of FAE activity of the recombinant strain. But, later stage-released FA was spontaneously converted into vanillin till 24 h. A total 4.01 g of crystal vanillin was separated from rice bran media after 24 h. FA consumption to vanillin production rate was observed many folds higher than native P. acidilactici BD16 strain. Recombinant enzyme expression in synthetic medium was different between P. acidilactici BD16 (fcs +/ech +) and E. coli top 10 (fcs +/ech +) as the enzymatic expression was observed in early hours (first 6 h) for E. coli top 10 (fcs +/ech +) and after 12 h for P. acidilactici BD16 (fcs +/ech +). FA consumption to vanillin production was more in P. acidilactici BD16 (fcs +/ech +).
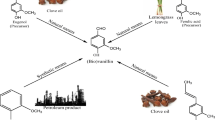
Four probable pathways, (a) reductive pathway, (b) CoA-dependent pathway, (c) CoA independent pathway, and (d) decarboxylase pathways for bioconversion of FA to vanillin, were proposed (Kaur and Chakraborty 2012). Of these, reductive pathway (FA → intermediate → dihydroferulic acid → homovanillic acid → vanillic acid → vanillin) and CoA-dependent pathway (FA → feruloyl CoA → vanillin) contributed significantly in vanillin production in the metabolic engineered LAB isolate of P. acidilactici BD16 (fcs +/ech +). Metabolite spectral analysis revealed abundance of FA, FA fragmentation products, cinnamic acid, and cinnamic acid fragmentation products in the early hours. But with increase in time with the production of FAE, FA, and dihydro FA was released and subsequently converted into vanillin. Unlike native strain, vanillin production took place through reductive or decarboxylation pathway in recombinant strain of P. acidilactici BD16 (fcs +/ech +). We can conclude that reductive pathway and enzyme carboxylic acid reductase (Car) contributed less in the vanillin production from rice bran which contributed most in case of native P. acidilactici BD16 isolate (Fig. 4). But after 12 h, CoA-dependent pathway involving reactions catalyzed by Fcs and Ech enzymes played a significant role in improving vanillin yield in recombinant LAB strain. Introduction of this metabolic pathway allowed a significant increase in consumption rate of FA, which was spontaneously converted into vanillin without its further fragmentation and at 24 h maximum vanillin production observed (Figs. 2 and 3 and Fig. S6). Throughout the study, major vanillin degradation was not observed indicating the stability of the produced vanillin after 24 h. Presence of vanillin degradation product like VAL and protocatechuic acid was not detected in the fermentation medium which is one of the major advantage of using recombinant P. acidilactici BD16 (fcs +/ech +) for vanillin biosynthesis gene expression. However, with E. coli top 10 (fcs +/ech +), vanillin production decreased after 6 h indicating a possible degradation of vanillin. In order to make biotransformation of FA to vanillin economically viable and to prevent further oxidation of vanillin to vanillic acid, recombinant P. acidilactici BD 16 (fcs +/ech +) strain can be a potential tool relevant for industrial production of vanillin.
In this study, P. acidilactici BD16 (fcs +/ech +) was used to convert the FA released from rice bran to vanillin. CoA-dependent pathway involving reactions catalyzed by Fcs and Ech enzymes played a significant role in improving vanillin yield in recombinant LAB strain by fourfold compared with its native strain P. acidilactici BD16 (MTCC 10973). In addition, vanillin degradation was not observed adding merit to the process. P. acidilactici BD16 (fcs +/ech +) strain can be used to prevent unwanted and toxic by-products and thus can reduce the downstream processing cost. Its productivity can be further improved by scaling up of the process to industrial level.
References
Achterholt S, Priefert H, Steinbüchel A (2000) Identification of Amycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 54:799–807
Barbosa ES, Perrone D, Amaral VAL, Ferriera LSG (2008) Vanillin production by Phanerochaete chrysosporium grown on green coconut agro-industrial husk in solid state fermentation. Bio Res 3(4):1042–1050
Barghini P, Gioia Di D, Fava F, Ruzzi M (2007) Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb Cell Factories 6(13). doi:10.1186/1475-2859-6-13
Bloem A, Bertrand A, Lonvaud-Funel A, Revel de G (2007) Vanillin production from simple phenols by wine-associated lactic acid bacteria. Lett Appl Microbiol 44(1):62–67
Brochado AR, Matos C, Moller BL, Hansen J, Mortensen UH, Patil KR (2010) Improved vanillin production in baker’s yeast through in silico design. Microb Cell Fact 9:84
Chakraborty D, Gupta G, Kaur B (2016) Metabolic engineering of E. coli top 10 for production of vanillin through FA catabolic pathway and bioprocess optimization using RSM. Protein Expr Purif 128:123–133
Cicero A F G and Derosa G (2005) Rice bran and its main components: potential role in the management of coronary risk factors. Curr Top Nutraceutical Res 3(1): 29–46
Converti A, Aliakbarian B, Dominguez JM, Bustos VG, Perego P (2010) Microbial production of biovanillin. Braz J Microbiol 41(3):519–530
Di Gioia DD, Luziatelli F, Andrea N, Ficca AG, Fava F, Ruzzi M (2011) Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J Biotechnol 156(4):309–316
Eugene SW (1958) Vanillin purification. US patent no: 3049566A. Retrive from http://www.google.co.in/patents/US3049566
Hansen EH, Moller BL, Kock GR (2009) De novo biosynthesis of vanillin in fission yeast Schizosaccharomyces pombe and baker’s yeast Saccharomyces cerevisiae. Appl Environ Microbiol 75:2765–2774
Kasana RC, Sharma UK, Sharma N, Sinha AK (2007) Isolation and identification of a novel strain of Pseudomonas chlororaphis capable of transforming isoeugenol to vanillin. Curr Microbiol 54:457–561
Kaur B, Chakraborty D (2012) Biotechnological and molecular approaches for vanillin production: a review. Appl Biochem Biotechnol 169(8):1353–1372
Kaur B, Chakraborty D (2013) Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. Ind J Exp Biol 51:935–943
Kaur B, Chakraborty D, Kaur G, Kaur G (2013a) Biotransformation of rice bran to ferulic acid by Pediococcal isolates. Appl Biochem Biotechnol 170(4):854–867
Kaur B, Chakraborty D, Kumar B (2013b) Phenolic biotransformations during conversion of ferulic acid to vanillin by lactic acid bacteria. BioMed Res Int Article ID 590359, 6 pages.
Kaur B, Chakraborty D, Kumar B (2014) Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl Microbiol Biotechnol 98(20):8539–8551
Landete M, Rodriguez H, Curiel JA, De Las Rivas B, Mancheno JM Munoz R (2010) Gene cloning, expression, and characterization of phenolic acid decarboxylase from Lactobacillus brevis RM84. J Indus Microbiol Biotechnol 37(6) 617–624
Lee EG, Yoon SH, Das A, Lee SH, Li C, Kim JY, Choi MS, Oh DK, Kim SW (2009) Directing vanillin production from ferulic acid by increased acetyl-CoA consumption in recombinant Escherichia coli. Biotechnol Bioeng 102(1):200–208
Lesage-Meessen L, Lomascolo A, Bonnin E, Thibault JF, Buleon A, Roller M, Asther M, Record E, Ceccaldi BC, Asther M (2002) A biotechnological process involving filamentous fungi to produce natural crystalline vanillin from maize bran. Appl Biochem Biotechnol 102-103:141–153
Lesage-Meessen L, Stentelaire C, Lomascolo A, Couteau D, Mi A, Moukha S, Record E, Sigoillot JC, Asther M (1999) Fungal transformation of FA from sugar beet pulp to natural vanillin. J Sci Food Agric 79:487–490
Liu Z, Rochfort S (2013) A fast liquid chromatography mass spectrometry (LCMS) method for quantification of major polar metabolites in plants. J Chromatography 912:8–15
Narbad A, Gasson MJ (1998) Metabolism of ferulic acid via vanillin using a novel CoA dependent pathway in a newly isolated strain of Pseudomonas fluorescens. Microbiol 144(5):1397–1405
Odoux E, Grisoni M (2010) Vanilla. In: Medicinal and aromatic plants: industrial profiles (Ed.)^(Eds.), p. 387. Taylor and Francis Group.
Overhage J, Priefert H, Rabenhorst J, Steinbüchel A (1999) Biotransformation of eugenol to vanillin by a mutant of Pseudomonas sp. strain HR199 constructed by disruption of the vanillin dehydrogenase (vdh) gene. Appl Microbiol Biotechnol 52(6):820–828
Overhage J, Priefert H, Rabenhorst J, Steinbüchel A (2000) Construction of production strains for producing substituted phenols by specifically inactivating genes of the eugenol and ferulic acid catabolism. Patent application no.WO0026355.
Overhage J, Steinbüchel A, Priefert H (2003) Highly efficient biotransformation of eugenol to ferulic acid and further conversion to vanillin in recombinant strains of Escherichia coli. Appl Environ Microbiol 69(11):6569–6576
Overhage J, Steinbüchel A, Priefert H (2006) Harnessing eugenol as a substrate for production of aromatic compounds with recombinant strains of Amycolatopsis sp. HR167. J Biotechnol 125(3):369–376
Plaggenborg R, Overhage J, Steinbüchel A, Priefert H (2003) Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Appl Microbiol Biotechnol 61:528–535
Plaggenborg R, Overhage J, Loos A, Archer JAC, Lessard P, Sinskey AJ, Steinbüchel A, Priefert H (2006) Potential of Rhodococcus strains for biotechnological vanillin production from ferulic acid and eugenol. Appl Microbiol Biotechnol 72(4):745–755
Ruzzi M, Luziatelli F, Matteo PD (2008) Genetic engineering of Escherichia coli to enhance biological production of vanillin from ferulic acid. Bull UASVM Animal Sci Biotechnol 65 (1–2).
Salleh NHM, Daud MZM, Arbain D, Ahmad MS (2011) Aromatic benzaldehyde from Oryzae sativa, International conference on food engineering and biotechnology, IPCBEE, 9: 141–144.
Song JW, Lee EG, Yoon SH, Lee SH, Lee JM, Lee SG, Kim SW (2009) Vanillin production enhanced by substrate channeling in recombinant E. coli. SIM annual meeting and exhibition. Indus. Microbiol. Biotechnol. 125. Poster no 125 (session 1), SIM annual meeting and exhibition Indus. Microbiol Biotechnol Westin harbor castle, Toronto ON, Canada.
Yang W, Tang H, Ni J, Wu Q, Hua D, Tao F, Xu P (2013) Characterization of two Streptomyces enzymes that convert ferulic acid to vanillin. PLoS One 8(6):e67339. doi:10.1371/journal.pone.0067339
Yoon SH, Lee EG, Das A, Lee SH, Li C, Ryu HK, Choi MS, Seo WT (2007) Enhanced vanillin production from recombinant E. coli using NTG mutagenesis and adsorbent resin. Biotechnol Prog 23(5):1143–1148
Yoon SH, Cui L, Lee YM, Lee SH, Kim SH, Choi MS, Seo WT, Yang JK, Kim JY, Kim SW (2005a) Production of vanillin from ferulic acid using recombinant strains of Escherichia coli. Biotechnol Bioprocess Eng 10:378–384
Yoon SH, Li C, Kim JE, Lee SH, Yoon JY, Choi MS, Seo WT, Yang JK, Kim JY, Kim SW (2005b) Production of vanillin by metabolically engineered Escherichia coli. Biotechnol Lett 27(22):1829–1832
Zheng L, Zheng P, Sun Z, Wang J, Guo X (2007) Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Bioresour Technol 98:1115–1119
Acknowledgements
Authors acknowledge University Grants Commission, New Delhi, India for providing UGC major research project to Dr. Baljinder Kaur and meritorious scholarship to Mr. Debkumar Chakraborty and Masafumi Noda for providing cloning vector pLES003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the authors mutually agree to submit the manuscript to Applied Microbiology and Biotechnology.
Funding
The authors acknowledge the UGC major research project entitled “Metabolic engineering of a lactic acid bacterial isolate for biotransformation of ferulic acid to vanillin” to Dr. Baljinder Kaur (F.39-271/2010(SR)).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
.
ESM 1
(PDF 1072 kb)
Rights and permissions
About this article
Cite this article
Chakraborty, D., Selvam, A., Kaur, B. et al. Application of recombinant Pediococcus acidilactici BD16 (fcs +/ech +) for bioconversion of agrowaste to vanillin. Appl Microbiol Biotechnol 101, 5615–5626 (2017). https://doi.org/10.1007/s00253-017-8283-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8283-8