Abstract
Occurrence of feruloyl-CoA synthetase (fcs) and enoyl-CoA hydratase (ech) genes responsible for the bioconversion of ferulic acid to vanillin have been reported and characterized from Amycolatopsis sp., Streptomyces sp., and Pseudomonas sp. Attempts have been made to express these genes in Escherichia coli DH5α, E. coli JM109, and Pseudomonas fluorescens. However, none of the lactic acid bacteria strain having GRAS status was previously proposed for heterologous expression of fcs and ech genes for production of vanillin through biotechnological process. Present study reports heterologous expression of vanillin synthetic gene cassette bearing fcs and ech genes in a dairy isolate Pediococcus acidilactici BD16. After metabolic engineering, statistical optimization of process parameters that influence ferulic acid to vanillin biotransformation in the recombinant strain was carried out using central composite design of response surface methodology. After scale-up of the process, 3.14 mM vanillin was recovered from 1.08 mM ferulic acid per milligram of recombinant cell biomass within 20 min of biotransformation. From LCMS-ESI spectral analysis, a metabolic pathway of phenolic biotransformations was predicted in the recombinant P. acidilactici BD16 (fcs +/ech +).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increasing consumer demand for natural food additives, biotechnological approaches for vanillin production have gained more interest in recent years, since they offer alternative way to produce this aromatic substance in a “natural” way. Until today, bioprocess for ferulic acid (FA) to vanillin biotransformation has been developed to an economically feasible scale (Overhage et al. 2003; Kaur and Chakraborty 2012). Vanillin production from FA has been reported in several microbial strains like Amycolatopsis sp. ATCC 39116 (Muhim et al. 2001; Devis et al. 2012), Amycolatopsis sp. HR167 (Achterholt et al. 2000), Pediococcus acidilactici (Kaur et al. 2013a, b), Pseudomonas fluorescens AN103 (Gasson et al. 1998), Pseudomonas sp. HR199 (Overhage et al. 1999b), Rhodococcus strains (Plaggenborg et al. 2006), Sphingomonas paucimobilis SYK-6 (Masai et al. 2002), Streptomyces setonii (Muhim and Lerch 1999), and Streptomyces sp. V1 (Yang et al. 2013). These strains generally use either coenzyme A-dependent, non-β-oxidative pathway or reductive pathway for FA bioconversion, which involves activity of feruloyl-CoA synthetase (Fcs) and enoyl-CoA hydratase/aldolase (Ech) enzymes. Vanillin production pathway was recently predicted in native P. acidilactici BD16 that indicates a key role of carboxylic acid reductase (Car) in vanillin production. Earlier, authors have reported use of ferulic acid esterase (Fae) producing P. acidilactici for the extraction of FA from rice bran and its further biotransformation to phenolic derivatives like vanillin (Kaur et al. 2013a, b). Native P. acidilactici BD16 is able to produce 1.26 g L−1 crude vanillin on RBM medium containing 150 g L−1 rice bran and 50 μg L−1 FA which is the highest yield reported so far in case of lactic acid bacteria (LAB) (Kaur and Chakraborty 2013). After extraction of the vanillin from the biotransformation medium, it was concentrated by rotary vacuum evaporation and crystallized. Using native P. acidilactici BD16, 1.04 g L−1 crystal vanillin can be recovered from the biotransformation medium containing FA (unpublished data).
Earlier, several engineered Escherichia coli strains were adopted for vanillin production at laboratory scale, but genetic instability of the recombinant strains is a major drawback as it causes rapid decline in levels of vanillin produced. Recently, native P. acidilactici BD16 was shown to exhibit vanillin tolerance up to 7 g L−1 and was reported to possess high FA retention capacity which means FA as well as vanillin are not metabolized in other native metabolic pathways of the strain as the strain is lacking vanillin dehydrogenase (Vdh) activity also (unpublished data). Vdh enzyme causes rapid decline in the levels of vanillin produced in many native microbial strains which overcome vanillin toxicity by converting it into vanillyl alcohol and other phenolic products (Di Gioia et al. 2011). The above-stated property of many microbial systems is another major drawback of their use at an industrial scale.
Therefore, present study was undertaken with the aim to engineer a natural vanillin tolerant strain of P. acidilactici BD16 by introducing synthetic vanillin gene cassette bearing fcs and ech genes using shuttle vector pLES003 and optimization of FA to vanillin biotransformation process using cell extracts of recombinant P. acidilactici BD16 (fcs +/ech +). Some important process parameters that influence bioconversion process (like substrate concentration, biomass concentration, temperature, and time) were statistically optimized using response surface methodology (RSM). Finally, optimized process conditions were used for scale-up of FA to vanillin biotransformation, using enzyme extracts of 1 mg biomass and cell-free supernatant for intracellular and extracellular vanillin biotransformation in the recombinant system.
Materials and methods
Microorganism and growth conditions
P. acidilactici BD16 MTCC 10973 was used as a host for expression of FA catabolic genes viz. fcs and ech. Recombinant P. acidilactici BD16 (fcs +/ech +) was grown in de Man’s Rogosa Sharpe Medium (MRS) broth (20 g L−1 dextrose, 10 g L−1 beef extract, 10 g L−1 peptone, 5 g L−1 sodium acetate, 5 g L−1 yeast extract, 2 g L−1 triammonium citrate, 2 g L−1 di-potassium hydrogen phosphate, 0.1 g L−1 magnesium sulfate, 0.05 g L−1 manganous sulfate, and 1 mL L−1 Tween 80, pH 5.6) under shaking conditions at 37 °C for 24 h. Erythromycin was added in the culture medium at a final concentration of 25 μg mL−1 for selection of recombinant strains.
Designing of synthetic vanillin gene cassette
Synthetic vanillin gene cassette containing genes encoding Fcs (converts FA to feruloyl-CoA) and Ech (converts feruloyl-CoA to vanillin) was designed by computer-assisted protocol using reference ech and fcs genes of Amycolatopsis sp. HR 167 vide GenBank accession number AJ290449.1 (Achterholt et al. 2000) and reverse translated as per codon choice of P. acidilactici by applying sequence manipulation suite 2 tools. In order to increase the activities of Fcs and Ech in recombinant P. acidilactici BD16 (fcs +/ech +) strain, an autoinducible Pediococcus promoter P289 (GenBank accession no. GQ214404) was integrated upstream to fcs gene. Other control regions such as RBS of Pediococcus sp., stop codons, and EcoR1 linkers were attached to the designed construct. Full-length sequence of vanillin biosynthesis gene cassette sent to GenScript Private Limited, USA, for its synthesis. Synthetic vanillin gene cassette (2,485 bp) was provided as a cloned construct on pCC1 vector in E. coli top10. It was deposited with GenBank database vide accession number KJ543568.
Subcloning of vanillin biosynthetic gene cassette and construction of recombinant plasmid pLES003 (P289/fcs +/ech +)
The shuttle vector pLES003 used for subcloning vanillin biosynthetic gene cassette was constructed in a previous report by Wada et al. (2009). Plasmid pCC1 (T7 /ech +/fcs +) was isolated from E. coli top10 using Geneaid High-Speed Plasmid Mini Kit. Further, a 2,485-bp vanillin biosynthetic gene cassette (P289/ech +/fcs +) containing synthetic fcs (1,473 bp) and ech (861 bp) genes flanked by EcoRI sites was subcloned into a shuttle vector pLES003 provided by Masafumi Noda (Assistant Professor, Hiroshima University, School of Life Science, Japan). Procedure involved simple EcoRI digestion of pCC1 (T7 /ech +/fcs +) and pLES003 using a reaction mixture containing 50 μg of plasmid DNA, 50 U of restriction enzyme and 1× EcoRI reaction buffer. Digestion was carried out at 37 °C for 3 h. Fragments of digested pCC1 (T7 /ech +/fcs +) were separated on 2 % agarose gel which was prepared by dissolving 1 g of agarose in 50 mL of 1× TBE buffer (89 mM Tris, 89 mM boric acid, 2 mM EDTA) using 1× TBE as running buffer. Vanillin biosynthetic gene fragment of 2,485 kb was extracted from gel by using Genei gel extraction kit. It was further ligated into EcoRI linearized pLES003 using T4 DNA ligase using 1 U enzyme per microgram of DNA at 25 °C overnight. Ligation was confirmed on 2 % agarose gel and visualized on UV transilluminator (Bio-Rad).
Preparation of competent cells of P. acidilactici BD16 and their transformation
P. acidilactici BD16 was inoculated in MRS broth and incubated at 37 °C for 24 h. After two subculturings, 1 % v/v of overnight grown pre-culture was transferred to 100-mL MRS medium. Cells were multiplied in MRS broth containing 3 % glycine and 50 mM dl-threonine for 2 to 4 h at 37 °C to achieve absorbance (OD600) of 0.4–0.6. Cultures were then kept on ice for approximately 15 min. Cells were harvested from 4 mL culture broth by centrifugation at 4,000 rpm for 5 min at 4 °C, washed twice in 2-mL washing solution (0.5 M sucrose and 10 % glycerol), and resuspended in 1 mL solution containing 0.6 M sucrose, 1 mM K2HPO4, 1 mM MgCl2, pH 7.0 for 30 min. After centrifugation at 5,000 rpm, cells were resuspended 1 mL of CaCl2 containing 15 % glycerol for storage at −70 °C in deep freezer (Caldwell et al. 1996).
One-hundred-microliter aliquot of competent cells were thawed and were mixed with 10 μL of recombinant plasmid DNA pLES003 (P289/ech +/fcs +) and incubated on ice for 20–30 min. Eppendorf was placed at 45 °C in water bath for 2 min to provide heat shock for facilitating transformation of pLES003 (P289/ech +/fcs +) into P. acidilactici BD16. One-milliliter outgrowth medium (MRS containing 0.5 M sucrose, 20 mM MgCl2, and 2 mM CaCl2) was added to eppendorf after heat shock and incubated at 37 °C for 1 h to active and to repair the cells. Recombinant P. acidilactici BD16 (ech + /fcs +) strains were selected on MRS agar media containing 25 μg/mL erythromycin.
Preparation of enzyme extracts
Recombinant strain of P. acidilactici BD16 (fcs +/ech +) was grown in MRS broth containing 25 μg mL−1 erythromycin for 24 h at 37 °C. Cells were harvested at regular intervals viz. 6 h (early log phage of 0.5 OD), 8 h (log phage of 1 OD), 12 h (late log phage of 1.5 OD), 16 h (stationary phase of 2 OD), and 18 h (late stationary phase of 2.5 OD) by centrifugation and pellets were resuspended in 20 mM phosphate buffer (pH 7). Cell-free extracts from these pellets were prepared by disrupting cells by sonication (1 min/mL of cell suspension with maximum amplitude of 100 μm) with Sonics Vibra Cell Ultrasonic Disintegrator for subsequent enzyme assay. Each cell-free extract contained 100 μg mL−1 soluble protein (adjusted after suspending in 20 mM phosphate buffer, pH 7), which were taken for intracellular enzymatic assays and vanillin production. Similarly, cell-free supernatant (CFS) containing 100 μg mL−1 soluble protein (prepared in 20 mM phosphate buffer, pH 7) was taken for extracellular enzymatic assays and vanillin production. Formation of feruloyl-CoA by heterologously expressed Fcs and Ech enzymes was measured using optical enzymatic test as described previously (Narbad and Gasson 1998). Vanillin production was estimated in each extract using standard protocol of Converti et al. (2010).
Optimization of vanillin production using RSM
RSM software (Statacase Design Expert version 8.0.7.1) was used to optimize the bioconversion process by studying the effect of biomass, temperature, time, and substrate concentration (FA). Four studied parameters, (a) substrate as FA, (b) biomass, (c) time of assay, and (d) temperature, were entered into the central composite design (CCD) which generated a full-factorial screening design with a total of 30 experiments including 6 replicate center points (Table 1). Results obtained in CCD were analyzed in terms of three vanillin responses as extracellular, intracellular, and total yield of vanillin (nM).
Scale-up studies
Runs giving maximum vanillin responses in all the three sets were selected from CCD design. Components were scaled up to 1 L to validate vanillin responses in recombinant P. acidilactici BD16 (fcs +/ech +). Biotransformation medium contained 100 mM potassium phosphate buffer (pH 7.0), 2.5 mM MgCl2, 2.5 mM ATP, and 0.2 mM coenzyme A. Extracellular activity was carried out according to run 11 (shown in Table 1) and intracellular activity carried out according to run 25 (shown in Table 1). For extracellular assay, 200 μg mL−1 FA, and for intracellular assay, 10 μg mL−1 FA was added in the reaction mixture containing 100 μg mL−1 protein containing CFS and cell-free extract of recombinant culture harvested after 16 h at 35 °C and 18 h at 25 °C for respective assays. Amounts of feruloyl-CoA/vanillin were estimated at 346 nm at 10-min intervals for 1 h. Confirmatory experiments were carried out in triplicate, and final data was presented taking mean of all the three responses.
Enzymatic assay
Enzymatic activity was measured in the reaction mixture (1 mL) containing 100 mM potassium phosphate buffer (pH 7.0), 100 mM tris-hydrochloric acid, 2.5 mM MgCl2, 10 μg mL−1 FA, 2.5 mM ATP, and 0.2 mM coenzyme A. As an enzyme source, 100 μg mL−1 protein extract containing 4.5 μL of supernatant and 7 μL of cell-free extract for extracellular and intracellular assays, respectively. The activity assay was started by the addition of ATP, and the initial increase in absorbance reflecting formation of feruloyl-CoA was measured at 346 nm using a UV-visible Thermo Fisher Scientific spectrophotometer using modified Narbad and Gasson 1998.
Kinetic study of Fcs
Specificity of enzyme toward different substrates is different. Some enzymes demonstrate high degree of specificity and some catalyze a reaction in only one direction (e.g., dehydrogenation), but some will carry out in both forward and reverse directions usually at different rates (e.g., hydrogenation in addition to dehydrogenation). That is why Km and Vmax, which represent specificity and rate of catalysis, are important factors for characterizing an enzymatic reaction. The above-stated reaction mixture containing different concentrations of FA (10 to 650 μg mL−1) were used to determine kinetic parameters (Km and Vmax) of cloned Fcs/Ech enzymes in triplicate. CFS or cell-free extracts containing 100 μg mL−1 protein were used in each assay which was carried out at 30 °C. From the slope of Lineweaver Burk plots, Km and Vmax values were calculated (Yang et al. 2013).
Extraction of phenolics and metabolic profiling of recombinant P. acidilactici BD16 (fcs + /ech +) culture by LCMS-ESI
After 20 min of biotransformation, biotransforming medium was acidified to pH 2 with 6 N HCl and phenolics were extracted from 1 L culture supernatant of recombinant P. acidilactici BD16 (fcs + /ech +), and metabolites were partitioned into an equal volume of ethyl acetate (1:1, v/v). Ethyl acetate fraction was separated and concentrated by rotary vacuum evaporation at 50 °C. Solvent was completely evaporated, and phenolics were recovered in 5 mL 80 % (v/v) methanol and then used for metabolite analysis. Phenolic characterization was carried out using liquid chromatography/mass spectrometry on a LCMS instrument (Thermo, model LTQ-XL) using positive electron spray ionization software (Kaur et al. 2013b).
Results
Designing of synthetic gene construct and construction of recombinant plasmid pLES003 (P289/fcs + /ech +)
From the recombinant vector pCC1 (T7/fcs + /ech +), synthetic vanillin gene cassette of 2,485 bp bearing fcs and ech genes was amplified by PCR and digested with EcoRI to generate sticky ends. Recombinant plasmid pLES003 (P289/fcs + /ech +) was constructed by ligating synthetic vanillin gene cassette with 6,134-bp long EcoRI linearized plasmid pLES003 (Fig. 1). During subcloning, a spontaneous mutation involving single base pair change (A to G) at nucleotide position 33 occurred in the promoter region of the synthetic construct. The sequence of the mutated vanillin gene construct in pLES003 (P289/fcs + /ech +) was deposited in GenBank database (GenBank accession no. KJ543569).
For genomic mapping, recombinant plasmid pLES003 (P289/fcs + /ech +) was digested with EcoRI and after electrophoresis, 2,485 bp synthetic fragment, native vector 6,134 bp, and total 8,619 bp recombinant vector were visualized in 0.8 % agarose gel (Fig. 2).
Optimization of vanillin production using RSM
Three-dimensional response surface curves were plotted for the vanillin in responses obtained in CCD design to study interaction between four response variables selected and to determine the optimum concentration of each for maximum vanillin yield.
Intracellular vanillin biosynthesis
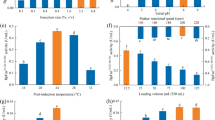
Maximum intracellular vanillin yield obtained was 104.2 nM (run 25, containing 10 μg mL−1 FA and cell extract from biomass of 1 OD/mL at 25 °C for 60 min). Time has maximum influence on intracellular vanillin production, and highest production was achieved after 1 h. Vanillin production increased as incubation proceeded up to 85 min, and 66.8 nM vanillin was estimated during biotransformation reaction performed as per run 21 (Table 2). Substrate concentration, temperature, and biomass influenced inversely but significantly in vanillin production as with increasing substrate concentration to 200 μg mL−1, temperature to 45 °C, and biomass of 2 OD/mL, the intracellular vanillin levels dropped to 17.6 nM (run 23), 31.5 nM (run 17), and 15.7 nM (run 22), respectively (Table 2, Fig. S1).
ANOVA analysis of intracellular vanillin response
The model F value of 4.17 implies that the model is significant. There is only a 0.48 % chances that a “model F value” this large could occur due to noise. Value of “prob>F” less than 0.05 indicates that the model terms are significant. In this case, model terms A, C, D, A2, C2, and D2 are significant as they have profound influence on vanillin synthesis. The “lack of fit F value” is significant at a level of 76.46 (Table S1).
Extracellular vanillin biosynthesis
Highest vanillin molar yield was achieved by extracellular activity of the cloned FA catabolism enzymes, namely, Fcs and Ech which accounted for the production of 984.4 nM of vanillin (run 11, containing 200 μg mL−1 FA, enzyme preparation extracted from biomass 2 OD/mL at 25 °C for 60 min). The temperature at which bioconversion assay was carried out markedly influenced vanillin yields. Among the three temperatures tested, highest vanillin synthesis was achieved at 25 °C after 1 h of incubation. Time, FA concentration, and biomass concentration are positively and significantly correlated with vanillin production. With decrease in time from 60 to 10 min (run 5), biomass at 1 OD (run 23), and substrate concentration of 10 μg mL−1 (run 22), vanillin levels dropped to 5, 365.2, and 29 nM, respectively. Temperature has a negative influence on vanillin production as with increase in incubation temperature from 25 to 45 °C; vanillin production decreased to 199 nM (Table 2, Fig. S2).
ANOVA analysis for extracellular vanillin response
F value of 4.16 indicates a significant CCD model of extracellular response. There is only a 0.48 % chances that a model F value this large could occur due to noise. In this case, A, C, A2, C2, and D2 are significant model terms as prob>F values of these terms found to be less than 0.05. The lack of fit F value of 13,045.5 is identified as significant (Table S2).
Total molar vanillin yields in recombinant P. acidilactici BD16 (fcs +/ech +)
A very high extracellular enzymatic activity of cloned genes was credited for maximum vanillin synthesis (984.4 nM) in recombinant cell extracts as obtained in run 11 (Table 2). The total extracellular and intracellular vanillin yields were also comparable in case of run 2, 4, 9, 12, 16, and 20 (861, 846.4, 850.6, 844.7, 843.6, and 847.8 nM, respectively) containing 105 μg mL−1 FA using enzyme preparation extracted from biomass of 1.5 OD/mL at 35 °C for 35 min of incubation. A higher cell density (1.5 OD/mL), when the cells are in log phase, was found to generate maximum vanillin. A decrease in vanillin formation by extracellular activity (551 nM) (run 3) was observed when the cells were harvested at higher OD600 (2.5; cells under stationary phase), and no vanillin formation was observed at lower OD600 (0.5; cells in early exponential growth phase) as observed in run 26 (Table 2, Figs. S1, S2 and 3). Intracellular activity of cloned enzymes was totally suppressed in the presence of 200 μg mL−1 FA whereas extracellular activity at this time was found to be maximum as indicated in run 11 (Figs. S1, S2, and 3). These results show that extracellular enzymatic activities of cloned genes have higher affinity for the substrate than enzyme expressed intracellularly. That is why vanillin production using extracellular enzymes extract was more than vanillin production using intracellular enzyme extract (Table 2).
ANOVA analysis of the final response
F value of 4.26 implies the model as significant and there is only a 0.43 % chances that a model F value this large could occur due to noise. Prob>F less than 0.05 value indicate significant model terms which are A, C, A2, C2, and D2 in this case. The lack of fit F value of 10,767.79 is significant as there is only a 0.01 % chance of getting a random response (Table 3).
Scale-up studies and validation of the model
In recombinant P. acidilactici (fcs +/ech +), optimized conditions for vanillin biosynthesis were scaled up and 2.62 mM vanillin was obtained after 10 min of incubation with CFS (extracellular), and 0.57 mM vanillin was obtained after 20 min of incubation with cell-free extracts (intracellular). Intracellular vanillin yield increased from 0.2 to 0.57 mM whereas extracellular vanillin yield increased from 0.92 to 2.58 mM as observed in Fig. 4. Overall, 3.14 mM of vanillin was obtained from 1.08 mM FA within 20 min using combined extracellular and intracellular responses which was 14.9 times higher than the native P. acidilactici strain BD16 (with 0.21 mM vanillin in 1 h and 20 min) and seven times more than recombinant E. coli top10 (fcs +/ech +) (with 0.45 mM vanillin within 20 min). Approximately, threefold higher vanillin production was reported in the recombinant P. acidilactici (fcs +/ech +) after scale-up of the process.
Enzyme activity of Fcs
Fcs enzyme activity was measured in cell suspension of recombinant P. acidilactici BD16 (fcs +/ech +) grown for 12 h, and extracellular and intracellular enzyme activities of Fcs were assayed in triplicate as per standard protocol (Narbad and Gasson 1998). An increase in absorbance at 346 nm was observed due to the formation of feruloyl-CoA with concomitant vanillin production reported after 30 and 10 min of incubation for intracellular and extracellular activity, respectively. Extracellular Fcs activity (12.63 IU mL−1) was found to be higher than intracellular activity (4.63 IU mg−1) as observed in this recombinant system.
Kinetic study of Fcs
Km and Vmax of extracellular and intracellular enzyme preparations were calculated through Lineweaver Burk plots. Km (16.2 μM) and Vmax (352 U mg−1) of intracellular enzyme was higher than extracellular enzyme (Km 4.07 μM and Vmax 274 U mg−1). Extracellular enzyme has lesser Km than intracellular enzyme which indicates its higher access to substrate provided than intracellular enzyme. That is why extracellular enzyme showed more activity than intracellular enzyme (Fig. S3).
LCMS-ESI-based metabolic profiling of recombinant P. acidilactici BD16 (fcs + /ech +)
Functional identification of the metabolites generated due to the activity of cloned Fcs and Ech enzymes was carried out using LCMS-based metabolic profiling technique. LCMS of methanolic reaction mixtures was performed using positive electron spray ionization, and phenolic metabolites were detected within mass range of 100 to 300. Vanillin with a mass of 156 kD (153 vanillin+3H+) (80 % relative abundance; retention time 0.34 min) was detected along with its fragmentation product at 79.27 kD (90 % relative abundance, retention time 0.12 min) in the extracellular extracts which was comparatively more than vanillin peak (58 % by relative abundance, retention time 0.35 min) and its fragmentation product (100 % relative abundance, retention time 0.12 min) detected in intracellular extract. Major products such as methoxycinnamic acid (100 % relative abundance, retention time 0.5 min) and fragmentation products of FA, caffeic acid, and trans-cinnamic acid were also found in abundance along with pyrogallol, caffeic acid, and trans-cinnamaldehyde in the both reaction mixtures. Sinapic acid was found in extracellular extract, but it was absent in intracellular extract. 4-Ethylphenol was not detected in any of the extract tested which symbolizes that the strain is lacking ferulic acid decarboxylase (fdc) activity as shown in Fig. 5.
Depending upon the metabolites characterized by LCMS-ESI, we can predict a metabolic pathway of FA to phenolic biotransformation which involves an intricate network of decarboxylation, hydroxylation, dehydroxylation, methylation, and demethylation reactions as summarized in Fig. 6. FA to pyrogallol formation takes place through different steps involving hydroxylation, methylation, demethylation, and decarboxylation, where 5-hydroxy ferulate, sinapic acid, and gallic acid were produced as main intermediates. Metamethoxycinnamic acid was the most abundant and stable metabolite found in the reaction mixture which confirms FA dehydroxylation as a frequent process during FA biotransformation. This compound after demethylation and dehydroxylation forms cinnamic acid. Bioconversion of FA to cinnamic acid proceeds through a separate pathway in which demethylation and dehydroxylation are found as key steps with the formation of caffeic acid and p-coumaric acid as key intermediates. Caffeic acid to cinnamic acid conversion involves dehydroxylation reaction that yields m-coumaric acid as an intermediate. p-Coumaric acid fragmentation and cinnamic acid to cinnamaldehyde reductions have been identified from their fragmentation product detected in the reaction mixture during LCMS-ESI-based metabolic profiling. FA to vanillin biotransformation through coenzyme A-dependant pathway has been confirmed in the metabolically engineered P. acidilactici BD16 (fcs +/ech +). The cloned enzymes Fcs and Ech successfully converted FA to vanillin through feruloyl-CoA and 4-hydroxy-3-methoxyphenyl-β-hydroxy propionyl-CoA as reaction intermediates. Decarboxylase activity is common in the wild-type LAB strain that results in formation of 4-vinylphenol followed by 4-ethylphenol as described previously by Kaur et al. (2013a, b). Formation of 4-vinylguiacol was not observed during biotransformation of FA into vanillin in both native as well as recombinant P. acidilactici BD 16 strains. 4-Vinylguiacol production during phenolic biotransformation was earlier reported only in few LAB strains like Pediococcus sp. (Bloem et al. 2007) and P. pentosaceus (Di Rivas et al. 2009).
Discussion
An increase in customer demand for natural vanillin has led to a growing interest of scientists for metabolic engineering of starter culture strains. Earlier, vanillin biosynthetic genes, namely, ech and fcs of Amycolatopsis sp. HR167, Amycolatopsis sp. HR104, D. acidovorans, Pseudomonas sp. HR199, and P. fluorescens BF13 have been cloned in E. coli using inducible expression systems (Table 4). Using metabolic engineered strains, vanillin production was found to have limited success due to longer bioconversion time required, genetic instability of the recombinants, and complicated downstream processing, etc. (Kaur and Chakraborty 2013).
In our laboratory, a natural vanillin producing P. acidilactici BD16 was isolated from a milk product and characterized for its biochemical and molecular properties (Kaur et al. 2013a). The wild-type strain was deposited with MTCC culture collection center, Institute of Microbial Technology, Chandigarh, India, vide culture deposit no. MTCC10973. P. acidilactici has already got GRAS status (GRN no. 000171, awarded by Food and Drug Administration); thus, it could be utilized as a probiotic food additive as per WHO guidelines on food and feed additives. A vanillin biosynthetic cassettes has been designed and cloned in vector pLES003 (pLES003-P289/fcs + /ech +) for expression in competent cells of P. acidilactici BD16 MTCC10973. Using the strategy of metabolic engineering, 2.58 mM extracellular vanillin was recovered within 10 min and 0.57 mM intracellular vanillin after 20 min of incubation in the recombinant P. acidilactici (fcs + /ech +) cell extracts. Overall, 3.14 mM vanillin was recovered from 1.08 mM FA with a molar conversion rate of approximately 100 %. Its native strain however did not convert FA efficiently into vanillin in which only 5.5 % molar conversion rate was observed. Engineered P. acidilactici (fcs + /ech +) showed higher affinity for FA, as well as a higher FA to vanillin molar conversion rate (approximately 100 %) in extracellular extracts, whereas only 22.63 % FA to vanillin molar conversion was achieved using intracellular extracts after scale-up. During LCMS-ESI-based metabolic profiling, vanillin degradation products like vanillyl alcohol and protocatechuic acid were not detected in cell extracts of recombinant P. acidilactici (fcs + /ech +). Results indicate its better suitability and acceptability than other recombinant microbial strains like Pseudomonas sp. HR199 (Overhage et al. 1999b) and E. coli JM109 (pBB1) (Barghini et al. 2007) as vanillin producing starter cultures for industrial processes. Recombinant P. acidilactici (fcs + /ech +) strain shows sevenfold more efficient FA to vanillin biotransformation than recombinant E. coli top10 strain (fcs + /ech +). Recently, Yang et al. (2013) reported that the cloned Fcs enzyme in recombinant E. coli (Km 0.35 mM mg−1 FA) is less substrate-specific than cloned Fcs enzyme in recombinant P. acidilactici (fcs + /ech +) (Km 4.07 μM mg−1 FA extracellular and 16.2 μM mg−1 FA intracellular). After statistical optimization of bioconversion reaction using CCD model of RSM, a 100 % molar conversion of FA to vanillin was reported for extracellular response (run 11).
In previous reports, Oenococcus oeni or Lactobacillus sp. (Bloem et al. 2007), E. coli strains (Yoon et al. 2005a, b; Lee et al. 2009; Luziatelli and Ruzzi 2008), Pseudomonas sp. (Plaggenborg et al. 2003), Streptomyces sp. (Achterholt et al. 2000; Hua et al. 2006), or Amycolatopsis sp. HR167 (Overhage et al. 2006) were used to optimize vanillin production in which longer incubation period and higher biomass utilization were affecting economic viability of the process (as indicated in Table 4). Production levels of vanillin in recombinant microbial systems ranged from 0.022 mM min−1 in E. coli (fcs+/ech+) to highest yield 0.16 mM min−1 in recombinant P. acidilactici (fcs + /ech +).
There are few metabolic engineering approaches reported so far in the literature utilizing host organisms other than E .coli. Bioconversion of FA to vanillin and vanillic acid was investigated by Narbad and Gasson (1998), and they found that 32 % of added FA (1 pmol) was converted to vanillin (molar conversion rate 18 %) and vanillic acid (molar conversion rate 14 %) within 4 h using crude extracts prepared from P. fluorescens AN103 cells. Supplementation of NAD in the incubation reaction mixture increased molar conversion rate of FA to vanillin by 68 %. When the incubation was prolonged, vanillin degradation to vanillic acid was reported. CoASH, ATP, and MgCl2 were found as absolute cofactors for bioconversion for FA biotransformation of FA to vanillin.
Another study reported that vanillin yields in P. fluorescens AN103 could be improved by disrupting vdh gene (Martinez-Cuesta et al. 2005). Barbosa et al. (2008) statistically optimized vanillin production by solid-state fermentation on green coconut husk in Phanerochaete chrysosporium. Plackett Burmen design was implemented to evaluate role of 19 variables including different N sources and 52.5 μg g−1 of vanillin was recovered after 24 h of incubation. In another report, vdh gene of P. fluorescens AN103 was disrupted and cloned it with fcs and ech genes on a low-copy plasmid pBB1 which produced 8.41 mM vanillin from FA acid (Di Gioia et al. 2011). In these cases, genetic instability of the recombinant strain was adjudged as a major drawback, and moreover, the use of Pseudomonas sp. is restricted in food products.
After critical analysis of the previous reports on vanillin production from FA (Table 4), higher FA to vanillin bioconversion rate was observed when enzyme extracts were used instead of whole cells. Extracellular extracts of recombinant P. acidilactici (fcs + /ech +) facilitated production of 2,576 μM vanillin within 10 min of incubation, and intracellular extracts yielded 565 μM vanillin within 20 min of incubation with the substrate, i.e., FA. This is the most efficient FA to vanillin bioconversion system reported so far using a recombinant microbial system. Earlier, there is a single report on recombinant E. coli (fcs+/ech+) where cloned Fcs and Ech enzymes resulted in production of 3.34 mg L−1 vanillin from 3.7 mM FA per minute with a molar conversion rate of 0.022 Μm min−1 mL−1 (Overhage et al. 1999a).
In our previous study, whole cells of native P. acidilactici BD16 were used for vanillin production on rice bran medium containing 150 g L−1 rice bran and 50 μg mL−1 FA and 1.269 g L−1 of crude vanillin was obtained (Kaur and Chakraborty 2013). However, 3.14 mM (478 mg L−1) vanillin was obtained in the present study using 1.08 mM (210 μg mL−1) of FA and enzyme extracts from 1 mg biomass of P. acidilactici BD16 (fcs+/ech+) cells. As rice bran is a rich source of FA and cinnamic acid derivatives, a higher production was reported in this biochemically unspecific media than synthetic medium used in the present study, where only FA was available for supporting growth as well as vanillin production in recombinant cells. If we follow a similar strategy in case of recombinant P. acidilactici BD16 (fcs +/ech +) also, vanillin productions will definitely increase. In our preliminary study, vanillin production was observed after 8 h in native P. acidilactici BD16, whereas in the present strategy, biotransformation period is reduced to 20 min. This work is still under progress. We can exploit this recombinant microorganism, especially because of its probiotic nature, as a starter culture in various dairy products, and process could also be optimized for in situ vanillin production in distillery, candies, chocolates, choco-eclairs, ice creams, yogurts, desserts, etc.
References
Achterholt S, Priefert H, Steinbüchel A (2000) Identification of Amycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 54:799–807
Barbosa ES, Perrone D, Amaral VAL, Ferriera LSG (2008) Vanillin production by Phanerochaete chrysosporium grown on green coconut agro-industrial husk in solid state fermentation. Bioresources 3(4):1042–1050
Barghini P, Di Gioia D, Fava F, Ruzzi M (2007) Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb Cell Factories 6:13. doi:10.1186/1475-2859-6-13
Bloem A, Bertrand A, Lonvaud-Funel A, de Revel G (2007) Vanillin production from simple phenols by wine-associated lactic acid bacteria. Lett Appl Microbiol 44(1):62–67
Caldwell SL, Mahon DJM, Oberg CJ, Broadbent JR (1996) Development and characterization of lactose-positive Pediococcus Species for milk fermentation. Appl Environ Microbiol 62(3):936–941
Converti A, Aliakbarian B, Dominguez JM, Bustos Vázquez G, Perego P (2010) Microbial production of biovanillin. Braz J Microbiol 41(3):519–530
Davis JR, Goodwin LA, Woyke T, Teshima H, Bruce D, Detter C, Tapia R, Han S, Han J, Pitluck S, Nolan M, Mikhailova N, Land ML, Sello JK (2012) Genome sequence of Amycolatopsis sp. strain ATCC 39116, a plant biomass-degrading actinomycete. J Bacteriol 194:2396–2397
De Las Rivas B, Rodriguez H, Curiel JA, Landete JM, Munoz R (2009) Molecular screening of wine lactic acid bacteria degrading hydroxycinnamic acids. J Agric Food Chem 57:490–494
del Martinez-Cuesta MC, Payne J, Hanniffy SB, Gasson MJ, Narbad A (2005) Functional analysis of the vanillin pathway in a vdh-negative mutant strain of Pseudomonas fluorescens AN103. Enzym Microb Technol 37(1):131–138
Di Gioia D, Luziatelli F, Andrea N, Ficca AG, Fava F, Ruzzi M (2011) Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J Biotechnol 156(4):309–316
Gasson MJ, Kitamura Y, McLauchlan WR, Narbad A, Parr AJ, Parsons EL, Payne J, Rhodes MJ, Walton NJ (1998) Metabolism of ferulic acid to vanillin. A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J Biol Chem 237:4163–4170
Hua D, Ma C, Lin S, Song L, Lin S, Zhang Z, Deng Z, Xu P (2006) Enhanced vanillin production from ferulic acid using adsorbent resin. Appl Microbiol Biotechnol 74(4):783–790
Kaur B, Chakraborty D (2012) Biotechnological and molecular approaches for vanillin production: a review. Appl Biochem Biotechnol 169(8):1353–1372
Kaur B, Chakraborty D (2013) Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. Ind J Exp Biol 51:935–943
Kaur B, Chakraborty D, Kaur G, Kaur G (2013a) Biotransformation of rice bran to ferulic acid by Pediococcal isolates. Appl Biochem Biotechnol 170(4):854–867
Kaur B, Chakraborty D, Kumar B (2013b) Phenolic biotransformations during conversion of ferulic acid to vanillin by lactic acid bacteria. BioMed Res Int Article ID 590359, 6 pages
Lee EG, Yoon SH, Das A, Lee SH, Li C, Kim JY, Choi MS, Oh DK, Kim SW (2009) Directing vanillin production from ferulic acid by increased acetyl-CoA consumption in recombinant Escherichia coli. Biotechnol Bioeng 102(1):200–208
Luziatelli F, Ruzzi M (2008) Genetic engineering of Escherichia Coli to enhance biological production of vanillin from ferulic acid. Bull UASVM Anim Sci Biotechnol 65:4–8
Masai E, Harada K, Kitayama H, Peng X, Katayama Y, Fukuda M (2002) Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl Environ Microbiol 68(9):4416–4424
Muheim A, Lerch K (1999) Towards a high-yield bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 51:456–461
Muheim A, Muller B, Munch T, Wetli M (2001) Microbiological process for producing vanillin. U.S. patent 6,235,507 B1
Narbad A, Gasson MJ (1998) Metabolism of ferulic acid via vanillin using a novel CoA dependent pathway in a newly isolated strain of Pseudomonas fluorescens. Microbiology 144(5):1397–1405
Overhage J, Priefert H, Steinbüchel A (1999a) Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl Environ Microbiol 65:4837–4847
Overhage J, Priefert H, Rabenhorst J, Steinbüchel A (1999b) Biotransformation of eugenol to vanillin by a mutant of Pseudomonas sp. strain HR199 constructed by disruption of the vanillin dehydrogenase (vdh) gene. Appl Microbiol Biotechnol 52(6):820–828
Overhage J, Steinbüchel A, Priefert H (2003) Highly efficient biotransformation of Eugenol to ferulic acid and further conversion to vanillin in recombinant strains of Escherichia coli. Appl Environ Microbiol 69(11):6569–6576
Overhage J, Steinbüchel A, Priefert H (2006) Harnessing eugenol as a substrate for production of aromatic compounds with recombinant strains of Amycolatopsis sp. HR167. J Biotechnol 125(3):369–376
Plaggenborg R, Overhage J, Steinbüchel A, Priefert H (2003) Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Appl Microbiol Biotechnol 61:528–535
Plaggenborg R, Overhage J, Loos A, Archer JAC, Lessard P, Sinskey AJ, Steinbüchel A, Priefert H (2006) Potential of Rhodococcus strains for biotechnological vanillin production from ferulic acid and eugenol. Appl Microbiol Biotechnol 72(4):745–755
Ruzzi M, Luziatelli F, Matteo PD (2008) Genetic engineering of Escherichia coli to enhance biological production of vanillin from ferulic acid. Bulletin UASVM. Anim Sci Biotechnol 65(1–2)
Song JW, Lee EG, Yoon SH, Lee SH, Lee JM, Lee SG, Kim SW (2009) Vanillin production enhanced by substrate channeling in recombinant E. coli. SIM annual meeting and exhibition. J Ind Microbiol Biotechnol 125
Wada T, Noda M, Kashiwabara F, Jeon HJ, Shirakawa A, Yabu H, Matoba Y, Kumagai T, Sugiyama M (2009) Characterization of four plasmids harboured in a Lactobacillus brevis strain encoding a novel bacteriocin, brevicin 925A, and construction of a shuttle vector for lactic acid bacteria and Escherichia coli. Microbiology 155(Pt 5):1726–1737
Yang W, Tang H, Jun N, Wu Q, Hua D, Tao F, Xu P (2013) Characterization of two Streptomyces enzymes that convert ferulic acid to vanillin. PLoS ONE 8(6):e67339. doi:10.1371/journal.pone.0067339
Yoon SH, Cui L, Lee YM, Lee SH, Kim SH, Choi MS, Seo WT, Yang JK, Kim SW (2005a) Production of vanillin from ferulic acid using recombinant strains of Escherichia coli. Biotechnol Bioprocess Eng 10:378–384
Yoon SH, Li C, Kim JE, Lee SH, Yoon JY, Choi MS, Seo WT, Yang JK, Kim JY, Kim SW (2005b) Production of vanillin by metabolically engineered Escherichia coli. Biotechnol Lett 27(22):1829–1832
Yoon SH, Lee EG, Das A, Lee SH, Li C, Ryu HK, Choi MS, Seo WT (2007) Enhanced vanillin production from recombinant E.coli using NTG mutagenesis and adsorbent resin. Biotechnol Prog 23(5):1143–1148
Acknowledgments
Authors acknowledge Masafumi Noda, Assistant Professor, Hiroshima University, Japan, for providing shuttle vector pLES003. Financial assistance is provided by UGC, New Delhi, India as a major research project entitled “Metabolic engineering of LAB isolate for biotransformation of ferulic acid to vanillin” to Dr. Baljinder Kaur and as a meritorious BSR fellowship to Mr. Debkumar Chakraborty.
Conflict of interest
We do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 802 kb)
Rights and permissions
About this article
Cite this article
Kaur, B., Chakraborty, D. & Kumar, B. Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl Microbiol Biotechnol 98, 8539–8551 (2014). https://doi.org/10.1007/s00253-014-5950-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5950-x