Abstract
Ferulic acid is a known precursor for vanillin production but the significance of agro waste as substrates for its extraction, in combination with microbes is a less explored option. Various lactic acid bacteria were screened for the production of ferulic acid esterase (FAE) and Enterococcus lactis SR1 was found to produce maximum FAE (7.54 ± 0.15 IU/ml) in the synthetic medium under submerged fermentation. To make the process cost effective, various lignocellulosic agroresidues were evaluated for the production of FAE from the bacterium. It was found that wheat bran serves as the best substrate for FAE production (4.18 ± 0.12 IU/ml) from E. lactis SR1. Further, optimization of fermentation conditions for FAE production from E. lactis SR1 using wheat bran as carbon source led to an increase in the enzyme production (7.09 ± 0.21 IU/ml) by 1.5 fold. The FAE produced was used alone or in combination with commercial holocellulase for biological release of FA from the tested agroresidues. The highest release of FA (mg/g) by enzymatic extraction occurred in sugarbeet pulp (2.56), followed by maize bran (1.45), wheat bran (1.39) and rice bran (0.87), when both the enzymes (FAE and holocellulase) were used together. Alkaline extraction and purification of ferulic acid (FA) from these agro residues also showed that sugarbeet pulp contains the highest amount of FA (5.5 mg/g) followed by maize bran (3.0 mg/g), wheat bran (2.8 mg/g) and rice bran (1.9 mg/g), similar to the trend obtained in biological/enzymatic extraction of FA from these residues. Furthermore, the substrates were found to release higher reducing sugars when both commercial holocellulase and FAE were used in combination than by the use of holocellulase alone. Thus, FAEs not only release FA but also enabled hemicellulase and cellulase to release more sugars from plant material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditional methods to produce vanilla flavor from vanilla pods are labor-intensive and time-consuming that increases its production cost, and the yields obtained are too low to meet market demand (Converti et al. 2010). As a result, chemical or synthetic vanillin is produced from fossil hydrocarbons, which is much cheaper compared to the naturally extracted vanillin (Chattopadhyay et al. 2018). Nevertheless, due to rising consumer preferences for natural additives in food products, research efforts in the area of development of natural flavors through alternative environment-friendly and cost-effective routes are being carried out globally (Kumar et al. 2017). In this regard, the biotechnological pathway for the production of vanillin is highly attractive as the products of this route are also considered natural, according to the European and US legislations (Perez-Rodriguez et al. 2016). Ferulic acid (3-methoxy-4-hydroxy cinnamic acid), which has a structural resemblance to vanillin has become the most popular precursor substrate for vanillin production as this phenolic acid in the plant cell wall can be released by chemical as well biological/enzymatic methods, and can be then converted to vanillin microbiologically (Kaur and Chakraborty 2013). The biological process for the production of vanillin (biovanillin) involves the conversion of ferulic acid (FA) to vanillin by some bacterial species, mainly Pseudomonas, Streptomyces as well as filamentous fungi viz. Aspergillus niger, Pycnoporous cinnabarinus and Phanerochaete chrysosporium (Kumar and Pruthi 2014). However, the free ferulic acid constitutes a small portion of total content in plant/cereal cell wall while the bound ferulic acid linked to arabinoxylan (ferulolylated arabinoxylans) is the predominant one (Malunga and Beta 2016). This arabinoxylan linked phenolic acid in the plant cell wall can be released by either chemical hydrolysis (alkali/acid treatment) or by the action of a microbial enzyme, ferulic acid esterase (FAE). Many microbial genera, for example, Streptomyces (Ferreira et al. 2007), Aspergillus (Ou et al. 2011), Fusarium (Xiros et al. 2009), Trichoderma (Long et al. 2018) possess the potential to release FA from plant cell walls by the action of their FAEs. Interestingly, many members of probiotic lactic acid bacteria (LAB) are also known to produce high amounts of FAE and de-esterify dietary fiber in the human and rumen gut, releasing hydroxycinnamates and its derivatives, which have been shown to have positive effects, including antioxidant, anti-inflammatory and antimicrobial activities (Faulds 2010). Similarly, it was found that enzymes and chemical secretions of the human upper gut model did not solubilize feruloyl groups from wheat bran, while the release of ferulic acid was demonstrated in the presence of human intestinal microflora (Kroon et al. 1997). On similar lines, FAE has been isolated from many LABs including L. gasseri (Couteau et al. 2001), L. acidophilus (Wang et al. 2004), L. helveticus (Guglielmetti et al. 2008), L. johnsonii (Lai et al. 2009), L. plantarum (Esteban-Torres et al. 2014), L. fermentum (Su et al. 2019). However, the release of sufficient FA from plant cell wall is the limiting factor in the economical and feasible production of biovanillin. Agricultural by-products, which possess little economic value provide an inexpensive and abundant source of ferulic acid, with potential to be used for biotransformation into vanillin. Among lignocellulosic agro-residues, sugarbeet pulp, maize bran, wheat bran, and rice bran are known to contain substantial amounts of ferulic acid (Fazary and Ju 2007). Cereal brans are one of the richest sources of dietary fibre, mainly arabinoxylans (Das et al. 2012), which comprises a linear (1–4)-β-D-xylopyranose chain that is substituted with L-arabinofuranose (Lequart et al. 1999). The arabinoxylans may be feruloylated with ferulic acid (FA) at the C-5 position of the arabinose units (Bunzel et al. 2006). In contrast, ferulic acid is mainly attached at the C-2 position of α-1,5-linked arabinofuranose residues in the hairy region of pectins of sugarbeet pulp (Micard et al. 1997). In the present study, lactic acid bacteria isolated from different sources (fermented foods and water channel of the dairy plant) were screened for the production of FAE. LAB generally flourish in the carbohydrate rich environment, such as plants, fermented foods, milk products, gastrointestinal and urogenital tracts of humans and animals, as well as in soil and water (Zhang et al., 2016; Ruiz Rodríguez et al. 2019). The selected LAB was used for FAE production under submerged fermentation conditions, with different lignocellulosic agro-residues as carbon source. The lignocellulosic substrate which gave maximum FAE production was chosen for optimization of physiological parameters (time, temp., pH, % inocula and % substrate concentration) for maximizing FAE production under submerged fermentation. The FAE produced was used in conjunction with commercial holocellulase for the release of ferulic acid and reducing sugars from four lignocellulosic agro-residues, namely wheat bran, rice bran, maize bran, and sugarbeet pulp. FA was also extracted from the same agro-residues using alkaline extraction method, and yields obtained were compared with that obtained by enzymatic method.
Materials and methods
Chemicals and raw materials
Trans-ferulic acid and ethyl ferulate were purchased from Merck, USA. Commercial holocellulase enzyme (Cat No. C2730, Merck, USA; consisting of exo and endoglucanase (FPase 10,500 IU/ml; CMCase 11,200 IU/ml, β-glucosidase 10,000 IU/ml) and xylanase (20,000 IU/ml) was used for saccharification of lignocellulosic biomass. Other chemicals and media components were purchased from SRL, Mumbai, India.
Sugarbeets were procured from ICAR-Central Arid Zone Research Institute, Jodhpur, Rajasthan, India. Maize bran was obtained from the Genetics Division, IARI, New Delhi, India, while, wheat bran and rice bran were purchased from the local market.
Processing of raw material
The sugarbeets were washed, peeled, diced and then boiled until beets were tender. The sugar-rich liquid (source of sugar) was separated and the beet pulp was collected in a strainer lined with a muslin cloth. The liquid from the beet pulp was pressed and the pulp was dried in an oven at 80 °C. The dried pulp was stored in airtight containers at room temperature before further use. The other types of cereal bran were thoroughly washed with water, dried in an oven and stored in airtight containers at room temperature before further use.
Lactobacillus strains and growth conditions
Lactobacillus species viz. (Lactobacillus fermentum, Pediococcus pentosaceus, Enterococus lactis, Enterococcus faecium, Lactobacillus farraginis) have been isolated and identified in our laboratory previously were maintained on MRS agar medium, containing the following ingredients (g/L): Proteose peptone 10; Beef extract 10; Yeast extract 5.0; Ammonium citrate 2; Sodium acetate 5.0; MgSO4.7H2O 0.1; MnSO4.4H2O 0.05; K2HPO4 2.0; agar 20.0, pH:6.5. The source and NCBI accession numbers of the LAB used in this study are given in Table 1.
Ferulic acid esterase activity of Lactobacillus strains
For screening of microbes that produce ferulic acid esterase (FAE), MRS agar without glucose (pH 6.5) was autoclaved, cooled to 55 °C followed by the addition of ethyl ferulate (1% w/v in dimethyl formamide). The media was mixed well and the plates were poured. One loopful of each Lactobacillus species was transferred from MRS-agar slant onto MRS-EF plates and incubated at 37 °C for 72 h. Bacterial species producing FAE were confirmed by visualization of a ring of clearance around the colonies on the plates.
Time course of FAE production from the bacterial isolates under submerged fermentation
The time course of FAE production from the five LAB was studied in MRS broth (without glucose) supplemented with ethyl ferulate (1% w/v in dimethylformamide) under submerged fermentation conditions. The medium was inoculated with the bacterial strains (0.2% v/v of 24 h grown culture) and incubated at 37 °C under static conditions for 120 h. Samples were withdrawn periodically at 24 h intervals and centrifuged at 10,000 rpm for 15 min at 4 °C, the cell-free supernatants were used as a source of crude enzyme for quantification of FAE activity.
FAE production from Enterococcus lactis SR1 using lignocellulosic wastes under submerged fermentation
FAE production from the selected bacterium Enterococcus lactis SR1 was studied under liquid submerged fermentation conditions using different lignocellulosic wastes as a carbon source. The inoculum of the bacterium was grown in MRS broth at 37 °C under static conditions for 16 h and inoculated @ 0.2% v/v in MRS-broth without glucose but supplemented with 1% w/v of different lignocellulosic wastes (sugarbeet pulp, maize bran, wheat bran, and rice bran) and incubated at 37 °C for 72 h. The cultures were then centrifuged at 10,000 rpm for 15 min and the supernatant was used as crude enzyme for estimation of FAE, carboxymethyl cellulases (CMCase) and xylanase activities.
Further optimization of various nutritional and physiological parameters for maximizing FAE production from E. lactis SR1 was done using wheat bran as a carbon source. The optimization factors included—incubation time (24 h–120 h), temperature (25–45 °C), pH (3.0–8.0), percent inoculum (0.5–2.5% v/v) and wheat bran concentration (0.5–2.5%).
Analytical methods
Assay for FAE was set up in 1.5 ml tubes (in triplicates), each containing 800 μl of 100 mM Na-Phosphate buffer (pH 6.5) and 15 μl of ethyl ferulate (10 mg/ml) dissolved in dimethylformamide as described by Liu et al. (2016). The reaction was initiated by adding 200 μl crude enzyme and incubated at 37 °C for 2 h. The reaction was stopped by keeping the tubes in a boiling water bath for 5 min. The concentration of ferulic acid in the reaction mixture was determined by HPLC (Waters) equipped with PDA detector 2998. A Biorad C-18 column (300 mm × 7.8 mm) was eluted with an isocratic mixture of (35:65) methanol: 0.3% acetic acid. The column oven was set at 37 °C. The elution was monitored at 310 nm, using a waters PDA detector 2998. One unit of enzyme is the amount of enzyme that is required to release one µM of ferulic acid in one minute. The standard curve of FA was prepared in the range of 25–250 mg/L. The substrate and enzyme blanks were also analyzed by HPLC and the readings were subtracted from the reading of the test samples.
The xylanase activity was estimated by measuring the release of xylose from birchwood xylan (1.0% w/v) in 50 mM sodium phosphate buffer (pH 6.0) after incubation at 50 °C for 10 min (Bailey et al. 1992). Carboxymethyl cellulase activity was assayed in accordance with the protocol given by Ghose (1987).
Reducing sugars were assayed using the DNSA method measuring absorbance at 540 nm (Miller 1959).
Determination of total alkali extractable ferulic acid present in agro-residues
Each substrate (0.1 g) used in the study (maize bran, wheat bran, rice bran, and sugarbeet pulp) was mixed separately with 6 ml of 0.5 N KOH and incubated at 50 °C for 6 h. The alkaline hydrolysates were centrifuged followed by acidification of supernatant (pH < 2) with 2 M HCl and the released FA was extracted twice using equal volumes of ethyl acetate. The organic fractions were collected and evaporated to dryness and the residues were dissolved in 1 ml of 80% methanol.
Purification of ferulic acid extracted chemically from agro-residues
Purification of FA was carried out using Amberlite XAD-4 resin as described by Tilay et al. (2008). The resin was washed with methanol:water (1:1) three times and packed in a 1 cm diameter column. The column was then equilibrated with 1 N HCl. The extracted ferulic acid (2 ml) from each sample was passed through the column at a flow rate of 1.0 ml/min. The saturated column was washed with 1 N HCl to remove unbound phenolics. Elution of absorbed FA was performed using an ethanolic solution of ammonium hydroxide (0.1%) with a flow rate of 1.0 ml/min. The eluted fractions showing maximum FA content were pooled, concentrated and dissolved in 1 ml of HPLC grade methanol. The purity of the extracted FA was checked using HPLC using conditions as described in Sect. 2.7.
Enzymatic release of ferulic acid and reducing sugars from agro-residues
The crude FAE from E. lactis SR1 was dialyzed against 0.1 M sodium phosphate buffer (pH 5.8) at 4 °C for 24 h and then used for the enzymatic release of FA from lignocellulosic residues namely; sugar beet pulp, maize bran, rice bran, and wheat bran. Substrates (0.1 g) was mixed with either of these combinations: a) 500 IU/g of crude FAE from E. lactis SR1; b) 500 IU/g of FAE from E. lactis SR1 + commercial holocellulase @ 50 FPU/g and 100 IU/g xylanase; c) commercial holocellulase @ 50 FPU/g and 100 IU/g xylanase in a total volume of 10 ml of 0.1 M sodium phosphate buffer, pH 5.8 and incubated at 50 °C, 120 rpm for 24 h. Sodium azide (0.1% w/v) was also added in each reaction mixture to prevent microbial contamination. Control samples without the addition of any enzyme were also run in parallel. Samples were collected at an interval of 8 h, centrifuged at 10,000 rpm for 10 min and boiled for inactivating the enzymes. Reducing sugars were determined in the samples using the DNSA method (Miller 1959). While ferulic acid was extracted from the samples with an equal volume of ethyl acetate and then analyzed for quantification of ferulic acid by HPLC. Control samples without the addition of enzyme were run in parallel.
Results and discussion
FAE activity of LAB on MRS-EF agar plates
All the five LAB strains used in the study were capable of growing under microaerophilic conditions on MRS agar medium without glucose but supplemented with 0.1% ethyl ferulate as carbon source. Further, the formation of the halo zone around the colonies for these five LAB strains indicated their capability to produce extracellular feruloyl esterase. However, the diameter of the zone of clearing varied with the maximum zone being observed for E. lactis SR1 (Table 1). Donaghy et al. (1998) developed agar plate assay for the detection of microbial esterases and found several Bacillus sp. as well as Lactobacillus plantarum can produce a halo around their colonies in MRS medium, in which carbon source was substituted with ethyl ferulate. Feruloyl esterases have been previously reported in lactic acid bacteria isolated from foods and human intestinal microbiota by many researchers. Couteau et al. (2001) isolated and characterized human fecal bacteria in chlorogenic based media and observed that six isolates belonging to genera E. coli, Bifidobacterium lactis and L. gasseri produced esterase. The high amounts of FAE produced by LAB help to de-esterify dietary fiber in human and ruminal digestion. Hydroxycinnamates, such as caffeic, ferulic and p-coumaric acids are commonly found as ester conjugates in dietary plants. Buchanan and co-workers (1996) observed that the feruloyl and p-coumaryl groups in spinach cell walls remain essentially unaltered in the upper gut of rats but occur partly as free acids in the colon, thus indicating the role of LAB colonizing the colon in the digestion and release of these functional groups. Similarly, enzymes and chemical secretions found in the upper gut were found to solubilize little or no feruloyl groups of wheat bran, while the release of ferulic acid was demonstrated in the presence of human intestinal microfora (Kroon et al. 1997). Likewise, the in-vitro release of ferulic acid from durum wheat dietary fibre (DWF) by intestinal microbes in a gut model of the human colon was also demonstrated by Napolitano et al. (2009). Xu et al. (2017) isolated four Lactobacillus strains (L. amylovorus CGMCC 11,056, L. acidophilus CCTCC AB2010208, L. farciminis CCTCC AB2016237 and L. fermentum CCTCC AB2010204) with feruloyl esterase activities by plate screening assay.
Time course study of FAE production using LAB strains under submerged fermentation
The five LAB strains were tested for FAE production under submerged fermentation conditions. All the isolates were found to produce FAE when grown in MRS-EF broth, with the highest being observed for E. lactis SR1 (7.54 IU/ml) after 72 h of submerged fermentation (Table 2). The amount of ferulic acid released in the reaction mixture after enzymatic assay of the 72 h culture filtrate of all the five strains grown in MRS-EF media with EF as the substrate is presented as HPLC chromatograms (Supplementary Fig. 1). Control samples (uninoculated media) did not exhibit any FAE activity. Liu and co-workers (2016) isolated FAE from L. fermentum NRRL B-1932 and expressed the recombinant protein in E. coli with FAE activities of 45.89 mU/mg of protein. Ding et al. (2019) showed FAE activities from L. plantarum A1 (33.56 mU/mg of protein) and L. brevis L3 (30.48 mU/mg of protein). In general, the activity of ferulic esterases is strain specific and varies according to genera and species.
Xylanase and CMCase activities in culture filtrate of E. lactis SR1
E. lactis SR1 was found to produce 2.61 ± 0.11 IU/ml of xylanase but did not produce any CMCase activities after 72 h of incubation under the conditions tested. Lee et al. (2019) reported specific xylanase activities in the range of 0.20–0.51 IU/mg and specific CMcase activities in the range of 0.81–0.58 U/mg from different strains of L. plantarum.
Ferulic acid esterase (FAE) production from E. lactis SR1 using lignocellulosic wastes under submerged fermentation
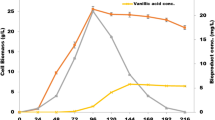
For the development of a cost-effective bioprocess for FAE production, the use of ethyl ferulate as a carbon source is not feasible, hence it is necessary to identify inexpensive substrates. Therefore, different lignocellulosic agro-residues were tested for their potential to serve as a carbon source for the production of FAE from E. lactis SR1. From the results, it was observed that E. lactis SR1 produces the highest FAE under submerged fermentation when grown on various lignocellulosic agro-residues as substrate (Fig. 1). However, among the agro-residues tested, maximum FAE production (4.51 ± 0.12 IU/ml) from E. lactis SR1 was observed on wheat bran as a carbon source (Fig. 1). In this regard, Mukherjee and co-workers (2007) produced FAE (2.0 mU/ml) from Streptomyces sp. using 1.5% wheat bran as substrate under agitated submerged fermentation. Similarly, Kheder et al. (2009) observed maximum FAE activity (0.22 mU/mg) from Streptomyces ambofaciens in complex medium containing wheat bran as carbon source followed by oat spelt xylan (0.21 mU/mg), maize bran (0.04 mU/mg) and beet pectin (non-quantifiable detectable amounts). Similarly, under solid-state fermentation conditions, wheat bran supported better xylanolytic (Nagar et al. 2011), cellulolytic (El-Shrishtawy et al. 2014), proteolytic (Meena et al. 2013) and phytase (Salmon et al. 2012) activities from microorganisms in comparison to other agro-residues. Thus, wheat bran is a richer source of nutrients that are required for microbial growth and subsequent enzyme production, compared to other lignocellulosic biomass (Katileviciute et al. 2019).
Further, from the results of optimization of physiological and nutritional parameters (incubation time, temp, pH, inoculum level and substrate concentration) as shown in Fig. 2, it was observed that maximum FAE (7.09 IU/ml) was produced from E. lactis SR1 at inoculum level of 0.5% v/v of 16-h old culture in production medium (pH 6.5) comprising MRS broth (without glucose) supplemented with 1% wheat bran as a carbon source at 37 °C after 72 h of incubation (Fig. 2).
Estimation of total alkali extractable ferulic acid present in different agro-residues
Alkaline extraction of ferulic acid from the lignocellulosic agro residues, followed by purification through amberlite XAD-4 resin indicated that sugarbeet pulp contains the highest amount of FA (5.5 ± 0.21 mg/g), followed by maize bran (3.0 ± 0.12 mg/g), wheat bran (2.8 ± 0.08 mg/g) and rice bran (1.9 ± 0.06 mg/g). Ferulic acids are known to be associated with cell walls of many plant products including maize bran, rice bran, wheat bran and sugarbeet pulp (Sakamoto et al. 2005). Fazary and Ju (2007) reported sugarbeet pulp (0.9%), maize bran (1.3%), wheat bran (0.5%) and rice bran oil (1.5%) as rich sources of ferulic acid, while Kheder et al. (2009) also reported similar values for alkali extractable FA content in wheat bran (0.5%) and maize bran (0.3%).
FAE mediated release of ferulic acid from agro-residues
The crude enzyme extract from E. lactis SR1 released substantial amounts of ferulic acid from different natural ferulic acid-rich raw materials after 16 h of enzymatic hydrolysis, as shown in Table 3. Among the agro-residues tested, the highest FAE mediated release of ferulic acid was observed from sugarbeet pulp (1.98 ± 0.03 mg/g) followed by wheat bran (1.18 ± 0.07 mg/g), maize bran (1.01 ± 0.05 mg/g) and rice bran (0.85 ± 0.01 mg/g), similar to the trend obtained in alkali extraction of ferulic acid from these agro-residues. Sakamoto et al. (2005) reported a release of 5.2 mg/g of FA from sugarbeet pulp using crude FAE of Penicillium chrysogenum. Szwajgier and Jakubczyk (2011) used an extracellular FAE from L. acidophilus K1 for the release of phenolic acids from barley malt. Similarly, Kaur and Chakraborty (2013) used LAB strains to release ferulic acid from rice bran.
No ferulic acid content was detected in control samples of each substrate and also no FA release was observed when the substrates were hydrolyzed with only the commercial holocellulase. Thus, the amount of ferulic acid released in the study is a consequence of a FAE-mediated hydrolysis of the substrates. However, the addition of commercial holocellulase along with FAE caused a considerable increase in the release of FA (29% in case of sugarbeet pulp, 43% in maize bran, 17.8% in wheat bran and 2.35% in rice bran) from the natural raw substrates (Table 2 and Supplementary Fig. 2). The % enzymatic release of FA from all the tested substrates was found to be in the range of 50–54%. However, the difference in the % increase in the release of FA from different substrates by the combined action of FAE and holocellulases than with FAE alone is dependent on the amounts of free and bound ferulic acid in the substrates. The addition of holocellulases aided in solubilization of xylan core of heteroxylans and feruloyated oligosaccharides of the natural substrates and thereby, efficiently releasing FA from them (Saulnier et al. 2001). Ferulic and p-coumaric acids occur as ester-linked to pectic side-chains in sugarbeet pulp, and to the arabinoxylans of cereal brans (Smith and Hartley 1983). It is known that approximately 85% of phenolic acids are in the bound form in maize, approximately 75% in wheat and 62% in rice (Adom and Liu 2002). It has been reported earlier that the generation of ferulic acid from lignocellulosic substrates is more effective when FAE and xylanase act together due to ‘heterosynergy’ between these enzymes. In the present study, the maximum increase in the release of ferulic acid from the substrates by the combined action of FAE and holocellulase is seen in maize bran (43%) followed by sugarbeet pulp (29%), wheat bran (17.8%) and rice bran (2.35%). Saulnier et al. (2001) used commercial enzyme Novozyme 342 containing cellulolytic, xylanolytic and ferulic acid esterase activities for the release of ~ 30% FA from cell walls of maize bran. Perez-Rodriguez et al. (2016) released 2.05 mg/g and 1.87 mg/g of ferulic acid from vine trimming shoots and corn cob, respectively, using crude enzyme extract of Aspergillus tereus containing both FAE and xylanase activities. They highlighted the relevance of high FAE/xylanase ratio in the release of optimal FA from raw plant substrates.
Release of reducing sugars by enzymatic hydrolysis of natural substrates
The highest reducing sugar release was observed from sugarbeet pulp (13.51 g/L) followed by wheat bran (10.81 g/L), maize bran (7.12 g/L), and rice bran (7.09 g/L), when both commercial holocellulase and FAE were used in combination to hydrolyze these substrates than with holocellulases alone as shown in Fig. 3. FAEs release ester cross-linked ferulic acid allowing accessibility of cellulase and hemicellulase to the substrate (Hunt et al. 2017). Thus, FAEs not only release FA but also enable the hemicellulase and cellulases to liberate sugars from plant materials more effectively. Synergism between FAEs, cellulases and xylanases in the release of reducing sugars from plant materials has been observed by researchers. Perez-Rodriguez et al. (2016) observed a relation between the higher release of FA and a high concentration of reducing sugars from corn cob by the action of FAE and xylanases. On the other hand, Li et al. (2019) observed that the application of FAE producing L. plantarum L1 to corn stalk silage improved the enzymatic digestibility of biomass and facilitated efficient subsequent enzymatic saccharification.
Conclusion
Ferulic acid is a high-value chemical, which acts as a precursor for bioproduction of vanillin. Sugarbeet pulp, a remnant of sugarbeet industries is a rich source of ferulic acid. Similarly, cereal brans also contain high amounts of FA. All the raw material used in the study viz. sugarbeet pulp, maize bran, wheat bran and rice bran were found to release considerable amounts of ferulic acid, both chemically (5.5–1.9 mg/g) as well as enzymatically using FAE from E. lactis SR1 in conjunction with commercial holocellulase (2.56–0.87 mg/g). Therefore, commercial production of ferulic acid from agroresidues can become a feasible process for novel biorefining applications. Additionally, higher amounts of reducing sugars were released from the agro-residues with commercial holocellulases in the presence of FAEs, than with holocellulases alone. This illustrates that FAEs can also be considered as an accessory enzyme to increase the yield of reducing sugars as well as to produce ferulic acid as a valuable end product in biorefineries. The study also provides unique insights into exploiting lactic acid bacteria and their enzymes to produce platform chemicals like ferulic acid from agrowastes, with an additional advantage of enhancing the yield of reducing sugars.
References
Adom KK, Liu RH (2002) Antioxidant activity of grains. J Agric Food Chem 50:6182–6187
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270. https://doi.org/10.1016/0168-1656(92)90074-J
Buchanan CJ, Wallace G, Fry SC, Eastwood MA (1996) In Vivo Release of 14C-Labelled phenolic groups from intact dietary spinach cell walls during passage through the rat intestine. J Sci Food Agri 71:459–469. https://doi.org/10.1002/(SICI)1097-0010(199608)71:4<459:AID-JSFA602>3.0.CO;2-H
Bunzel M, Ralph J, Brüning P, Steinhart H (2006) Structural identification of dehydrotriferulic and dehydrotetraferulic acids isolated from insoluble maize bran fiber. J Agric Food Chem 54:6409–6418. https://doi.org/10.1021/jf061196a
Chattopadhyay P, Banerjee G, Sen SK (2018) Cleaner production of vanillin through biotransformation of ferulic acid esters from agroresidue by Streptomyces sannanensis. J Clean Prod 182:272–279. https://doi.org/10.1016/j.jclepro.2018.02.043
Converti A, Aliakbarian B, Domínguez JM et al (2010) Microbial production of biovanillin. Braz J Microbiol 41:519–530. https://doi.org/10.1590/S1517-83822010000300001
Couteau D, McCartney AL, Gibson GR et al (2001) Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J Appl Microbiol 90:873–881. https://doi.org/10.1046/j.1365-2672.2001.01316.x
Das A, Raychaudhuri U, Chakraborty R (2012) Cereal based functional food of Indian subcontinent: a review. J Food Sci Technol 49:665–672. https://doi.org/10.1007/s13197-011-0474-1
Ding ZT, Xu DM, Bai J et al (2019) Characterization and identification of ferulic acid esterase-producing Lactobacillus species isolated from Elymus nutans silage and their application in ensiled alfalfa. J Appl Microbiol 127:985–995. https://doi.org/10.1111/jam.14374
Donaghy J, Kelly PF, McKay AM (1998) Detection of ferulic acid esterase production by Bacillus spp. and lactobacilli. Appl Microbiol Biotechnol 50:257–260. https://doi.org/10.1007/s002530051286
El-Shishtawy RM, Mohamed SA, Asiri AM, Gomaa AB, Ibrahim IH, Al-Talhi HA (2014) Solid fermentation of wheat bran for hydrolytic enzymes production and saccharification content by a local isolate Bacillus megatherium. BMC Biotechnol 14:29
Esteban-Torres M, Mancheño JM, de Las Rivas B, Muñoz R (2014) Production and characterization of a tributyrin esterase from Lactobacillus plantarum suitable for cheese lipolysis. J Dairy Sci 97:6737–6744. https://doi.org/10.3168/jds.2014-8234
Faulds CB (2010) What can feruloyl esterases do for us? Phytochem Rev 9:121–132. https://doi.org/10.1007/s11101-009-9156-2
Fazary AE, Ju Y-H (2007) Feruloyl esterases as biotechnological tools: current and future perspectives. Acta Biochim Biophys Sin (Shanghai) 39:811–828. https://doi.org/10.1111/j.1745-7270.2007.00348.x
Ferreira P, Diez N, Faulds CB et al (2007) Release of ferulic acid and feruloylated oligosaccharides from sugar beet pulp by Streptomyces tendae. Bioresour Technol 98:1522–1528. https://doi.org/10.1016/j.biortech.2006.06.004
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Guglielmetti S, De Noni I, Caracciolo F et al (2008) Bacterial cinnamoyl esterase activity screening for the production of a novel functional food Product. Appl Environ Microbiol 74:1284–1288. https://doi.org/10.1128/AEM.02093-07
Hunt CJ, Antonopoulou I, Tanksale A et al (2017) Insights into substrate binding of ferulic acid esterases by arabinose and methyl hydroxycinnamate esters and molecular docking. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-17260-x
Katileviciute A, Plakys G, Budreviciute A et al (2019) A sight to wheat bran: High value-added products. Biomol 9:887
Kaur B, Chakraborty D (2013) Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. Indian J Exp Biol 51(11):935–943
Kheder F, Delaunay S, Abo-Chameh G et al (2009) Production and biochemical characterization of a type B ferulic acid esterase from Streptomyces ambofaciens. Can J Microbiol 55:729–738. https://doi.org/10.1139/W09-027
Kroon PA, Faulds CB, Ryden P et al (1997) Release of covalently bound ferulic acid from fiber in the human colon. J Agric Food Chem 45:661–667. https://doi.org/10.1021/jf9604403
Kumar K, Yadav AN, Kumar V et al (2017) Food waste: a potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour Bioprocess 4:18. https://doi.org/10.1186/s40643-017-0148-6
Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep (Amst) 4:86–93. https://doi.org/10.1016/j.btre.2014.09.002
Lai KK, Lorca GL, Gonzalez CF (2009) Biochemical properties of two cinnamoyl esterases purified from a Lactobacillus johnsonii strain isolated from stool samples of diabetes-resistant rats. Appl Environ Microbiol 75:5018–5024. https://doi.org/10.1128/AEM.02837-08
Lee FH, Wan SY, Foo HL et al (2019) Comparative study of extracellular proteolytic, cellulolytic, and hemicellulolytic enzyme activities and biotransformation of palm kernel cake biomass by lactic acid bacteria isolated from Malaysian foods. IJMS 20:4979. https://doi.org/10.3390/ijms20204979
Lequart C, Nuzillard J-M, Kurek B, Debeire P (1999) Hydrolysis of wheat bran and straw by an endoxylanase: production and structural characterization of cinnamoyl-oligosaccharides. Carbohyd Res 319:102–111. https://doi.org/10.1016/S0008-6215(99)00110-X
Li F, Ding Z, Ke W et al (2019) Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification. Biores Technol 282:211–221. https://doi.org/10.1016/j.biortech.2019.03.022
Liu S, Bischoff KM, Anderson AM, Rich JO (2016) Novel feruloyl esterase from Lactobacillus fermentum NRRL B-1932 and analysis of the recombinant enzyme produced in Escherichia coli. Appl Environ Microbiol 82:5068–5076. https://doi.org/10.1128/AEM.01029-16
Long L, Tian D, Zhai R et al (2018) Thermostable xylanase-aided two-stage hydrolysis approach enhances sugar release of pretreated lignocellulosic biomass. Bioresour Technol 257:334–338. https://doi.org/10.1016/j.biortech.2018.02.104
Malunga LN, Beta T (2016) Isolation and identification of feruloylated arabinoxylan mono- and oligosaccharides from undigested and digested maize and wheat. Heliyon 2:e00106. https://doi.org/10.1016/j.heliyon.2016.e00106
Meena P, Tripathi A, Srivastava S, Jha A (2013) Utilization of agro-industrial waste (wheat bran) for alkaline protease production by Pseudomonas aeruginosa in SSF using Taguchi (DOE) methodology. Biocatal Agric Biotechnol 2:210–216
Micard V, Grabber JH, Ralph J, Renard CMGC, Thibault JF (1997) Dehydroferulic acids from sugarbeet pulp. Phytochem 44:1365–1368
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chem 31:426–428
Mukherjee G, Singh RK, Mitra A, Sen SK (2007) Ferulic acid esterase production by Streptomyces sp. Biores Technol 98:211–213. https://doi.org/10.1016/j.biortech.2005.12.001
Nagar S, Mittal A, Kumar D, Kumar L, Kuhad RC, Gupta VK (2011) Hyper production of alkali stable xylanase in lesser duration by Bacillus pumilus SV-85S using wheat bran under solid state fermentation. New Biotechnol 28:581–587
Napolitano A, Costabile A, Martin-Pelaez S et al (2009) Potential prebiotic activity of oligosaccharides obtained by enzymatic conversion of durum wheat insoluble dietary fibre into soluble dietary fibre. Nutr Metab Cardiovas Dis 19:283–290. https://doi.org/10.1016/j.numecd.2008.07.005
Ou S, Zhang J, Wang Y, Zhang N (2011) Production of feruloyl esterase from Aspergillus niger by solid-state fermentation on different carbon sources. In: Enzyme Research. https://www.hindawi.com/journals/er/2011/848939/. Accessed 10 Apr 2020
Perez-Rodriguez N, Moreira CD, Torrado Agrasar A, Dominguez JM (2016) Feruloyl esterase production by Aspergillus terreus CECT 2808 and subsequent application to enzymatic hydrolysis. Enz Microb Technol 91:52–58
Ruiz Rodríguez LG, Mohamed F, Bleckwedel J et al (2019) Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in Northern Argentina. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01091
Sakamoto T, Nishimura S, Kato T et al (2005) Efficient extraction of ferulic acid from sugar beet pulp using the culture supernatant of Penicillium chrysogenum. J Appl Glycosci 52:115–120. https://doi.org/10.5458/jag.52.115
Salmon DN, Piva LC, Binati RL, Rodrigues C, Vandenberghe LP, Soccol CR, Spier MR (2012) A bioprocess for the production of phytase from Schizophyllum commune: Studies of its optimization, profile of fermentation parameters, characterization and stability. Bioprocess Biosyst 35:1067–1079
Saulnier L, Marot C, Elgorriaga M et al (2001) Thermal and enzymatic treatments for the release of free ferulic acid from maize bran. Carbohyd Polym 45:269–275. https://doi.org/10.1016/S0144-8617(00)00259-9
Smith MM, Hartley RD (1983) Occurrence and nature of ferulic acid substitution of cell-wall polysaccharides in graminaceous plants. Carbohyd Res 118:65–80. https://doi.org/10.1016/0008-6215(83)88036-7
Su R, Ni K, Wang T et al (2019) Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ. https://doi.org/10.7717/peerj.7712
Szwajgier D, Jakubczyk A (2011) Production of extracellular ferulic cid esterases by Lactobacillus strains using natural and synthetic carbon sources. Acta Sci Pol Technol Aliment 10(3):287–302
Tilay A, Bule M, Kishenkumar J, Annapure U (2008) Preparation of ferulic acid from agricultural wastes: Its improved extraction and purification. J Agric Food Chem 56:7644–7648. https://doi.org/10.1021/jf801536t
Wang X, Geng X, Egashira Y, Sanada H (2004) Purification and characterization of a feruloyl esterase from the intestinal bacterium Lactobacillus acidophilus. Appl Environ Microbiol 70:2367–2372. https://doi.org/10.1128/AEM.70.4.2367-2372.2004
Xiros C, Moukouli M, Topakas E, Christakopoulos P (2009) Factors affecting ferulic acid release from Brewer’s spent grain by Fusarium oxysporum enzymatic system. Biores Technol 100:5917–5921. https://doi.org/10.1016/j.biortech.2009.06.018
Xu Z, He H, Zhang S et al (2017) Characterization of feruloyl esterases produced by the four Lactobacillus Species: L. amylovorus, L. acidophilus, L. farciminis and L. fermentum, isolated from ensiled corn stover. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00941
Zhang Q, Li X, Zhao M, Yu Z (2016) Lactic acid bacteria strains for enhancing the fermentation quality and aerobic stability of Leymus chinensis silage. Grass Forage Sci 71:472–481. https://doi.org/10.1111/gfs.12190
Acknowledgements
Abha Sharma acknowledges fellowship received from Department of Science and Technology (File No. LS/700/2016) under Wos-A scheme. All the authors thank ICAR-IARI, Pusa, New Delhi for providing essential facilities for the research work. The authors acknowledge Dr. Radha Prasanna, Professor, IARI, New Delhi for English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, A., Sharma, A., Singh, J. et al. A biorefinery approach for the production of ferulic acid from agroresidues through ferulic acid esterase of lactic acid bacteria. 3 Biotech 10, 367 (2020). https://doi.org/10.1007/s13205-020-02360-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02360-9