Abstract
Rare sugars have recently drawn attention because of their potential applications and huge market demands in the food and pharmaceutical industries. All l-hexoses are considered rare sugars, as they rarely occur in nature and are thus very expensive. l-Hexoses are important components of biologically relevant compounds as well as being used as precursors for certain pharmaceutical drugs and thus play an important role in the pharmaceutical industry. Many general strategies have been established for the synthesis of l-hexoses; however, the only one used in the biotechnology industry is the Izumoring strategy. In hexose Izumoring, four entrances link the d- to l-enantiomers, ketose 3-epimerases catalyze the C-3 epimerization of l-ketohexoses, and aldose isomerases catalyze the specific bioconversion of l-ketohexoses and the corresponding l-aldohexoses. In this article, recent studies on the enzymatic production of various l-hexoses are reviewed based on the Izumoring strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
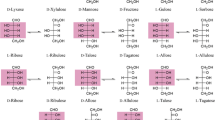
Hexose is a typical monosaccharide and classified into the d- or l-configuration, with the chemical formula C6H12O6. The d-form is usually the isomer found in nature, and the biotechnological production of various d-hexoses has recently been reviewed in detail elsewhere (Mu et al. 2015). Recently, l-hexoses have recently garnered attention because of their potential applications in the pharmaceutical industry (Table 1). l-Hexoses can be categorized to include four l-ketohexoses including l-sorbose, l-psicose, l-tagatose, and l-fructose, and each l-ketohexose corresponds to two l-aldohexoses, giving rise to eight different sugars including l-gulose, l-idose, l-galactose, l-talose, l-altrose, l-allose, l-mannose, and l-glucose.
l-Sugars have often been recognized as important components of biologically relevant compounds and precursors of certain pharmaceutical drug molecules (Ahmed 2001). l-Sorbose has been used as an intermediate for industrial synthesis of l-ascorbic acid (vitamin C) for decades (Pappenberger and Hohmann 2014). l-Tagatose is used as a starting material for synthesis of deoxygalactonojirimycin (DGJ), a pharmacological chaperone, with synergistic activity that has potential implications for treating lysosomal storage disorders (Jenkinson et al. 2011). l-Fructose is a potential nonnutritive sweetener (Levin et al. 1995) and an effective inhibitor of various glycosidases (Muniruzzaman et al. 1996). l-Gulose is an important component of Bleomycin A2, which is employed clinically as an anti-tumor antibiotic (Saitta et al. 2008), and its 6-deoxy derivative, 6-dexoy-l-gulose, is contained in yet another anti-tumor antibiotic, zorbamycin (Wang et al. 2007), while l-gulosamine is a key component of adenomycin, a potential antibacterial agent (Hung et al. 2001). The l-idose derivative l-iduronic acid is an important component of the repeating unit of some glycosaminoglycans (GAGs), including heparin, heparin sulfate, and dermatan sulfate (Seeberger and Werz 2007). l-Talose has been used as substrate to synthesize 9-α-l-talopyranosyladenine, a slow-reacting substrate for calf intestinal adenosine deaminase (EC 3.5.4.4), which inhibits the growth of leukemia L1210 cells in vitro (Lerner and Mennitt 1994). l-Glucose is a major component of littoralisone, a natural bioactive product from Verbena littoralis, which plays a role in enhancing nerve growth factor (NGF)-induced neurite outgrowth in PC12D cells (Li et al. 2001). These findings have greatly increased the interest in research regarding the synthesis of l-hexoses.
By definition, all l-hexoses are classified as rare sugars and are thus very expensive (Granstrom et al. 2004). Limited availability and high costs limit the research and application of l-hexoses. General methodologies for l-hexose synthesis have been widely studied. Chemical synthesis of l-hexoses has recently been reviewed in detail (D’Alonzo et al. 2009; Frihed et al. 2015), while the Izumoring strategy is the preferred method in the biotechnology industry (Granstrom et al. 2004; Izumori 2002). In this article, recent advances in the studies of enzymatic production of various l-hexoses are reviewed. However, due to the scope of this review, l-hexitols were not included.
Entrances to form l-hexoses from d-hexoses
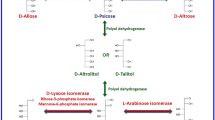
Based on the hexose Izumoring strategy, there are four entrances to form l- from d-hexoses via four d, l-series of hexitols, including d-glucitol (l-gulitol, d-sorbitol), allitol (d-allitol, l-allitol), galactitol (d-galactitol, l-galactitol, dulcitol), and d-gulitol (l-glucitol, l-sorbitol) (Izumori 2006). These four hexitols can be converted to l-ketohexoses by polyol dehydrogenases and are then inevitable passing points to enter the l-hexose world (Fig. 1).
l-Sorbose production from d-sorbitol
l-Sorbose is the most studied l-hexose not only because it is used as an important intermediate for l-ascorbic acid (Pappenberger and Hohmann 2014) but also because the substrate for l-sorbose synthesis, d-sorbitol, is very cheap and easy to obtain. d-Sorbitol can be industrially produced from d-glucose, with theoretically 100 % yield by simple chemical hydrogenation (Zhang et al. 2013a).
l-Sorbose production was firstly reported by Reichstein et al., stating that l-sorbose could be biotechnically synthesized by oxidation of d-sorbitol using Gluconobacter oxydans (previously called Acetobacter suboxydans) pyrroloquinoline quinone (PQQ) enzymes (Reichstein et al. 1933). Since then, bulk l-sorbose production has been widely studied using microbial fermentation by G. oxydans (Giridhar and Srivastava 2001a; Giridhar and Srivastava 2001b; Macauley-Patrick et al. 2005). Furthermore, d-sorbitol 2-dehydrogenase (EC 1.1.1.14) is very responsible for converting d-sorbose to l-sorbose (Shinjoh et al. 2002). Immobilized whole cells were used for efficient production of l-sorbose from d-sorbitol (Kim et al. 1999; Spassov et al. 1995; Wang et al. 2013), and the fermentation of l-sorbose could be significantly enhanced by improving the mRNA abundance of d-sorbitol 2-dehydrogenase in these cells (Xu et al. 2014).
l-Psicose production from allitol
Through specific polyol dehydrogenase, l-psicose could be produced from allitol, which is a scare hexitol and converted from d-psicose. Enzymatic oxidation of allitol to l-psicose was first reported using Acetomonas oxydans (Carr et al. 1968). However, the process was lengthy and required 2 weeks for l-psicose production. Consequently, Takeshita et al. developed a highly efficient production of l-psicose from allitol using the whole cells of Gluconobacter frateurii IFO 3254. The conversion rate was approximately 98 % when 100-g L−1 allitol was used; however, the enzyme responsible for catalyzing this reaction is still unknown (Takeshita et al. 1996).
l-Tagatose production from galactitol
Bioconversion of galactitol to l-tagatose is the third of the four entrances to form l- from d-hexoses (Fig. 1). However, only two publications have focused on l-tagatose production from galactitol. Shimonishi et al. used an isolated l-tagatose-producing bacterial strain from soil, Klebsiella pneumoniae strain 40b, to produce l-tagatose, with a yield of higher than 60 % from 0.5 to 2 % galactitol (Shimonishi et al. 1995). Huwig et al. developed a strategy for enzymatic production of l-tagatose from galactitol using a specific galactitol dehydrogenase from Rhodobacter sphaeroides D, with NADH regeneration by l-lactate dehydrogenase (EC 1.1.1.27) and with the overall yield of l-tagatose reaching 78 % (Huwig et al. 1998).
l-Fructose production from l-glucitol
l-Fructose can theoretically be produced from l-glucitol by enzymatic dehydrogenation and the latter can be produced by dehydrogenation of d-sorbose (Fig. 1). Unfortunately, there are no reports concerning these two steps, but interestingly, the reverse reactions of these two steps have been documented. The l-glucitol dehydrogenase responsible for catalyzing the conversion of l-glucitol to d-sorbose was first identified from Pseudomonas sp. Ac (Mayerskuntzer et al. 1994), and the whole cells produced d-sorbose from l-glucitol, with a yield of higher than 95 % (Huwig et al. 1996). Through hydrogenation, Aureobasidium pullulans LP23, isolated from a soy sauce mash, could convert l-fructose to l-glucitol (Sasahara and Izumori 2005).
C-3 epimerization between l-ketohexoses
Ketose 3-epimerase catalyzes the epimerization of various ketoses at the C-3 position to produce their C-3 epimers. The enzyme generally exhibits a broad specificity toward many ketoses including ketohexoses and ketopentoses, and theoretically, is able to catalyze the epimerization between l-sorbose and l-tagatose and between l-psicose and l-fructose.
In 1993, the enzyme was first reported by Izumori et al., which they isolated from Pseudomonas cichorii (Izumori et al. 1993). The purified enzyme showed C-3 epimerization activity toward various ketoses with the optimum substrate being d-tagatose, and thus, it was classified as d-tagatose 3-epimerase (EC 5.1.3.31) (Itoh et al. 1994). Twelve years later, a second ketose 3-epimerase was identified from Agrobacterium tumefaciens (Kim et al. 2006a), showing the highest activity toward d-psicose and was named d-psicose 3-epimerase (EC 5.1.3.30). In 2009, the third ketose 3-epimerase, with d-fructose being the optimum substrate, was characterized from R. sphaeroides (Zhang et al. 2009). Recently, d-psicose 3-epimerases were identified from Clostridium cellulolyticum (Mu et al. 2011), Ruminococcus sp. 5_1_39BFAA (Zhu et al. 2012), Clostridium scindens (Zhang et al. 2013b), Desmospora sp. 8437 (Zhang et al. 2013c), Clostridium sp. BNL1100 (Mu et al. 2013), Clostridium bolteae (Jia et al. 2014), Dorea sp. CAG317 (Zhang et al. 2015), and Treponema primitia (Zhang et al. 2016). Finally, a ketose 3-epimerase with the optimum substrate of l-ribulose, named l-ribulose 3-epimerase (EC 5.1.3.31), was first identified from Mesorhizobium loti (Uechi et al. 2013b).
Ketose 3-epimerases have attracted considerable attention, because they have a very important role in the Izumoring strategy (Izumori 2006) and in the biological production of d-psicose (Mu et al. 2012). These enzymes are also used for the production of deoxyketohexoses (Gullapalli et al. 2010; Rao et al. 2009), methyl-ketohexoses (Jones et al. 2008; Rao et al. 2008), and some other rare sugars, including d-sorbose (Itoh et al. 1995), l-xylulose (Uechi et al. 2013b), l-tagatose, and l-fructose (Itoh and Izumori 1996). Although they exhibit broad substrate specificity toward various ketoses, the activities of many ketose 3-epimerases toward l-sugars have not been measured, probably due to the difficulty in obtaining l-sugars as substrates commercially. Only the d-psicose 3-epimerase from P. cichorii (Izumori et al. 1993) and the l-ribulose 3-epimerase from M. loti (Uechi et al. 2013b), identified by the Rare Sugar Research Center at Kagawa University, have been used to measure the epimerization activity toward l-ketohexoses. The epimerase isolated from P. cichorii not only displayed 20 % of the relative activity toward l-psicose, compared to the optimum substrate d-tagatose, but also showed trace activity toward l-tagatose, l-sorbose, and l-fructose (Itoh et al. 1994). The epimerase isolated from M. loti exhibited specific activities of 31, 10, and 5.5 U mg−1 toward l-psicose, l-fructose, and l-tagatose, respectively, and by comparison, showed a specific activity of 230 U mg−1 toward the optimum substrate l-ribulose and only trace activity toward l-tagatose (Uechi et al. 2013b).
Except for the oxidative dehydrogenation by polyol dehydrogenase, C-3 epimerization is an efficient approach to biological production of l-ketohexoses. However, only isolated P. cichorii has been used for the enzymatic production of l-ketohexoses. The enzyme was first immobilized on Chitopearl beads BCW 2510, and the immobilized enzyme produced 20 % l-tagatose and 65 % l-fructose from 40 g L−1 of l-sorbose and 20 g L−1 of l-psicose, respectively (Itoh and Izumori 1996). The crystal structure of ketose 3-epimerases from P. cichorii (PDB: 2QUL) (Yoshida et al. 2007), A. tumefaciens (PDB: 2HK0) (Kim et al. 2006b), C. cellulolyticum (PDB: 3VNI) (Chan et al. 2012), and M. loti (PDB: 3VYL) (Uechi et al. 2013a) has been determined and is available in the Protein Data Bank. Based on already known crystal structure information, a molecular modification made to these epimerases would be expected to improve the substrate specificity and enzymatic activity toward l-ketohexoses, and thus, the enzymatic production of l-ketohexoses could be more efficient and widely utilized in the near future.
Isomerization between l-ketohexoses and l-aldohexoses
l-Ketohexoses can be biologically produced through the same entrances from d-hexoses mentioned above. Enzymatic isomerization is an important approach in the production of l-aldohexoses from l-ketohexoses (Fig. 1). Each l-ketohexose corresponds to two different l-ketoaldoses by isomerization. Many aldose isomerases and aldose phosphate isomerases have been identified to catalyze the isomerization between various ketoses and their corresponding aldoses. Unfortunately, none of the reported isomerases shows a high activity toward l-hexoses. Still, some aldose isomerases have been used for l-aldohexose production, including l-rhamnose isomerase (EC 5.3.1.14) (Bhuiyan et al. 1997; Leang et al. 2004a; Takata et al. 2011), l-ribose isomerase (EC 5.3.1.B3) (Terami et al. 2015), and d-arabinose isomerase (EC 5.3.1.3) (Leang et al. 2003). Some aldose phosphate isomerases have also shown potential for the application of l-aldohexose production, including d-ribose-5-phosphate isomerase (EC 5.3.1.6) (Park et al. 2010), d-glucose-6-phosphate isomerase (EC 5.3.1.9) (Yoon et al. 2009b), d-mannose-6-phosphate isomerase (EC 5.3.1.8) (Yeom et al. 2009a), and d-galactose-6-phosphate isomerase (EC 5.3.1.26) (Park et al. 2007). However, for practical industrial applications, it is essential to obtain enzymes that utilize l-hexoses as an optimum substrate, or the specific activity needs to be vastly improved using molecular modification.
Isomerization of l-sorbose to l-gulose and l-idose
To the best of our knowledge, there is no existing literature concerning the biological production of l-gulose and l-idose from l-sorbose. However, it was discovered that the Pyrococcus furiosus d-glucose-6-phosphate isomerase (Yoon et al. 2009b), the Serratia proteamaculans d-lyxose isomerase (EC 5.3.1.15) (Park et al. 2010), and the Cellulomonas parahominis l-ribose isomerase (Morimoto et al. 2013) exhibit isomerization activity toward l-sorbose. Using l-sorbose as the substrate, the P. furiosus d-glucose-6-phosphate isomerase could convert l-sorbose to l-idose and l-gulose by a two-step isomerization mechanism. The final equilibrium ratio of l-sorbose/l-idose/l-gulose is 60:26:14. (Yoon et al. 2009b). In contrast, the S. proteamaculans d-lyxose isomerase and C. parahominis l-ribose isomerase only catalyze one-step isomerization between l-gulose and l-sorbose (Park et al. 2010; Morimoto et al. 2013).
Isomerization of l-tagatose to l-talose and l-galactose
Many aldose and aldose phosphate isomerases can catalyze the isomerization between l-tagatose and l-talose (Table 2). Most of the isomerases produce l-talose as a sole product from l-tagatose. However, P. furiosus d-glucose-6-phosphate isomerase produces two aldo-isomers from l-tagatose, giving a conversion ratio of l-tagatose/l-talose/l-galactose of 80:15:5 (Yoon et al. 2009b). The l-rhamnose isomerase from Pseudomonas stutzeri also produces both l-talose and l-galactose from l-tagatose (Leang et al. 2004b). However, the M. loti l-rhamnose isomerase only catalyzes one-step isomerization between l-tagatose and l-talose (Takata et al. 2011).
Production of l-tagatose from l-talose
The P. furiosus d-glucose-6-phosphate isomerase shows the highest activity toward l-talose among all the tested aldoses, giving the specific activity of 620 U mg−1, and therefore has great potential for l-tagatose production (Yoon et al. 2009b). Streptococcus pneumoniae d-ribose-5-phosphate isomerase, another potential l-tagatose-producing enzyme, catalyzed the isomerization between l-talose and l-tagatose, which could produce 450 g L−1 of l-tagatose from 500 g L−1 of l-talose after a 5-h reaction (Park et al. 2011).
Production of l-talose from l-tagatose
l-Talose production was performed by an M. loti l-rhamnose isomerase immobilized on Chitopearl beads BCW 2510, which converted 300 g L−1 of l-tagatose to 30 g L−1 of l-talose without any byproducts after 12 h of reaction (Takata et al. 2011). The P. stutzeri l-rhamnose isomerase immobilized on BCW 2603 beads was also used for l-talose production, and it produced l-talose from 200 g L−1 of l-tagatose, with a conversion ratio of 12 % (Bhuiyan et al. 1999). However, the P. stutzeri l-rhamnose isomerase normally produces both l-talose and l-galactose from l-tagatose (Leang et al. 2004b), which makes it an unlikely candidate in the production of l-talose on the industrial level.
Production of l-galactose from l-tagatose
The P. stutzeri l-rhamnose isomerase has also been used for l-galactose production. The enzyme immobilized on BCW 2510 beads produced approximately 7.5 g L−1 of l-galactose from 25 g L−1 of l-tagatose, with l-talose production as a minor byproduct during the reactions (Leang et al. 2004a).
Isomerization of l-psicose
Isomerization between l-psicose and l-altrose
There are scarce reports in the literature regarding the isomerization between l-psicose and l-altrose. The P. furiosus d-glucose-6-phosphate isomerase can catalyze the conversion between l-psicose and l-altrose, with specific activities of 251 and 67 U mg−1, respectively. However, the reactions with both substrates produced l-allose as a very minor byproduct (Yoon et al. 2009b).
Isomerization between l-psicose and l-allose
l-Ribose isomerases have potential to be used in the isomerization between l-psicose and l-allose. An l-ribose isomerase isolated from C. parahominis catalyzed l-psicose/l-allose isomerization, with an equilibrium ratio of 65:32 (Morimoto et al. 2013). The enzyme was also used for l-allose production from l-psicose via immobilization on DIAION HPA25L beads. The immobilized enzyme produced 33 g L−1 of l-allose from 100 g L−1 of l-psicose after a 1-day reaction (Terami et al. 2015).
Isomerization of l-fructose
Isomerization between l-fructose and l-mannose
Most of the reported l-rhamnose isomerases prefer l-rhamnose and l-lyxose, respectively, as the first and second aldose substrates, with the third preference toward the production of l-fructose from l-mannose. The specific activities of the enzymes isolated from P. stutzeri, Caldicellulosiruptor saccharolyticus (Lin et al. 2011), Thermotoga maritime (Park et al. 2010), Thermoanaerobacterium saccharolyticum (Hung et al. 2014), Bacillus pallidus (Poonperm et al. 2007), M. loti (Takata et al. 2011), and Bacillus subtilis (Park 2014) toward l-mannose were 81.6, 38, 15, 6, 4.52, 2.27, and 0.92 U mg−1, respectively. In addition, l-fructose has also been produced from l-mannose with a conversion ratio of approximately 25 % by an enzyme reactor packed with cross-linked Streptomyces rubiginosus d-glucose isomerase crystals (Jokela et al. 2002).
Certain l-rhamnose isomerases are also used for l-mannose production. The enzyme isolated from P. stutzeri converted l-fructose to l-mannose, with a yield of 30 % via immobilization on BCW 2603 beads (Bhuiyan et al. 1997). The free enzyme from B. subtilis produced 25 g L−1 of l-mannose from 100 g L−1 of l-fructose after a reaction of 80 min (Park et al. 2010), and the enzyme isolated from T. maritime produced 175 g L−1 of l-mannose from 500 g L−1 of l-fructose after 5 h (Park 2014).
Isomerization between l-fructose and l-glucose
There have been few reports regarding the enzymatic isomerization between l-fructose and l-glucose. An l-glucose-producing d-arabinose isomerase was isolated from Klebsiella pneumoniae, and the enzyme empirically produced 0.35 g of l-glucose from 1.0 g of l-fructose, with an overall yield of 35 % (Leang et al. 2003).
References
Ahmed Z (2001) Production of natural and rare pentoses using microorganisms and their enzymes. Electron J Biotechnol 4(2):13–14
Bhuiyan SH, Itami Y, Izumori K (1997) Immobilization of l-rhamnose isomerase and its application in l-mannose production from l-fructose. J Ferment Bioeng 84(6):558–562
Bhuiyan SH, Itami Y, Takada G, Izumori K (1999) Preparation of l-talose and d-gulose from l-tagatose and d-sorbose, respectively, using immobilized l-rhamnose isomerase. J Biosci Bioeng 88(5):567–570
Carr JG, Coggins RA, Hough L, Stacey BE, Whiting GC (1968) Micro-biological oxidation of allitol to l-ribo-hexulose by Acetomonas oxydans. Phytochemistry 7(1):1–4
Chan HC, Zhu Y, Hu Y, Ko TP, Huang CH, Ren F, Chen CC, Ma Y, Guo RT, Sun Y (2012) Crystal structures of d-psicose 3-epimerase from Clostridium cellulolyticum H10 and its complex with ketohexose sugars. Protein Cell 3(2):123–131
D’Alonzo D, Guaragna A, Palumbo G (2009) Recent advances in monosaccharide synthesis: a journey into l-hexose world. Curr Org Chem 13(1):71–98
Ferreira F, Kenne L, Cotta MA, Stack RJ (1997) Structural studies of the extracellular polysaccharide from Butyrivibrio fibrisolvens strain CF3. Carbohydr Res 301(3–4):193–203
Frihed TG, Bols M, Pedersen CM (2015) Synthesis of l-hexoses. Chem Rev 115(9):3615–3676
Giridhar R, Srivastava AK (2001a) Computer coupled substrate feeding strategies for efficient conversion of d-sorbitol to l-sorbose in fed-batch culture. Process Biochem 36(8–9):829–834
Giridhar R, Srivastava AK (2001b) l-sorbose production in a continuous fermenter with and without cell recycle. World J Microbiol Biotechnol 17(2):185–189
Granstrom TB, Takata G, Tokuda M, Izumori K (2004) Izumoring: a novel and complete strategy for bioproduction of rare sugars. J Biosci Bioeng 97(2):89–94
Gullapalli P, Yoshihara A, Morimoto K, Rao D, Akimitsu K, Jenkinson SF, Fleet GWJ, Izumori K (2010) Conversion of l-rhamnose into ten of the sixteen 1- and 6-deoxyketohexoses in water with three reagents: d-tagatose-3-epimerase equilibrates C3 epimers of deoxyketoses. Tetrahedron Lett 51(6):895–898
Hallak LK, Collins PL, Knudson W, Peeples ME (2000) Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology 271(2):264–275
Hung SC, Wang CC, Chaag SW, Chen CS (2001) 1,6-Anhydro-β-l-hexopyranoses as valuable building blocks toward the synthesis of l-gulosamine and l-altrose derivatives. Tetrahedron Lett 42(7):1321–1324
Hung XG, Tseng WC, Liu SM, Tzou WS, Fang TY (2014) Characterization of a thermophilic l-arabinose isomerase from Thermoanaerobacterium saccharolyticum NTOU1. Biochem Eng J 83:121–128
Huwig A, Emmel S, Giffhorn F (1996) Preparation of d-sorbose from l-glucitol by bioconversion with Pseudomonas sp Ac. Carbohydr Res 281(1):183–186
Huwig A, Emmel S, Jakel G, Giffhorn F (1998) Enzymatic synthesis of l-tagatose from galactitol with galactitol dehydrogenase from Rhodobacter sphaeroides D. Carbohydr Res 305(3):337–339
Itoh H, Izumori K (1996) Enzymatic production of l-tagatose and l-fructose from l-sorbose and l-psicose, respectively. J Ferment Bioeng 81(4):351–353
Itoh H, Okaya H, Khan AR, Tajima S, Hayakawa S, Izumori K (1994) Purification and characterization of d-tagatose 3-epimerase from Pseudomonas sp. ST-24. Biosci Biotechnol Biochem 58(12):2168–2171
Itoh H, Sato T, Takeuchi T, Khan AR, Izumori K (1995) Preparation of d-sorbose from d-tagatose by immobilized d-tagatose 3-epimerase. J Ferment Bioeng 79(2):184–185
Izumori K (2002) Bioproduction strategies for rare hexose sugars. Naturwissenschaften 89(3):120–124
Izumori K (2006) Izumoring: a strategy for bioproduction of all hexoses. J Biotechnol 124(4):717–722
Izumori K, Khan AR, Okaya H, Tsumura T (1993) A new enzyme, d-ketohexose 3-epimerase, from Pseudomonas sp. ST-24. Biosci Biotechnol Biochem 57(6):1037–1039
Jenkinson SF, Fleet GWJ, Nash RJ, Koike Y, Adachi I, Yoshihara A, Morimoto K, Izumori K, Kato A (2011) Looking-glass synergistic pharmacological chaperones: DGJ and l-DGJ from the enantiomers of tagatose. Org Lett 13(15):4064–4067
Jia M, Mu W, Chu F, Zhang X, Jiang B, Zhou LL, Zhang T (2014) A d-psicose 3-epimerase with neutral pH optimum from Clostridium bolteae for d-psicose production: cloning, expression, purification, and characterization. Appl Microbiol Biotechnol 98(2):717–725
Jokela J, Pastinen O, Leisola M (2002) Isomerization of pentose and hexose sugars by an enzyme reactor packed with cross-linked xylose isomerase crystals. Enzym Microb Technol 31(1):67–76
Jones NA, Rao D, Yoshihara A, Gullapalli P, Morimoto K, Takata G, Hunter SJ, Wormald MR, Dwek RA, Izumori K, Fleet GWJ (2008) Green syntheses of new 2-C-methyl aldohexoses and 5-C-methyl ketohexoses: d-tagatose-3-epimerase (DTE)-a promiscuous enzyme. Tetrahedron Asymmetry 19(16):1904–1918
Kim HJ, Hyun EK, Kim YS, Lee YJ, Oh DK (2006a) Characterization of an Agrobacterium tumefaciens d-psicose 3-epimerase that converts d-fructose to d-psicose. Appl Environ Microbiol 72(2):981–985
Kim HJ, Kim JH, Shin CS (1999) Conversion of d-sorbitol to l-sorbose by Gluconobacter suboxydans cells co-immobilized with oxygen-carriers in alginate beads. Process Biochem 35(3):243–248
Kim K, Kim HJ, Oh DK, Cha SS, Rhee S (2006b) Crystal structure of d-psicose 3-epimerase from Agrobacterium tumefaciens and its complex with true substrate d-fructose: a pivotal role of metal in catalysis, an active site for the non-phosphorylated substrate, and its conformational changes. J Mol Biol 361(5):920–931
Leang K, Maekawa K, Menavuvu BT, Morimoto K, Granstrom TB, Takada G, Izumori K (2004a) A novel enzymatic approach to the massproduction of l-galactose from l-sorbose. J Biosci Bioeng 97(6):383–388
Leang K, Sultana I, Takada G, Izumori K (2003) A novel bioconversion of l-fructose to l-glucose by Klebsiella pneumoniae. J Biosci Bioeng 95(3):310–312
Leang K, Takada G, Fukai Y, Morimoto K, Granstrom TB, Izumori K (2004b) Novel reactions of l-rhanmose isomerase from Pseudomonas stutzeri and its relation with d-xylose isomerase via substrate specificity. Biochim Biophys Acta Gen Subj 1674(1):68–77
Lerner LM, Mennitt G (1994) A new synthesis of l-talose and preparation of its adenine nucleosides. Carbohydr Res 259(2):191–200
Levin GV, Zehner LR, Saunders JP, Beadle JR (1995) Sugar substitutes: their energy values, bulk characteristics, and potential health benefits. Am J Clin Nutr 62(5):1161–1168
Li YS, Matsunaga K, Ishibashi M, Ohizumi Y (2001) Littoralisone, a novel neuritogenic iridolactone having an unprecedented heptacyclic skeleton including four- and nine-membered rings consisting of glucose from Verbena littoralis. J Org Chem 66(6):2165–2167
Lin CJ, Tseng WC, Fang TY (2011) Characterization of a thermophilic l-rhamnose isomerase from Caldicellulosiruptor saccharolyticus ATCC 43494. J Agric Food Chem 59(16):8702–8708
Macauley-Patrick S, McNeil B, Harvey LA (2005) By-product formation in the d-sorbitol to l-sorbose biotransformation by Gluconobacter suboxydans ATCC 621 in batch and continuous cultures. Process Biochem 40(6):2113–2122
Mayerskuntzer H, Reichert A, Schneider KH, Giffhorn F (1994) Isolation and characterization of a l-glucitol dehydrogenase from the newly isolated bacterium Pseudomonas sp Ac. J Biotechnol 36(2):157–164
Morimoto K, Terami Y, Y-i M, Yoshihara A, Takata G, Izumori K (2013) Cloning and characterization of the l-ribose isomerase gene from Cellulomonas parahominis MB426. J Biosci Bioeng 115(4):377–381
Mu W, Chu F, Xing Q, Yu S, Zhou L, Jiang B (2011) Cloning, expression, and characterization of a d-psicose 3-epimerase from Clostridium cellulolyticum H10. J Agric Food Chem 59(14):7785–7792
Mu W, Yu L, Zhang W, Zhang T, Jiang B (2015) Isomerases for biotransformation of d-hexoses. Appl Microbiol Biotechnol 99(16):6571–6584
Mu W, Zhang W, Fang D, Zhou L, Jiang B, Zhang T (2013) Characterization of a d-psicose-producing enzyme, d-psicose 3-epimerase, from Clostridium sp. Biotechnol Lett 35(9):1481–1486
Mu W, Zhang W, Feng Y, Jiang B, Zhou L (2012) Recent advances on applications and biotechnological production of d-psicose. Appl Microbiol Biotechnol 94(6):1461–1467
Muniruzzaman S, Pan YT, Zeng Y, Atkins B, Izumori K, Elbein AD (1996) Inhibition of glycoprotein processing by l-fructose and l-xylulose. Glycobiology 6(8):795–803
O’Neill MA, Ishii T, Albersheim P, Darvill AG (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55:109–139
Pappenberger G, Hohmann HP (2014) Industrial production of l-ascorbic acid (vitamin C) and d-isoascorbic acid. Biotechnol Food Feed Addit, Springer, Berlin Heidelberg, pp 143–188
Park CS (2014) Characterization of a recombinant l-rhamnose isomerase from Bacillus subtilis and its application on production of l-lyxose and l-mannose. Biotechnol Bioprocess Eng 19(1):18–25
Park CS, Yeom SJ, Lim YR, Kim YS, Oh DK (2010) Characterization of a recombinant thermostable l-rhamnose isomerase from Thermotoga maritima ATCC 43589 and its application in the production of l-lyxose and l-mannose. Biotechnol Lett 32(12):1947–1953
Park CS, Yeom SJ, Lim YR, Kim YS, Oh DK (2011) Substrate specificity of a recombinant ribose-5-phosphate isomerase from Streptococcus pneumoniae and its application in the production of l-lyxose and l-tagatose. World J Microbiol Biotechnol 27(4):743–750
Park HY, Park CS, Kim HJ, Oh DK (2007) Substrate specificity of a galactose 6-phosphate isomerase from Lactococcus lactis that produces d-allose from d-psicose. J Biotechnol 132(1):88–95
Poonperm W, Takata G, Okada H, Morimoto K, Granstrom TB, Izumori K (2007) Cloning, sequencing, overexpression and characterization of l-rhamnose isomerase from Bacillus pallidus Y25 for rare sugar production. Appl Microbiol Biotechnol 76(6):1297–1307
Rao D, Best D, Yoshihara A, Gullapalli P, Morimoto K, Wormald MR, Wilson FX, Izumori K, Fleet GWJ (2009) A concise approach to the synthesis of all twelve 5-deoxyhexoses: d-tagatose-3-epimerase—a reagent that is both specific and general. Tetrahedron Lett 50(26):3559–3563
Rao D, Yoshihara A, Gullapalli P, Morinioto K, Takata G, da Cruz FP, Jenkinson SF, Wormald MR, Dwek RA, Fleet GWJ, Izumori K (2008) Towards the biotechnological isomerization of branched sugars: d-tagatose-3-epimerase equilibrates both enantiomers of 4-C-methyl-ribulose with both enantiomers of 4-C-methyl-xylulose. Tetrahedron Lett 49(20):3316–3321
Reichstein T, Grüssner A, Oppenauer R (1933) Synthese der d- und l-ascorbinsäure (C-vitamin). Helv Chim Acta 16(1):1019–1033
Saitta P, Krishnamurthy K, Brown LH (2008) Bleomycin in dermatology: a review of intralesional applications. Dermatol Surg 34(10):1299–1313
Sasahara H, Izumori K (2005) Production of l-sorbitol from l-fructose by Aureobasidium pullulans LP23 isolated from soy sauce mash. J Biosci Bioeng 100(2):223–226
Sasamori H, Reddy KS, Kirkup MP, Shabanowitz J, Lynn DG, Hecht SM, Woode KA, Bryan RF, Campbell J, Lynn WS, Egert E, Sheldrick GM (1983) New cytotoxic principles from Datisca glomerata. J Chem Soc Perkin Trans 1:1333–1347
Seeberger PH, Werz DB (2007) Synthesis and medical applications of oligosaccharides. Nature 446(7139):1046–1051
Shimonishi T, Okumura Y, Izumori K (1995) Production of l-tagatose from galactitol by Klebsiella pneumoniae strain 40b. J Ferment Bioeng 79(6):620–622
Shinjoh M, Tazoe M, Hoshino T (2002) NADPH-dependent l-sorbose reductase is responsible for l-sorbose assimilation in Gluconobacter suboxydans IFO 3291. J Bacteriol 184(3):861–863
Spassov G, Christov P, Pramatarova V (1995) Conversion of d-sorbit to l-sorbose by cells of Acetobacter suboxydans immobilized in sintered glass. Appl Microbiol Biotechnol 43(1):35–37
Takata G, Uechi K, Taniguchi E, Kanbara Y, Yoshihara A, Morimoto K, Izumori K (2011) Characterization of Mesorhizobium loti l-rhamnose isomerase and its application to l-talose production. Biosci Biotechnol Biochem 75(5):1006–1009
Takeshita K, Shimonishi T, Izumori K (1996) Production of l-psicose from allitol by Gluconobacter frateurii IFO 3254. J Ferment Bioeng 81(3):212–215
Terami Y, Uechi K, Nomura S, Okamoto N, Morimoto K, Takata G (2015) Production of l-allose and d-talose from l-psicose and d-tagatose by l-ribose isomerase. Biosci Biotechnol Biochem 79(10):1725–1729
Uechi K, Sakuraba H, Yoshihara A, Morimoto K, Takata G (2013a) Structural insight into l-ribulose 3-epimerase from Mesorhizobium loti. Acta Crystallogr Sect D 69(12):2330–2339
Uechi K, Takata G, Fukai Y, Yoshihara A, Morimoto K (2013b) Gene cloning and characterization of l-ribulose 3-epimerase from Mesorhizobium loti and its application to rare sugar production. Biosci Biotechnol Biochem 77(3):511–515
Wang LY, Yun BS, George NP, Wendt-Pienkowski E, Galm U, Oh TJ, Coughlin JM, Zhang GD, Tao MF, Shen B (2007) Glycopeptide antitumor antibiotic zorbamycin from Streptomyces flavoviridis ATCC 21892: strain improvement and structure elucidation. J Nat Prod 70(3):402–406
Wang XB, Liu J, Du GC, Zhou JW, Chen J (2013) Efficient production of l-sorbose from d-sorbitol by whole cell immobilization of Gluconobacter oxydans WSH-003. Biochem Eng J 77:171–176
Xu S, Wang XB, Du GC, Zhou JW, Chen J (2014) Enhanced production of l-sorbose from d-sorbitol by improving the mRNA abundance of sorbitol dehydrogenase in Gluconobacter oxydans WSH-003. Microb Cell Factories 13(1):1
Yamazaki M, Thorne L, Mikolajczak M, Armentrout RW, Pollock TJ (1996) Linkage of genes essential for synthesis of a polysaccharide capsule in Sphingomonas strain S88. J Bacteriol 178(9):2676–2687
Yeom SJ, Ji JH, Kim NH, Park CS, Oh DK (2009a) Substrate specificity of a mannose-6-phosphate isomerase from Bacillus subtilis and its application in the production of l-ribose. Appl Environ Microbiol 75(14):4705–4710
Yeom SJ, Kim NH, Yoon RY, Kwon HJ, Park CS, Oh DK (2009b) Characterization of a mannose-6-phosphate isomerase from Geobacillus thermodenitrificans that converts monosaccharides. Biotechnol Lett 31(8):1273–1278
Yoon RY, Yeom SJ, Kim HJ, Oh DK (2009a) Novel substrates of a ribose-5-phosphate isomerase from Clostridium thermocellum. J Biotechnol 139(1):26–32
Yoon RY, Yeom SJ, Park CS, Oh DK (2009b) Substrate specificity of a glucose-6-phosphate isomerase from Pyrococcus furiosus for monosaccharides. Appl Microbiol Biotechnol 83(2):295–303
Yoshida H, Yamada M, Nishitani T, Takada G, Izumori K, Kamitori S (2007) Crystal structures of d-tagatose 3-epimerase from Pseudomonas cichorii and its complexes with d-tagatose and d-fructose. J Mol Biol 374(2):443–453
Zhang L, Mu W, Jiang B, Zhang T (2009) Characterization of d-tagatose-3-epimerase from Rhodobacter sphaeroides that converts d-fructose into d-psicose. Biotechnol Lett 31(6):857–862
Zhang W, Fang D, Xing Q, Zhou L, Jiang B, Mu W (2013a) Characterization of a novel metal-dependent d-psicose 3-epimerase from Clostridium scindens 35704. PLoS One 8(4):e62987
Zhang W, Fang D, Zhang T, Zhou L, Jiang B, Mu W (2013b) Characterization of a metal-dependent d-psicose 3-epimerase from a novel strain, Desmospora sp. 8437. J Agric Food Chem 61(47):11468–11476
Zhang W, Li H, Zhang T, Jiang B, Zhou L, Mu W (2015) Characterization of a d-psicose 3-epimerase from Dorea sp. CAG317 with an acidic pH optimum and a high specific activity. J Mol Catal B Enzym 120:68–74
Zhang W, Zhang T, Jiang B, Mu W (2016) Biochemical characterization of a d-psicose 3-epimerase from Treponema primitia ZAS-1 and its application on enzymatic production of d-psicose. J Sci Food Agric 96(1):49–56
Zhu Y, Men Y, Bai W, Li X, Zhang L, Sun Y, Ma Y (2012) Overexpression of d-psicose 3-epimerase from Ruminococcus sp. in Escherichia coli and its potential application in d-psicose production. Biotechnol Lett 34(10):1901–1906
Acknowledgments
This work was supported by the NSFC Project (No. 21276001), the 863 Project (No. 2013AA102102), the Support Project of Jiangsu Province (No. BK20130001 and 2015-SWYY-009), and the project of Outstanding Scientific and Technological Innovation Group of Jiangsu Province (Jing Wu).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, Z., Zhang, W., Zhang, T. et al. Advances in the enzymatic production of l-hexoses. Appl Microbiol Biotechnol 100, 6971–6979 (2016). https://doi.org/10.1007/s00253-016-7694-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7694-2