Abstract
Catalpa bungei is an economically important native hardwood in China whose hygroscopic behavior is vital for industrial applications as it influences the final product’s dimensional stability and mechanical properties. In this study, the adsorption and desorption behavior of earlywood and latewood in the same growth ring of C. bungei wood samples was documented using a dynamic vapor sorption resolution and analyzed using the Guggenheim–Andersen–de Boer (GAB) model. The earlywood and latewood exhibited varying sorption isotherms and hysteresis degrees, and the reasons were analyzed in terms of structure and chemical composition (mainly hemicellulose and lignin). The influence of benzene–alcohol extracts and vessels on sorption characteristics was also examined. The GAB model perfectly fits the experimental data (R2 ≥ 99.7%) over the full relative humidity range. Specifically, parameters such as the internal specific surface area of wood can be obtained from the GAB model to help explain the differences in sorption properties between earlywood and latewood. The maximum water content bound to the primary sites for earlywood and latewood is 6.87% and 7.47%, respectively. Correspondingly, two internal specific surface areas are 261 m2/g and 284 m2/g, respectively. The adsorption isotherms of earlywood and latewood in C. bungei cannot be fully classified as type II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Moisture content is an important factor affecting wood’s performance and utility. The wood–water relation, including the adsorption/desorption behavior, is still the focus of research both from theoretical and practical points of view (Hill and Xie 2011; Hill et al. 2015; Salmén and Larsson 2018; Chen et al. 2020; Hou et al. 2022). Meanwhile, the wood structure is highly heterogeneous and hierarchically organized; therefore, the moisture sorption behavior of wood in response to relative humidity (RH) variations is complex (Bonnet et al. 2017). Several studies have shown that the sorption isotherm differs between wood species (Popper and Niemz 2009; Albrektas and Ukvalbergienė 2015; Gao et al. 2019; Ouertani et al. 2022). Moreover, the sorption characteristics of different sampling positions of the same wood species and the sorption behavior between juvenile and mature wood also differ (Lenth and Kamke 2001; Majka and Olek 2008; Esteban et al. 2015; Lopes et al. 2022; Garcia et al. 2022). The heartwood and sapwood in wood can also influence the sorption isotherm (Ball et al. 2001; Obataya et al. 2006; Broda et al. 2019; Quartey et al. 2021; Lopes et al. 2022). Experiments have also revealed differences in hygroscopicity between normal and compression (tension) wood (Gorišek and Straze 2006; Huda et al. 2018; Zhan et al. 2021; Majka et al. 2022a). The hygroscopicity differences of samples studied above are due to variations in structure and chemical composition. Earlywood and latewood are derived from the same cambial initial cells in the cambium layer, while their cell morphology and cell wall structure differ dramatically according to the season. Earlywood cells are produced during the spring growth period and have expanded lumens and relatively thin cell walls. Latewood tracheid has smaller lumens with thicker cell walls that provide the mechanical strength to support the large tree size (Kurata et al. 2018). Therefore, theoretically, the structure and chemical composition of earlywood and latewood will be different, affecting their sorption properties.
As early as the 1970s, Ahlgren et al. (1972) began to study the differences in sorption characteristics between earlywood and latewood. The fiber saturation point (FSP) was determined to be higher in earlywood than in latewood in Douglas fir (Pseudotsuga menziesii) and aspen (Populus tremuloides) by the exclusion solute method. However, due to the technical level constraints, the measured FSP was too high, and the accuracy remains to be discussed. Kärenlampi et al. (2005) showed that the equilibrium moisture content (EMC) of spruce latewood was slightly higher than that of earlywood, and the EMC of the two did not change after multiple moisture sorption cycles. Derome et al. (2011) and Patera et al. (2013) also showed that the EMC of Norway spruce (Picea abies) latewood was higher than that of earlywood at the same RH, attributed to the thicker S2 layer of latewood. Their article focused on the swelling and shrinkage of wood, and did not deeply explore the sorption characteristics of earlywood and latewood. Hill et al. (2015) used a dynamic vapor sorption (DVS) analyzer to study the sorption characteristics of earlywood and latewood in different annual rings of Japanese larch wood (Larix kaempferi), confirming the difference between the two, showing that the hygroscopicity of earlywood was stronger than that of latewood in the same growth ring, which became more pronounced as the distance from the pith increased. In addition, the results indicated that the sorption isotherms of earlywood had good reproducibility after two sorption cycles, while the sorption isotherms of latewood showed significant differences. Research by Bonnet et al. (2017) showed that the difference in sorption isotherms between earlywood and latewood was caused by the difference in sorption capacity, and the environment and role of bound water in earlywood and latewood was similar. However, not all studies were as speculated; that is, the sorption characteristics of earlywood and latewood were different. Neimsuwan et al. (2008), Sargent et al. (2010), Sharratt et al. (2010) and Hill et al. (2011) studied the sorption isotherms of earlywood and latewood of loblolly pine (Pinus taeda), radiata pine (Pinus radiata), Scots pine (Pinus sylvestris) and Sitka spruce (Picea sitchensis), respectively. The results indicated that the sorption isotherms of earlywood and latewood almost overlapped under any RH condition. Compared with latewood, earlywood responded more rapidly to changes in environment RH, and the sorption rate of earlywood was higher than that of latewood. Therefore, it is still controversial whether the sorption characteristics of earlywood and latewood are different, and further discussion and research are needed.
Desorption gives higher EMC than adsorption at equal environmental conditions, a phenomenon termed sorption hysteresis. Hysteresis can be interpreted by the ‘ink-bottle’ theory (McBain 1935), the hydroxyl groups concentrations participating in adsorption and desorption (Urquhart 1960), or the ‘contact angle’ theory (Chen and Wangaard 1968), changes in the free volume in the glassy state of the polymer (Vrentas and Vrentas 1996) and the formation of metastable states of adsorbate in fixed pores (Sander et al. 2005). Both the ‘ink-bottle’ and ‘contact angle’ theories assume the presence of liquid water, that is, capillary water. Based on the conceptual framework of Vrentas and Vrentas (1996), researchers proposed that the sorption hysteresis of wood is related to changes in the softening properties of the constituent wood polymers during water vapor sorption; in other words, adsorption and desorption take place in materials with different physical properties (Hill et al. 2010; Engelund et al. 2013; Fredriksson and Thybring 2018; Salmén and Larsson 2018). Although the sorption hysteresis behavior of wood has been documented, the investigations on the sorption hysteresis behavior of earlywood and latewood were very limited.
The aim of this study was to investigate the sorption behavior of earlywood and latewood in the same growth ring of C. bungei and explain mechanisms in terms of their sorption behavior. Adsorption and desorption behavior of earlywood and latewood in the same growth ring of C. bungei wood samples was documented using a dynamic vapor sorption (DVS) resolution. The results were analyzed using the Guggenheim–Andersen–de Boer (GAB) model, and the sorption isotherms and hysteresis of C. bungei earlywood and latewood were compared. The effects of structure and chemical components on earlywood and latewood’s sorption isotherms and hysteresis were discussed.
Materials and methods
Sample preparations
Clear wood samples without any visible defects or knots were cut from the 32nd growth ring of the heartwood of a 44-year-old C. bungei tree. The earlywood and latewood from the 32nd growth ring were used because they have representative oven-dry density. Most importantly, the 32nd growth ring is wider than other growth rings allowing obtaining an equal volume of earlywood and latewood samples. Earlywood and latewood samples were rectangular solids within 2.5 (radial) × 4 (tangential) × 4 (longitudinal) mm3 dimensions. All samples were dried in a sealed container with phosphorus pentoxide at room temperature until a constant mass was achieved. The oven-dry density of earlywood and latewood was 330 kg/m3 and 410 kg/m3, respectively. The average values of five replicates per sample are reported.
Determination of chemical components
The contents of cellulose, hemicellulose and lignin in earlywood and latewood of C. bungei were determined by Van Soest’s analytical method, and the results are shown in Table 1. The Soxhlet extraction-rotary evaporation method was used to determine the benzene–alcohol extract content, and the results are shown in Table 2.
Dynamic water vapor sorption
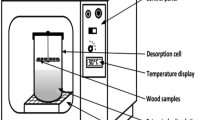
Wood samples’ water vapor sorption behavior was determined using a dynamic vapor sorption apparatus (DVS Resolution, Surface Measurement Systems, UK). The samples were exposed to relative humidity (RH) variations for adsorption and desorption, as shown in Fig. 1. The adsorption and desorption periods were taken at a constant temperature of 25 ± 0.1 °C for approximately 9 days. Adsorption started at 0% RH and increased in 10% RH increments up to 95% RH, and desorption decreased to 0% RH, also in 10% RH decrements. Referring to the findings of Glass et al. (2017, 2018), combined with the authors’ previous experimental experience (Ouyang et al. 2022), samples were maintained at a constant RH, until the weight change was less than 0.002% min−1 for 10 min and then continued at the current RH for 180 min. Data on weight changes were acquired every minute.
Guggenheim–Andersen–de Boer (GAB) model
The GAB model, frequently applied to modeling the sorption isotherms of bamboo and wood (Bratasz et al. 2012; Olek et al. 2013; Florisson et al. 2020; Su et al. 2020; Charupeng and Kunthong 2022; Majka et al. 2022b), was used to describe the water vapor sorption isotherms of earlywood and latewood samples because it is capable of describing the full shape of type II isotherm and yields meaningful physical parameters (Hartley 2000; Timmermann 2003), and the GAB parameters can provide the internal specific surface area of water. The GAB model is based on the theoretical concept of multilayer sorption. The model describes water molecules bonding to sorption sites to form a monomolecular layer; water molecules in the monolayer become secondary sorption sites, and additional water layers are formed (multilayer sorption) (Basu et al. 2006). Briefly, the model equations were as follows:
where EMC refers to the equilibrium moisture content of earlywood and latewood (%); Xm is the monolayer capacity (%); C is the equilibrium constant related to the monolayer sorption; K is the equilibrium constant related to the multilayer sorption; and RH is the relative humidity (%). Origin 2018 (Origin Lab Corporation, Northampton, MA, USA) analysis software was used to obtain the models’ parameters by the least square method and fit isothermal adsorption data.
Results and discussion
Adsorption and desorption isotherms
The water vapor adsorption and desorption isotherms for earlywood and latewood samples reported as EMC against RH are depicted in Fig. 2. The adsorption–desorption isotherms for all samples formed a closed loop. Meanwhile, all samples showed noticeable moisture sorption hysteresis over the full RH range. The EMC of each sample in the adsorption isotherm was lower than that in the desorption isotherm. However, the EMC values of the two sample types differ during the sorption process. During adsorption (0–95% RH) or desorption (95–0% RH), the EMC of the latewood sample was higher than that of the earlywood under any RH. At the highest RH (95%), earlywood and latewood samples reached EMCs of 14.54% and 15.66%, respectively. Structurally, earlywood and latewood densities were 330 kg/m3 and 480 kg/m3, respectively. On the one hand, earlywood contains a larger lumen and thinner cell wall, while latewood consists of thick-walled cells with small lumens. On the other hand, hygroscopic behavior has also shown the difference in the main components of the cell wall, as hemicelluloses provide the highest number of OH groups available for water sorption, followed by cellulose and lignin (Christensen and Kelsey 1959). It can be seen from Table 1 that the content of cellulose and hemicellulose in latewood was higher than that in earlywood, and the content of lignin was lower than that in earlywood. Therefore, compared with earlywood, latewood has a higher density, higher cellulose content, higher hemicellulose content and lower lignin content, so it has more accessible OH groups (Bertaud and Holmbom 2004; Bonnet et al. 2017; Kurata et al. 2018). While the large earlywood vessels commonly become embolized or occluded with tyloses by the end of the growing season in ring-porous tree species, blocking the sorption of the earlywood cell wall (Li et al. 2019). In contrast, the smaller and safer latewood vessels may remain functional for many years (Kitin and Funada 2016).
In addition, during adsorption at 80–95% RH, the sorption rate of the earlywood and latewood suddenly increased. The reason for this sudden increase in the upper end of the hygroscopic range was unknown. The different EMC values between earlywood and latewood under the same RH during moisture adsorption (desorption) are shown in Fig. 3. In water adsorption or desorption, the different EMC values between earlywood and latewood increased first and then decreased. The turning point was 80% RH. It could be speculated that the number of micro–nano-pores in the earlywood is higher than in latewood (Engelund et al. 2013); more capillary condensed water was formed when the RH was higher than 80%, hence the phenomenon shown in Fig. 3.
Sorption hysteresis
Absolute hysteresis (obtained by subtracting the EMC of adsorption from the EMC of desorption isotherm at a constant RH) and the hysteresis coefficient (the EMC for adsorption to EMC for desorption ratio at constant RH) are two common moisture sorption hysteresis characterization methods (Olek et al. 2013; Zhang et al. 2018; Ouyang et al. 2022). The changes in the absolute moisture sorption hysteresis and moisture sorption hysteresis coefficients are shown in Fig. 4. As shown in Fig. 4a, the absolute hysteresis values of the earlywood and latewood samples were 1.05–2.78% and 0.92–2.56%, respectively. Regardless of earlywood or latewood, when the RH was 10–70%, the absolute hysteresis increased with the increase in RH; when the RH was 70–90%, the absolute hysteresis decreased with the increase in RH. Many previous studies (Bertolin et al. 2020; Chen et al. 2020; García-Iruela et al. 2020) had also observed this phenomenon. The absolute hysteresis of samples decreased at 70% RH, most likely due to hemicelluloses softening (Olsson and Salmén 2003). One explanation is that it crosses the glass transition point at this moisture range at room temperature, allowing the accommodation of more water molecules within the wood’s cell wall (Engelund et al. 2013). Another explanation is that when a glassy solid absorbs or desorbs water, it is affected by the “rigidity” of the macromolecules. It is likely that molecular chains cannot be quickly arranged to adapt to the entry and exit of water molecules, so the adsorption and desorption processes occur in different physical environments and cause hysteresis (Hill et al. 2009, 2010); while RH is higher than 70%, the molecule is in a rubbery state, it is more flexible and can respond immediately to the entry and exit of water molecules. Hence, the hysteresis weakens or even disappears (Hou et al. 2022). In addition, under any RH condition, earlywood absolute hysteresis was larger than that of latewood, which is also due to earlywood lignin content being higher than that of latewood (27.38% > 21.95%); higher lignin content will cause more absolute hysteresis in the wood cell wall (Hill et al. 2009; Kulasinski et al. 2015; Derome et al. 2018; Yang et al. 2018). There are many unsaturated groups in the molecular structure of lignin, reducing the lignin molecules’ flexibility and increasing hysteresis (Lu and Pignatello 2004). Hemicellulose also affects absolute hysteresis. Zhou et al. (2016) found that the higher the hemicellulose content, the greater the absolute hysteresis. According to Hou et al. (2022), this was because the complex network formed between hemicellulose and lignin reduces the mobility of hemicellulose; the molecular chain cannot be arranged quickly enough to adapt to the entry and exit of water molecules, showing an absolute hysteresis increase. However, Table 3 shows that the lignin content of latewood was lower than in earlywood (21.95% < 27.38%), while the hemicellulose content was higher than in earlywood (16.16% > 12.13%). Moreover, from previous studies (Hill et al. 2009; Kulasinski et al. 2015; Zhou et al. 2016; Derome et al. 2018; Yang et al. 2018; Hou et al. 2022), lignin and hemicellulose contents were positively correlated with the absolute hysteresis. Therefore, it is speculated that lignin has a more pronounced influence on absolute hysteresis than hemicellulose. Furthermore, many tyloses in vessels of earlywood can also increase absolute hysteresis. The existence of many tyloses in the vessels of earlywood exerts a physical barrier effect on the moisture sorption of earlywood (Li et al. 2019).
As shown in Fig. 4b, the hysteresis coefficient ranges of earlywood and latewood were 0.56–0.91 and 0.65–0.95, respectively. Under any RH condition, the hysteresis coefficient of latewood was larger than that of earlywood. Zhang et al. (2018) calculated the hysteresis coefficient of fourteen kinds of bamboos, and the hysteresis coefficient with an average of 0.88; the average hysteresis coefficient of rattan was 0.80 (Yang et al. 2021). However, hysteresis coefficients in earlywood and latewood of C. bungei were 0.70 and 0.77, respectively. Compared with rattan and bamboo, hysteresis coefficient of C. bungei was relatively small, which might be related to its low extract content, as shown in Table 2. It is known that the presence of extractives in wood can influence the sorption isotherm (Popper et al. 2007). The presence of extractives clogs pores and other channels inside the material preventing the free entry and exit of water molecules (Kymäläinen et al. 2018). Therefore, the hysteresis coefficient increased. It could also be seen that the extract content of earlywood was the lowest, so its hysteresis coefficient was less than that of latewood.
The water sorption hysteresis variation of different samples seems to be a very complex phenomenon. Hou et al. (2022) showed in their research that the interaction and cross-linking between wood components influenced hysteresis. Hence, a more detailed study is required to explain the difference in the water sorption hysteresis behavior of different samples.
Fitting the GAB model to the date
The results of fitting the GAB model to the data of the earlywood and latewood samples are presented in Table 3. The GAB model perfectly fits the experimental data (R2 ≥ 99.7%) over the full RH range. The fits were valid as all the R2 values were above 99.0% (Esteban et al. 2015). GAB parameters can be used to compare the hygroscopic properties of the earlywood and latewood samples. Among them, the Xm obtained from the adsorption branch refers to the moisture content when the monolayer is full and can be used to estimate the internal specific surface area corresponding to the monolayer capacity. The internal specific surface area (SGAB) is defined as
where ρ is water density; NA is the Avogadro number, 6.022 × 1023; σ is the average area where water occupies the complete monolayer (0.114 nm2 was used in this study), and M is the molar mass of water, 18 g/mol. As shown in Table 3, Xm of earlywood and latewood samples is 6.87% and 7.47% for adsorption, respectively. Correspondingly, SGAB of the two is 261 m2/g and 284 m2/g, respectively. Both Xm and SGAB are higher in the latewood samples than in the earlywood samples, corresponding to the greater cellulose and hemicellulose content in latewood than in earlywood (Table 1), indicating that both the hydrophilic group content and the monolayer adsorption capacity are greater in the latewood than in the earlywood. As shown in Table 3, Xm and SGAB of the earlywood and latewood of the hardwood C. bungei are similar to those of rattan and higher than bamboo and some softwoods, indicating that their hydrophilic group content is greater than that of bamboo and some softwoods. The Xm values were consistently lower for adsorption than desorption processes (Olek et al. 2013; Majka et al. 2022b). This is consistent with the results reported by Krupińska et al. (2007) and indicates that during adsorption, the binding energy between the active sites and multilayer water molecules is higher than during desorption.
The values of the C in Table 3 are higher than 2 for earlywood and latewood samples. Therefore, the necessary condition for classifying the isotherms as type II was satisfied (Olek et al. 2013; Majka et al. 2022b). Lewicki (1997) states that type II isotherms should also satisfy the following two inequalities, where 5.57 ≤ C < ∞ and 0.24 < K ≤ 1. These additional conditions were met for desorption isotherms only. That means, the adsorption isotherms cannot be fully classified as type II. Except for bamboo, higher C values were noticed during desorption than adsorption (Table 3). Hess et al. (2018) attributed differences in C values for desorption and adsorption to sorption hysteresis. The additional thermodynamic analysis of the GAB model was made by Pradas et al. (2004), and the so-called jamming phenomenon was found. The phenomenon was related to forming the first layer of the adsorbed water. It was indicated that not all sorption sites were occupied during adsorption, not even at saturation. The sufficient condition for the jamming phenomenon was given by the relation K (equilibrium constant related to the multilayer sorption) < 1. The fraction of the total number of sorption sites occupied at saturation f was defined as:
For all adsorption processes analyzed in the present study, K was always lower than 1 (Table 3). The f for earlywood and latewood was 0.84 and 0.86, respectively, i.e., the proportion of the total number of sorption sites occupied by latewood samples in the saturated state was high. The observed values of C were always significantly higher than K in corresponding isotherms (Table 3). This suggests that monolayer water molecules might be much stronger bound than those with multilayer bonding (Hess et al. 2018).
Conclusion
The following conclusions could be drawn from the sorption isotherm behavior of homogenous catalpa wood samples documented by DVS Resolution:
-
(1)
During adsorption or desorption, the EMC of latewood was higher than in earlywood under any RH: meanwhile, the EMC difference between earlywood and latewood increased first and then decreased, and the turning point was 80% RH, related to the different number of micro–nano-pores in earlywood and latewood.
-
(2)
Adsorption–desorption isotherms formed a closed loop, all samples showed noticeable water sorption hysteresis over the full RH range, and the absolute hysteresis increased and then decreased due to hemicelluloses softening, with an inflection point at 70% RH. The absolute hysteresis of earlywood was larger than that of latewood, while its hysteresis coefficient was smaller, related to the differences in structure and chemical composition.
-
(3)
The GAB model could predict the sorption isotherms of earlywood and latewood. The Xm for earlywood and latewood was 6.87% and 7.47%, respectively. In particular, the GAB model confirmed more SGAB in latewood than in earlywood, indicating greater hydrophilic group content and monolayer adsorption capacity in latewood than in earlywood. The adsorption isotherms of earlywood and latewood in C. bungei cannot be fully classified as type II. The f for earlywood and latewood was 0.84 and 0.86, respectively, i.e., the proportion of the total number of sorption sites occupied by latewood samples in the saturated state was high.
References
Ahlgren PA, Wood JR, Goring DAI (1972) The fiber saturation point of various morphological subdivisions of Douglas-fir and aspen wood. Wood Sci Technol 6:81–84
Albrektas D, Ukvalbergienė K (2015) Impact of wood species, dimensions and drying temperature on sorption behaviour of wood. Drv Ind 66:1–10
Ball RD, Simpson IG, Pang S (2001) Measurement, modeling and prediction of equilibrium moisture content in Pinus radiate heartwood and sapwood. Holz Roh Werskst 59:457–462
Basu S, Shivhare US, Mujumdar AS (2006) Models for sorption isotherms for food: a review. Dry Technol 24:917–930
Bertaud F, Holmbom B (2004) Chemical composition of earlywood and latewood in Norway spruce heartwood, sapwood and transition zone wood. Wood Sci Technol 38:245–256
Bertolin C, Ferri L, Strojecki M (2020) Application of the Guggenheim, Anderson, de Boer (GAB) equation to study the impact of sealing treatments on pine wood sorption characteristics. Mater Des Process Commun 3:e189
Bonnet M, Courtier-Murias D, Faure P, Rodts S, Care S (2017) NMR determination of sorption isotherms in earlywood and latewood of Douglas fir. Identification of bound water components related to their local environment. Holzforschung 71:481–490
Bratasz Ł, Kozłowska A, Kozłowski R (2012) Analysis of water adsorption by wood using the Guggenheim–Anderson–de Boer equation. Eur J Wood Prod 70:445–451
Broda M, Curling SF, Spear MJ, Hill CAS (2019) Effect of methyltrimethoxysilane impregnation on the cell wall porosity and water vapour sorption of archaeological waterlogged oak. Wood Sci Technol 53:703–726
Charupeng N, Kunthong P (2022) A novel space–time finite element algorithm to investigate the hygro-mechanical behaviours of wood fiber-polymer composites. Math Model Eng Probl 9:117–128
Chen CM, Wangaard FF (1968) Wettability and the hysteresis effect in the sorption of water vapour by wood. Wood Sci Technol 2:177
Chen Q, Wang G, Ma XX, Chen ML, Fang CH, Fei BH (2020) The effect of graded fibrous structure of bamboo (Phyllostachys edulis) on its water vapor sorption isotherms. Ind Crops Prod 151:112467
Christensen GN, Kelsey KE (1959) The rate of sorption of water vapor by wood. Holz Roh Werkst 17:178–188
Derome D, Griffa M, Koebel M, Carmeliet J (2011) Hysteretic swelling of wood at cellular scale probed by phase-contrast X-ray tomography. J Struct Biol 173:180–190
Derome D, Kulasinski K, Zhang C, Chen M, Carmeliet J (2018) Using modeling to understand the hygromechanical and hysteretic behavior of the S2 cell wall layer of wood. In: Geitmann A, Gril J (eds) Plant biomechanics: from structure to function at multiple scales. Springer, Cham, pp 247–269
Engelund ET, Thygesen LG, Svensson S, Hill CAS (2013) A critical discussion of the physics of wood-water interactions. Wood Sci Technol 47:141–161
Esteban LG, Simón C, Fernández FG, Palacios PD, Martín-Sampedro R, Eugenio ME, Hosseinpourpia R (2015) Juvenile and mature wood of Abies pinsapo Boissier: sorption and thermodynamic properties. Wood Sci Technol 49:725–738
Florisson S, Vessby J, Mmari W, Ormarsson S (2020) Three-dimensional orthotropic nonlinear transient moisture simulation for wood: analysis on the effect of scanning curves and nonlinearity. Wood Sci Technol 54:1197–1222
Fredriksson M, Thybring EE (2018) Scanning or desorption isotherms? Characterising sorption hysteresis of wood. Cellulose 25:4477–4485
Gao X, Zhou F, Fu Z, Zhou Y (2018) Sorption isotherms characteristics of high temperature heat-treated Picea abies and Pseudotsuga menziesii. J For Eng 3:25–29
Gao X, Zhou F, Zhou YD (2019) Sorption isotherms characteristics of high temperature heat-treated wood. Sci Silvae Sin 55:119–127
Garcia RA, Rosero-Alvarado J, Hernández RE (2022) Moisture-induced strains in earlywood and latewood of mature and juvenile woods in jack pine from 3D-DIC measurements. Wood Mater Sci Eng. https://doi.org/10.1080/17480272.2022.2056714
García-Iruela A, García Esteban L, García Fernández F, de Palacios P, Rodriguez-Navarro AB, Sánchez LG, Hosseinpourpia R (2020) Effect of degradation on wood hygroscopicity: the case of a 400-year-old coffin. Forests 11:712
Glass SV, Boardman CR, Zelinka SL (2017) Short hold times in dynamic vapor sorption measurements mischaracterize the equilibrium moisture content of wood. Wood Sci Technol 51:243–260
Glass SV, Boardman CR, Thybring EE, Zelinka SL (2018) Quantifying and reducing errors in equilibrium moisture content measurements with dynamic vapor sorption (DVS) experiments. Wood Sci Technol 52:909–927
Gorišek Z, Straže A (2006) Sorption and swelling characteristics of normal and tension beech wood (Fagus sylvatica L.). In: Kurjatko S, Kudela J, Lagana R (eds) Wood structure and properties ’06. Zvolen, Arbora, pp 227–231
Hartley ID (2000) Application of the Guggenheim–Anderson–de Boer sorption isotherm model to Klinki pine (Araucaria klinkii Lauterb.). Holzforschung 54:661–663
Hess KM, Killgore JP, Srubar WV (2018) Nanoscale hygromechanical behavior of lignin. Cellulose 2511:6345–6360
Hill CAS, Xie YJ (2011) The dynamic water vapour sorption properties of natural fibres and viscoelastic behaviour of the cell wall: is there a link between sorption kinetics and hysteresis? J Mater Sci 46:3738–3748
Hill CAS, Norton AJ, Newman G (2009) The water vapor sorption behavior of natural fibers. J Appl Polym Sci 112:1524–1537
Hill CAS, Norton AJ, Newman G (2010) The water vapour sorption properties of Sitka spruce determined using a dynamic vapour sorption apparatus. Wood Sci Technol 44:497–514
Hill CAS, Moore JR, Jalaludin Z, Leveneu M, Mahrdt E (2011) Influence of earlywood/latewood and ring position upon water vapour sorption properties of Sitka spruce. Int Wood Prod J 2:12–19
Hill CAS, Ramsay J, Gardiner B (2015) Variability in water vapour sorption isotherm in Japanese Larch (Larix kaempferi Lamb.) -earlywood and latewood influences. Int Wood Prod J 6:53–59
Hou S, Wang J, Yin F, Qi C, Mu J (2022) Moisture sorption isotherms and hysteresis of cellulose, hemicelluloses and lignin isolated from birch wood and their effects on wood hygroscopicity. Wood Sci Technol 56:1087–1102
Huda ASMA, Koubaa A, Cloutier A, Hernández RE, Pierre P, Fortin Y (2018) Phenotypic and genotypic correlations for wood properties of hybrid poplar clones of Southern Quebec. Forests 9:140–157
Kärenlampi PP, Tynjälä P, Ström P (2005) Phase transformations of wood cell wall water. J Wood Sci 51:118–123
Kitin P, Funada R (2016) Earlywood vessels in ring-porous trees become functional for water transport after bud burst and before the maturation of the current-year leaves. IAWA J 37:315–331
Krupińska B, Strømmen I, Pakowski Z, Eikevik TM (2007) Modeling of sorption isotherms of various kinds of wood, at different temperature conditions. Dry Technol 25:1459–1466
Kulasinski K, Guyer R, Derome D, Carmeliet J (2015) Water adsorption in wood microfibril-hemicel-lulose system: role of the crystalline–amorphous interface. Biomacromolecules 16:2972–2978
Kurata Y, Mori Y, Ishida A, Nakajima M, Ito N, Hamada M, Yamashita K, Fujiwara T, Tonosaki M, Katayama Y (2018) Variation in hemicellulose structure and assembly in the cell wall associated with the transition from earlywood to latewood in Cryptomeria japonica. J Wood Chem Technol 38:254–263
Kymäläinen M, Mlouka SB, Belt T, Merk V, Liljeström V, Hänninen T, Uimonen T, Kostiainen M, Rautkari L (2018) Chemical, water vapour sorption and ultrastructural analysis of Scots pine wood thermally modified in high-pressure reactor under saturated steam. J Mater Sci 53:3027–3037
Lenth CA, Kamke FA (2001) Equilibrium moisture content of wood in high temperature pressurized environments. Wood Fiber Sci 33:104–118
Lewicki PP (1997) The applicability of the GAB model to food water sorption isotherms. Int J Food Sci Technol 32(6):553–557
Li S, Li X, Link R, Li R, Deng LP, Schuldt B, Jiang XM, Zhao RJ, Zheng JM, Li S, Yin YF (2019) Influence of cambial age and axial height on the spatial patterns of xylem traits in Catalpa bungei, a ring-porous tree species native to China. Forests 10:662–678
Lopes JDO, Cáceres CB, Hernández RE, Garcia RA (2022) Effect of the thermal treatment on the chemical components, sorption, and shrinkage properties of Tectona grandis juvenile wood. Maderas Cienc y Tecnol 24:1–16
Lu Y, Pignatello JJ (2004) History-dependent sorption in humic acids and a lignite in the context of a polymer model for natural organic matter. Environ Sci Technol 38:5853–5862
Majka J, Olek W (2008) Sorption properties of mature and juvenile lime wood (Tilia sp.). Folia For Pol B 39:65–75
Majka J, Sydor M, Prentki J, Zborowska M (2022a) Initial desorption of reaction beech wood. Drv Ind 73:299–308
Majka J, Rogoziński T, Olek W (2022b) Sorption and diffusion properties of untreated and thermally modified beech wood dust. Wood Sci Technol 56:7–23
McBain JW (1935) An explanation of hysteresis in the hydration and dehydration of gels. J Am Chem Soc 57:699–700
Neimsuwan T, Wang S, Taylor AM, Rials TG (2008) Statics and kinetics of water vapour sorption of small loblolly pine samples. Wood Sci Technol 42:493–506
Obataya E, Shibutani S, Hanata K, Doi S (2006) Effects of high temperature kiln drying on the practical perfomances of Japanese cedar wood (Cryptomeria japonica) I: changes in hygroscopicity due to heating. J Wood Sci 52:33–38
Olek W, Majka J, Czajkowski Ł (2013) Sorption isotherms of thermally modified wood. Holzforschung 67:183–191
Olsson A-M, Salmén L (2003) The softening behavior of hemicelluloses related to moisture. In: Tenkanen M, Gatenholm P (eds) Science and technology. American Chemical Society, Hemicelluloses, pp 184-197
Ouertani S, Simo-Tagne M, Rémond R (2022) Sorption isotherms and moisture transfer properties of seven Central Africa hardwood species. Wood Mater Sci Eng. https://doi.org/10.1080/17480272.2022.2051736
Ouyang B, Yin FY, Li Z, Jiang JL (2022) Study on the moisture-induced swelling/shrinkage and hysteresis of Catalpa bungei wood across the growth ring. Holzforschung 76:711–721
Patera A, Derome D, Griffa M, Carmeliet J (2013) Hysteresis in swelling and in sorption of wood tissue. J Struct Biol 182:226–234
Popper R, Niemz P (2009) Water sorption behaviour of selected domestic and overseas wood species. Bauphysik 31:117–121
Popper R, Niemz P, Eberle G, Torres MH (2007) Influence of extractives on water vapour sorption by the example of wood species from Chile. Wood Res Slovak 52:57–68
Pradas MM, Sánchez MS, Ferrer GG, Ribelles JJG (2004) Thermodynamics and statistical mechanics of multilayer adsorption. J Chem Phys 121:8524–8531
Quartey GA, Eshun JF, Sakyiama B (2021) Effect of microstructure on the permeability and sorption of wood of Sterculia rhinopetala and Albizia ferruginea. Adv Technol 1:523–535
Salmén L, Larsson PA (2018) On the origin of sorption hysteresis in cellulosic materials. Carbohydr Polym 182:15–20
Sander M, Lu YF, Pignatello IJ (2005) A thermodynamically based method to quantify true sorption hysteresis. J Environ Qual 34:1063–1107
Sargent R, Riley S, Schöttle L (2010) Measurement of dynamic sorption behaviour of small specimens of Pinus radiate-influence of wood type and moisture content on diffusion rate. Maderas Cienc y Tecnol 12:93–103
Sharratt V, Hill CAS, Zaihan J, Kint DPR (2010) Photodegradation and weathering effects on timber surface moisture profiles as studied using dynamic vapour sorption. Polym Degrad Stab 95:2659–2662
Su N, Fang C, Zhou H, Tong T, Zhang S, Fei B (2020) Hydrophobic treatment of bamboo with rosin. Constr Build Mater 271:121507
Timmermann EO (2003) Multilayer sorption parameters: BET or GAB values? Coll Surf A Eng Asp 220:235–260
Urquhart AR (1960) Sorption isotherms. Wiley, New York
Vrentas JS, Vrentas CM (1996) Hysteresis effects for sorption in glassy polymers. Macromolecules 29:4391–4396
Wu YZ (2007) Chemical composition of three kinds of rattan canes. Sci Silvae Sin 43:155–158
Yang TT, Ma EN, Cao JZ (2018) Effects of lignin in wood on moisture sorption and hygroexpansion tested under dynamic conditions. Holzforschung 72:943–950
Yang LM, Liu XE, Jiang ZH, Tian GL, Yang SM, Shang LL (2021) Water adsorption characteristics of Calamus simplicifolius cane. Sci Silvae Sin 57:150–157
Zhan TY, Lyu JX, Eder M (2021) In situ observation of shrinking and swelling of normal and compression Chinese fir wood at the tissue, cell and cell wall level. Wood Sci Technol 55:1–19
Zhang QS, Guan MJ, Ji WL (2002) Variation of moso bamboo chemical compositions during mature growing period. J Nanjing For Univ Nat Sci Edit 26:7–10
Zhang XX, Li J, Yu Y, Wang HK (2018) Investigating the water vapor sorption behavior of bamboo with two sorption models. J Mater Sci 53:8241–8249
Zhou HZ, Xu R, Ma EN (2016) Effects of removal of chemical components on moisture adsorption by wood. BioResources 11:3110–3122
Funding
This study was funded by the Fundamental Research Funds of the Chinese Academy of Forestry (CAFYBB2020MA001) and the National Natural Science Foundation of China (No. 32071689) sponsored this research.
Author information
Authors and Affiliations
Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, F., Du, Y., Li, Z. et al. Water vapor sorption characteristics and hysteresis of earlywood and latewood within the same growth ring of Catalpa bungei. Wood Sci Technol 57, 507–521 (2023). https://doi.org/10.1007/s00226-023-01457-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-023-01457-7