Abstract
A better understanding of the sorption behavior of different wood structures could be useful in protecting wood against wood deterioration and fungal attack. The purpose of this study was to investigate the effect of differences among earlywood, latewood, and tree ring location within the stem cross-section of loblolly pine (Pinus taeda) on the sorption kinetics and statics of water vapor under ambient conditions. The water vapor sorption of earlywood and latewood in different tree rings was recorded using a dynamic contact angle analyzer under relative humidity changes from 11 to 89%, as provided by saturated salt solutions. Earlywood had higher sorption rates and diffusion coefficients than latewood, while outer tree rings had higher sorption rates and diffusion coefficients than inner tree rings. The sorption isotherms of earlywood, latewood, and different tree ring locations within the stem cross-section were fitted very well by a Hailwood–Horrobin model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sorption kinetics and statics are used in many fields of science and engineering, e.g., food science, material science, soil science, pharmaceutical science, and wood science. Sorption behavior and diffusion processes of solid wood are of interest for many reasons, including as a method of evaluating its in-service performance to help understand mold growth on wood, and for evaluating drying kinetics in wood drying (Efremov 2002; Greethamma and Thomas 2005; Pham et al. 1999). Sorption studies can also be used to gather information about composites manufacturing, such as the influence of moisture sorption on resin curing and gluing properties (Kim et al. 2006; Uysal 2006).
Wood is a heterogeneous material and its anatomy and properties vary with position in the tree (Panshin and DeZeeuw 1980). Moisture sorption could vary with changes in anatomy and position in the tree because sorption behavior is affected by several parameters such as the size and distribution of cell cavities, intercellular spaces and pit membrane pores, and moisture content of the wood (Choong 1963).
Surface properties may affect water vapor sorption, especially in the initial sorption period. One of the most important surface properties is wettability, which can be represented by contact angle. There are inconsistent reports about the relative wettability of earlywood and latewood. Hse (1972) found that the earlywood was wetted more easily than latewood in southern pine, while Shupe et al. (1998) did not observe any effect of earlywood and latewood differences on the wettability of loblolly pine. Maldas and Kamdem (1999) found that latewood wetted more easily than earlywood in loblolly pine.
Wood species and wood grain direction influence water vapor sorption and diffusion coefficients. For Scots pine in the longitudinal direction, moisture diffusion is six times higher than that for spruce while there is no difference in the radial direction (Fakhouri et al. 1993). Moisture movement in the tangential direction of spruce is higher than that of Scots pine (Fakhouri et al. 1993). However, few studies have investigated sorption behavior of different wood tree ring locations within the stem cross-section. The purpose of this study was to investigate the effect of differences among earlywood, latewood, and tree ring location within the stem cross-section of loblolly pine (Pinus taeda) on water vapor sorption statics and kinetics under ambient conditions.
Materials and methods
Wood specimen and basic properties
The wood samples used in this experiment were loblolly pines (Pinus taeda) originating from a field in southern Arkansas. Two-foot long bolts were cut from three loblolly pine trees every 16 feet along the length of the main stem. These bolts were cut into longitudinal strips at each compass heading. Specimens containing three growth rings were cut from the wood strips to produce the bending specimens. Although the specimens were too small to be covered by the second method of ASTM standard for smaller clear specimens, they comprised a number of growth rings to reduce the influence of earlywood and latewood differences. These specimens were oven dried at 105°C and reconditioned at 65% relative humidity (RH) and 23 ± 2°C before testing. The small amount of volatile extractives possibly lost during oven drying was neglected. The moduli of elasticity (MOE) and moduli of rupture (MOR) were tested according to the ASTM standard for three-point bending (ASTM 1999). The specific gravity was also calculated from the bending specimens, using a weighted average of the earlywood and latewood. Bending specimens were milled separately for each growth ring and screened until they passed a 20 mesh screen. The earlywood and latewood of each growth ring was milled together due to the small amount of material. The chemical composition was analyzed from the milled specimens using ASTM standard methods for whole biomass analysis (ASTM 1999). National Institute of Standards and Technology (NIST) pine standard reference material #8493 was used as a reference to determine carbohydrate, extractives and lignin content (NIST 1993).
A set of radial strips was cut from the bolts for the sorption studies. These strips were divided into tree ring no. 2, 5, 9, 16, 32 and 50 from the pith to bark. These specimens were visually separated into earlywood and latewood, which were cut from the wood strips into slivers 5 mm (longitudinal) × 5 mm (tangential) × 0.5 mm (radial) for sorption isotherm and sorption kinetics experiments. A diagram of bending sample and sliver preparation from the loblolly pine bolts is shown in Fig. 1.
Sorption isotherms
Five replications of earlywood and latewood from different tree rings were conditioned in desiccators above one of the six saturated salt solutions listed in Table 1. All specimens were weighed to the nearest 0.0001 g once a day until equilibrium was reached (weight change of less than 1% from previous measurement). After the last run, oven-dried weight was measured and moisture content was calculated based on oven-dried weight. All salt solutions were maintained at a constant laboratory temperature of 23 ± 2°C.
Sorption kinetics
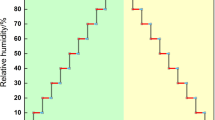
The measurement of sorption behavior was performed by a vapor sorption method. A second set of specimens, which had the same dimensions as the first set of earlywood and latewood specimens from different tree rings, were prepared from pine trees as described above. The sorption process was performed by changing the RH from 11 to 89%. The weight change of the specimen during the sorption process was continuously measured and recorded within 4 h using a Dynamic Contact Angle Analyzer DCA 332 (Thermo Cahn Corp. Madison, WI, USA). The temperature was kept constant at 23 ± 2°C by a temperature-controlled electric heater and the salt solutions were bubbled in order to keep the RH at the target level at ambient pressure. The air velocity was 0.5 l/min. A data logger kept a record of the climate during the sorption period. The humidity sensor takes only a few seconds to start recording RH changes. Five replications of each condition were used. The experimental set-up of the climate control instrument is shown in Fig. 2. The specimens were oven-dried after the last weight measurement and moisture content was calculated based on oven-dried weight.
Evaluation of sorption isotherms
The sorption data obtained in the study at each RH were fitted with an ideal solution equation (Hailwood and Horrobin 1946). From ideal solution behavior, the following equation was obtained:
where M is the equilibrium moisture content; h is RH; M h is the moisture content of the hydrate water (monomolecular sorption); M d is the moisture content of the dissolved water (polymolecular sorption); K 1 is the equilibrium constant of the hydrate formed from dissolved water and dry wood; K 2 is the equilibrium constant between water vapor and dissolved water; W is the molecular weight of dry wood per mole of water sorption sites. For convenience in fitting to the experimental data, the polynomial form was obtained:
where:
Equation 2 shows a parabolic relationship between the ratio of h/M and h. The A, B and C are regression coefficients, which contain the equilibrium constant (K 1, K 2) and W values. The values of K 1, K 2, and W can be calculated as follows:
Evaluation of diffusion coefficient
The calculation of diffusion coefficients was based on the solution of a practical problem such as non-perfect RH-step. Therefore, the final part of the sorption curve was used due to the non-perfect RH-step. The accuracy of this calculation was usually best around 60–80% of total sorption (Wadso 1994). The equation to calculate diffusion coefficient is expressed as follows:
where D is the diffusion coefficient [m²/s]; l is half of thickness of specimen [m]; t is time [s]; E is the dimensionless sorption. The calculation of dimensionless sorption is as follows:
where ΔM i is the weight change at time t[s]; ΔM ∞ is the weight change at final equilibrium. This diffusion coefficient is approximated with the following assumptions: the diffusion coefficient is constant, the initial moisture is uniform within the specimen, the instantaneous equilibrium is reached with RH at the cell surface, and there is symmetrical transport of moisture through the cell wall. It was also assumed that the sorption through the edges can be neglected due to a small surface-to-thickness ratio. Therefore, in this experiment diffusion was considered to be unidirectional. The diffusion coefficient calculated based on a graphical analysis of fractional change in moisture content is shown in Fig. 3.
Evaluation of surface resistance
Surface resistance is a limited mass transfer coefficient, which hinders transport. In wood science, the term “surface emission (σ)”, which is the inverse of the surface resistance (S), is used. Liu (1989) proposed a method for the determination of surface emission coefficients. The equation to calculate the surface emission is expressed as follows:
Evaluation of sorption rate
The sorption rate was determined from the slope of the plot of moisture content against time (Wang and Liau 1998). The early stage was linearly governed by Fickian sorption, while the second stage was non-linear, due to the slow sorption of the cell wall. The initial sorption rate (K 1) was calculated from the slope within five minutes, while the second slope (K 2) was calculated from the second linear slope within another 5 min.
Results and discussions
Sorption isotherms
The sorption behavior of wood in the different tree rings was analyzed using the Hailwood–Horrobin (H–H) model. The values of A, B, C, the coefficient of determination (R 2), K 1, K 2 and W of wood at various tree rings are listed in Table 2. The equilibrium constants of the EL values were determined by means of a regression analysis with a weighted average from the entire earlywood (EW) and latewood (LW) dataset. The degree of fit was described by the coefficient of determination (R 2) and it ranged from 0.912 to 0.996, indicating a good fit to the experimental results. The H–H model was divided into the moisture sorption of the monomolecular component and the polymolecular component. The constant W describes the molecular weight of the dry wood polymer per mole of water sorption sites. The constant K 1 describes the equilibrium constant where the hydrate is formed from dissolved water and dry wood. The K 1 values varied between 159.18 and 750.64. Most of the K 1 values were higher for earlywood and outer tree rings. Latewood of some tree rings had higher K 1 values. The constant K 2 describes the equilibrium constant between dissolved water and water vapor, or the activity of dissolved water per unit relative vapor pressure. This value should be unity if it has the same activity as liquid water. The K 2 values varied between 0.63 and 0.78, indicating that the dissolved water shows a lower activity than the liquid water. The value of W did not show significant differences among the samples.
Sorption rate
The moisture content of earlywood and latewood was plotted as a function of the square root of time, as shown in Fig. 4. Each data point was the average of the 30 samples from all the tree rings. The sorption curve showed the moisture content changing linearly with the square root of time for the initial period of sorption, although a non-perfect RH-step before 500 s was observed. The sorption plot of earlywood and latewood had a similar shape, which is a linear rate at initial sorption, followed by a gradual decrease in sorption rate until moisture content is constant. The plot of the moisture content of wood from the various tree rings is shown in Fig. 5. Each value was the average of ten samples of earlywood and latewood in each tree ring. The highest constant MC values from each plot were considered to be the pseudo-equilibrium values because the true equilibrium cannot be reached in such short time periods. The initial sorption rate and second slope are shown in Table 3. The sorption rate of ring no. 16, 32 and 50 was higher than the sorption rate of ring no. 2, 5 and 9, where ring no. 2 refers to the second tree ring from the center (pith) of the bolt. Tree ring no. 50 had a higher sorption rate than tree ring no. 2. Although differences in the sorption rate of the outer tree rings and inner tree rings were not explicitly studied in this experiment, the sorption rate of outer tree rings was generally higher than that of inner tree rings. The variation of outer tree ring and inner tree rings may have been due to the transition from sapwood to heartwood, although sapwood and heartwood were not identified in these samples. This sapwood-to-heartwood transition is generally accompanied by an increase in extractive content and/or a change in extractive chemistry. Heartwood extractives can reduce permeability, make heartwood dry slowly, make heartwood hard to impregnate with chemical preservatives, make the wood resistant to fungi or insect attack, increasing stability under changing moisture conditions and increase density slightly (USDA Forest Service 1974).
A comparison of the initial sorption rate of earlywood and latewood at different tree rings is shown in Fig. 6. The initial sorption rate of earlywood was always higher than latewood. This may be due to different anatomical structure between earlywood and latewood. Earlywood is characterized by cells with relatively large cavities and thin walls, while latewood has smaller cavities and thicker cell walls (Bodig and Jayne 1993). In general, moisture easily penetrates the empty spaces in wood structures such as the lumen of cells. Pit membranes and the cell wall play an important role in inhibiting moisture flow (Siau 1995). Therefore, the initial sorption rate is highly dependent on wood structural features such as tracheid length in addition to the temperature, density, microfibril angle and moisture content of the wood (Comstock 1963).
All plots showed that the sorption behavior was divided into two stages: the first stage is diffusion-controlled and the second stage is a slow partial relaxation of the swelling stress, which increases the amount of sorbed water (Downes and Mackay 1958). This phenomenon was described as a surface resistance, which hindered the water sorption. The first stage ended at the point after which Fick’s law cannot be applied. It is usually about 30% of the total sorption. In the second stage, it is believed to be controlled by sorption in the cell wall. It is about 50% of total sorption (Wadso 1993). The second slope values obtained in this experiment are shown in Table 3 and Fig. 7. The initial sorption rate was 2–4 times higher than the second slope. However, the second slope of earlywood was always lower than latewood, except for tree ring no. 2. The second slope tended to be higher for outer rings than for inner rings.
Sorption diffusion
The diffusion coefficients of earlywood and latewood at different tree rings are shown in Fig. 8. A non-perfect RH-step was noted; however, only the final part of the sorption curve was used to calculate diffusion coefficient. The diffusion coefficients at 50% (D 0.5) and 60% (D 0.6) of total sorption, surface emission coefficients (σ), and surface resistance coefficients (S) of earlywood and latewood in different tree rings are shown in Table 3. The diffusion coefficient of water vapor at 50% of total sorption was between 0.45 × 10−10 and 7.63 × 10−10 m2/s, while it was between 0.48 × 10−10 and 8.55 × 10−10 m2/s at 60% of total sorption. The diffusion coefficient of earlywood was approximately 2–4 times higher than latewood. The diffusion coefficients of outer tree rings were significantly higher than that of inner tree rings. These data suggest that moisture can diffuse more rapidly in earlywood and outer tree rings than in latewood and inner tree rings.
In the sorption process, the direct interaction between water vapor and the surface at the monolayer was called chemi-sorption while the interaction of water vapor at the multiple layers was called physi-sorption (Hartley et al. 1992). When moisture content reaches a constant, it is believed that this point represents equilibrated moisture content governed by the wood surface. If it is assumed that wood cannot be equilibrated in short time periods, the moisture content at this point can be called pseudo-equilibrium. The influence of surface emission is closely related to diffusion coefficient as well as to internal surface resistance, which is the inverse of the surface emission. The higher surface resistance coefficient of latewood and inner tree rings appears to correspond to slower sorption processes (Table 3). The calculation of the diffusion coefficient by maximum slope and 80% of total sorption is not appropriate due to the internal surface resistance during the initial sorption process, and non-Fickian effects at the end of the sorption process, respectively. Therefore, the calculation of the diffusion coefficient at 60% of total sorption can be used instead of the traditional method based on a calculation from the maximum slope.
Chemical analysis
Table 4 shows physical properties, mechanical properties and chemical composition of the loblolly pine samples in different tree rings. Wood near the bark had higher density than wood near the pith. This density difference was probably due to the relative amount of earlywood and latewood changing from pith to bark. The outer tree rings had higher strength and stiffness than inner tree rings, which likely was mostly a function of differences in density. Siau (1995) stated that wood density was indirectly related to water vapor sorption. However, the relation of density to water vapor sorption is unclear. Other factors may influence sorption such as tracheid length and microfibril angle. The hemicelluloses usually have higher sorptive capacity than celluloses and lignin (Christensen and Kelsey 1958). The hemicelluloses and lignin content were only slightly different along the tree radius, while cellulose content was lower, and extractive content was higher, in the inner tree rings than in the outer tree rings. Although the lower moisture sorption of inner tree rings seems to be correlated with the lower content of (hydrophilic) cellulose and higher amounts of extractives, the degree to which the chemical differences influenced moisture sorption is uncertain.
Conclusions
The differences due to earlywood, latewood, and tree ring location within the stem cross-section on the sorption kinetics and sorption statics of water vapor were observed in loblolly pine (Pinus taeda) trees. Earlywood had higher sorption rates and diffusion coefficients than latewood, while outer tree rings had higher sorption rates and diffusion coefficients than inner tree rings. The sorption isotherms of earlywood, latewood, and different tree ring locations with the stem cross-section were fitted very well by Hailwood–Horrobin model.
References
American Society for Testing Materials (ASTM) (1999) Standard test methods for evaluating properties of wood-base fiber and particle panel materials. ASTM D 1037-99. ASTM, Philadelphia
Bodig J, Jayne AJ (1993) Mechanics of wood and wood composites. Van Nostrand Reinhold, New York
Choong ET (1963) Movement of moisture through a softwood in the hygroscopic range. For Prod J 13(11):489–498
Christensen GN, Kelsey KE (1958) The sorption of water vapour by the constituents of wood: determination of sorption isotherms. Austr J Appl Sci 9:265–282
Comstock LG (1963) Moisture diffusion coefficients in wood as calculated from adsorption, desorption, and steady state data. For Prod J 13(3):97–103
Downes JG, Mackay BH (1958) Sorption kinetics of water vapor in wool fibers. J Polym Sci 28:45–67
Efremov GI (2002) Drying kinetics derived from diffusion equation with flux-type boundary condition. Drying Technol 20(1):55–66
Fakhouri B, Mounji M, Vergnaud JM (1993) Comparison of the adsorption and desorption of water between Scots pine and spruce after submersion in water. Holzforschung 47:271–277
Greethamma VG, Thomas S (2005) Diffusion of water and artificial seawater through coir fiber reinforced natural rubber composites. Polym Comp 26:136–143
Hailwood AJ, Horrobin S (1946) Absorption of water by polymers: analysis in terms of simple model. Trans Faraday Soc 42B:84–102
Hartley ID, Kamke FA, Peemoeller H (1992) Cluster theory for water sorption in wood. Wood Sci Technol 26:83–99
Hse CY (1972) Wettability of southern pine veneer by phenol formaldehyde wood adhesives. For Prod J 22(1):51–56
Kim JW, Carlborn K, Matuana LM, Heiden PA (2006) Thermoplastic modification of urea-formaldehyde wood adhesives to improve moisture resistance. J Appl Polym Sci 101:4222–4229
Lide DR (1994) CRC handbook of chemistry and physics, 75th edn. CRC Press, Boca Raton
Liu JY (1989) A new method for separating diffusion coefficient and surface emission coefficient. Wood Fiber Sci 21:133–141
Maldas DC, Kamdem DP (1999) Wettability of extracted southern pine. For Prod J 49(11/12):91–93
National Institute of Standards, Technology (NIST) (1993) Pine standard reference material-8493. NIST, Gaithersburg
Panshin AJ, DeZeeuw C (1980) Textbook of wood technology. McGraw-Hill, New York
Pham X, Vittadini E, Levin RE, Chinachoni P (1999) Role of water mobility on mold spore germination. J Agric Food Chem 47:4976–4983
Shupe TE, Hse CY, Choong ET, Groom LH (1998) Effect of wood grain and veneer side of loblolly pine veneer wettability. For Prod J 46(6):95–97
Siau JF (1995) Wood: influence of moisture on physical properties. Department of Wood Science and Forest Products, Virginia Tech, Virginia
US Department of Agriculture, Forest Service (USDA Forest Service) (1974) Wood handbook: wood as an engineering material. Forest Products Laboratory, Washington DC
Uysal B (2006) Effects of the steam test on bonding strength of laminated veneer lumbers manufactured by using different adhesives. J Appl Polym Sci 99:2973–2977
Wadso L (1993) Measurements of water vapour sorption in wood. Part 2. Results. Wood Sci Technol 28:59–65
Wadso L (1994) A test of different methods to evaluate the diffusivity from unsteady-state sorption measurement. Drying Technol 12(8):1863–1876
Wang SY, Liau CF (1998) Assessment of hygroscopic conditioning performance of interior decorative materials. IV. Sorption characteristics of wood under high relative humidity condition. J Wood Sci 44:260–266
Acknowledgments
The authors would like to acknowledge financial supports from the USDA Wood Utilization Research Program and the Tennessee Agricultural Experiment Station (Project TEN000MS-96). The authors also thank Dr. Steve Kelley for providing wood samples and their related properties.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neimsuwan, T., Wang, S., Taylor, A.M. et al. Statics and kinetics of water vapor sorption of small loblolly pine samples. Wood Sci Technol 42, 493–506 (2008). https://doi.org/10.1007/s00226-007-0165-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-007-0165-2