Abstract
Thermal modification of wood results in improved dimensional stability and increases the end-use possibilities of wood. Modification under saturated steam is reported to result in higher performance when compared to more traditional thermal modification methods. This study analyses the chemical and ultrastructural changes, as well as water vapour sorption properties of Scots pine modified thermally in a high-pressure reactor under saturated steam. The aim is to reveal important chemical and sorption-related changes in wood modified under saturated steam. Chemical composition, water vapour sorption properties, accessibility and concentration of cellulosic hydroxyl groups, as well as evolution of cell wall are discussed. At a temperature of 180 °C, clear cell wall delamination and distortion were observed. In nanoscale, the results indicated opening of microfibril bundles, which leads to higher surface area and theoretically, a higher accessibility. However, a decrease in the equilibrium moisture content and accessibility of both extracted and unextracted samples were observed, but the decrease was less obvious in extracted samples.Hence, it was concluded that extractives and degradation products play an important role during thermal modification by blocking porosity and therefore decreasing accessibility and reducing sorption of thermally modified samples. The changes in hysteresis behaviour after extraction also support this outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal modification of wood is known to produce desirable features, such as increased durability and dimensional stability that increase the end-use possibilities of wood [1,2,3,4,5,6]. There are several options for thermal modification, and depending on the desired result an open or closed system, wet or dry process with different heating media and atmospheres can be used. The ThermoWood process dominates the European markets and holds the highest production numbers globally [3]. The process is based on modification under superheated steam, while other processes use, e.g., a combination of thermo-hydrolysis at elevated pressure followed by a dry high-temperature curing step [5], dry heat modification under a nitrogen atmosphere [6] or immersion in hot oil [6]. More recently, modification under saturated steam has been brought forward, reportedly preserving the wood structure to a higher degree than the more traditional modification methods [7].
Regardless of the process, thermal modification of wood leads to degradation of hemicelluloses [8, 9], while cellulose resists [10]. The aryl–alkyl–ether bonds of lignin are altered to some extent already below 200 °C [11], but the temperature range of lignin degradation is much wider than that of carbohydrates or cellulose [12]. The effect of thermal modification on wood extractives depends on the wood species [13]. The mechanical properties of thermally modified wood are reported to decrease after heat treatment, resulting in brittle character with poor tensile, flexural and impact strength [3, 5, 14]. Naturally, the treatment process and material characteristics significantly affect the result. In contrast to these adverse effects, other properties of wood are enhanced by thermal modification. Hygroscopicity is reduced, which in addition to chemical changes such as cross-linking reactions increases the resistance to biological degradation and increases the dimensional stability [11, 15, 16], the magnitude depending on the process parameters.
Moisture properties significantly affect the performance of wood products, and it is therefore highly beneficial to reach a low-equilibrium moisture content (EMC). Thermal degradation of wood first leads to volatilisation of the thermally labile polysaccharides. As hemicelluloses and amorphous cellulose host the largest amount of hydroxyl groups out of the constituents of wood, the EMC of the product is permanently lowered [4, 17,18,19]. Other mechanisms also contribute to the reduced EMC: cellulose crystallinity is often reported to increase following thermal modification, due to preferential degradation of less-ordered amorphous regions, but also because of rearrangement or reorientation of cellulose molecules [20]. However, the magnitude is highly dependent on the treatment temperature and atmosphere [20,21,22]. The crystalline areas of cellulose naturally inhibit the access of water molecules inside the cellulose matrix. The formation of cross-links within the lignin–carbohydrate complex during thermal modification also contributes to reduced hygroscopicity, as the stiffer structure resists swelling and therefore the access of water molecules. According to Dwianto et al. [21], cross-linking reactions are dominant under a dry atmosphere and crystallisation under a moist one. Hornification, or the irreversible aggregation of cellulose microfibrils, is a well-known concept within the pulp industry, but has not been unarguably proven for thermally treated wood, though indication of such a phenomenon has been found [23, 24]. Hornified fibres are stiffer than virgin fibres and may lead to better dimensional stability of thermally modified wood [25]. Concerning hornification in wood modified in high-temperature-saturated steam, a specific literature is missing.
Modification under saturated steam is reported to produce wood material with improved properties compared to processes that include a drying step. As wood stays moist throughout the modification, cellular damage related to high-temperature drying is avoided and mechanical performance should be higher than in wood modified in atmospheric or sub-atmospheric conditions [7]. This study intends to further investigate the properties of the materials obtained in a previous study [14], where mechanical properties of Scots pine modified under saturated steam atmosphere were investigated. The goal is to reveal the important changes taking place in material modified in this manner. Accessibility of the hydroxyl groups of modified Scots pine is measured, and the role of extractives on the water vapour sorption behaviour is identified. Furthermore, structural and chemical changes are analysed using, for example GC–MS (gas chromatography–mass spectrometry), SEM (scanning electron microscope) and SAXS (small-angle X-ray scattering).
Materials and methods
Scots pine (Pinus sylvestris L.) heartwood boards obtained from Southeast Finland with average density of 490 kg/m3 were used in this study. Kiln dried wood samples were dried in three steps, at 40, 70 and 103 °C for 24 h each, at ambient relative humidity (RH). This drying process was selected as it was expected to be mild enough to avoid any cell wall damage. Paired specimens of clear wood with dimensions of 25 × 25 mm and 300 mm in length were cut from the middle of the board. Half of the specimens were thermally modified under a saturated steam atmosphere in a high-pressure reactor for 3 h. The specimens were modified at 150 and 180 °C with corresponding steam pressures of 4.7 and 9.8 bars, respectively. The materials and modification processes are described more in detail in an earlier study [13]. Prior to the analysis, the materials were milled using a Wiley Mill (20 mesh) and stored at 30% RH, 20 °C.
The chemical composition of the samples was determined using a modified version of NREL/TP-510-42618. The ground samples (2–5 g each) were Soxhlet extracted with acetone, air-dried, and then hydrolysed with sulphuric acid (approx 0.3 g of sample as dry mass reacted with 3 ml 72 m-% sulphuric acid for 60 min, then autoclaved for 60 min after addition 84 ml of deionised water). All samples were processed and analysed in duplicate. After hydrolysis, the liquors were filtered through medium porosity filtering crucibles. The obtained solids were washed and dried and used for gravimetric Klason lignin determination. A 0.5-ml-aliquot of each filtered liquor was removed and diluted (1:100) with deionised water for carbohydrate analysis by HPAEC (Dionex ICS-3000, Sunnyvale, California) with pulsed amperometric detection (PAD) and a CarboPac PA20 column, with water as the eluent (0.37 ml/min), and pure monosaccharides as standards. Each sample was analysed twice.
To determine the chemical composition of the acetone extracts, the individual extractives compounds were first identified by GC–MS (ISQ single quadrupole MS + Trace 1300 GC, Thermo Fischer Scientific, Waltham, Massachusetts) and then quantified by GC (GC-2010 Plus, Shimadzu Corp., Tokyo, Japan). The dry extracts were re-dissolved in acetone (10 mg/ml), silylated (0.1 ml of sample added to 0.7 ml of pyridine and 0.25 ml of BSTFA with 5% TMSC) and analysed with a 30 m × 0.25 mm i.d. column coated with trifluoropropylmethyl polysiloxane (TraceGOLD TG-200MS, 0.25 μm film thickness). The oven temperature programme was 2 min at 100 °C, 30 °C/min to 260 °C and 15 min at 250 °C. Helium (1 ml/min) was used as carrier gas in GC–MS and hydrogen (2 ml/min) in GC. Mass spectra were recorded in the 50–700 (m/z) range at an ionisation energy of 70 eV. Heneicosanoic acid was used as the internal standard for quantification. All samples were analysed in duplicate.
Water vapour sorption analysis was performed using an Intrinsic DVS apparatus (Surface Measurement System Ltd., London, UK). The sorption cycle was 0–95% RH with 5% RH steps and the desorption cycle employing a reverse sequence. The temperature was set at 25 °C, and the criteria of mass equilibrium for each step were fixed to dm/dt 0.002% for 10 min. Accessibility of hydroxyl groups was analysed by deuterium exchange in an ET1 DVS (Surface measurement system Ltd., London, UK), as also described in [26, 27]. The change in mass directly gives the amount of accessible sites as protium ions are exchanged with heavier deuterium ions. Approximately, 10 mg of milled particles were placed in the sample pan and preconditioned at 0% RH using dry nitrogen. The sequence included a drying step at 60 °C for 6 h using a pre-heater (and cooling to 25 °C), followed by an impregnation step at 95% RH D2O to replace hydrogen atoms with deuterium ones. This step lasted for 10 h and was followed by a last step of 6 h at 0% RH and 60 °C and cooling step until 25 °C reached. Accessibility is given as the difference between the dry mass after the first step and the last. The sorption rate reveals the rate at which materials absorb water molecules. The data were obtained from the sorption analysis. After each step, there is a response in the specimen, seen as a new EMC within a specific duration. Sorption rate was calculated by dividing mass change (%) with consumed time (min), as in Xie et al. [28].
The samples for SAXS analysis were milled using Wiley mill and sieved using 200-µm-mesh. The SAXS was measured using a rotating anode Microstar microfocus X-ray source (Cu K-α radiation, 1.54 Å) (Bruker, Billerica, Massachusetts). The beam was monochromated and focused by a Montel multilayer focusing monochromator (Incoatec, Germany). The X-ray beam was further collimated by a set of four slits (JJ X-Ray) resulting in the final spot size of less than 1 mm at the sample position. The SAXS is measured for two different overlapping q-ranges (q = 0.009–0.14 Å−1 and q = 0.024–0.37 Å−1) for the samples.
Cross-sections of wood samples were prepared using a rotary microtome (Leica Ultracut, Germany). The block faces were imaged with a FEI Quanta 600 scanning electron microscope (FEI Co., Hillsboro, Oregon) in the low-vacuum mode (water vapour, 0.53 Torr) driven by an acceleration voltage of 20 kV using a backscattered electron detector.
Results and discussion
In a previous study [14], the mass loss of the materials also used here was determined to be 2.3 and 13.9% for modification at 150 and 180 °C, respectively. Table 1 shows that some hemicellulosic sugars (arabinose, rhamnose and galactose) are completely removed at 180 °C and the amounts of xylose and mannose have decreased. Lignin and cellulose have their relative proportions increased. The values are approximate, as it is possible that some components remained in the material after Soxhlet extraction in addition to measurement error. The colour of the extraction solution changed at 180 °C, indicating the presence of degradation products of hemicelluloses. Hemicelluloses are the first components degrading during exposure to heat. The relative proportion of lignin increased, as previously reported by several studies [4, 13, 29, 30], and polycondesation reactions leading to further cross-linking also increase the relative lignin content [4]. Increase of Klason lignin may also be due to the formation of lignin-like, aromatic compounds through polysaccharide recondensation reactions. These reactions are more dominant at higher temperatures (i.e. over 220 °C) or with longer treatments times (several hours) [31]. The increase in glucose is attributed to relative increase of more resilient cellulose, namely the crystalline fraction because of loss of more labile amorphous polysaccharides.
The composition of the acetone-soluble extractives is presented in Table 2. It is observed that the higher the temperature, the more sugars are present in the extractives. It is also evident that pinosylvins have degraded, and the amount of resin and fatty acids has increased. A study by Nuopponen et al. [32] described a flux of resin acid, through the resin canals of Scots pine modified with the ThermoWood process. Fatty acid esters had their peak below 160 °C, and at temperatures above 200 °C, resin acids disappeared. Fats and waxes begin migrating to the surface already at quite mild temperatures, which can be well below 100 °C [32]. Esteves et al. [33] found that under superheated and saturated steam the extractives amount of hardwood first increased but then decreased when modification resulted in a mass loss of 6.1%. After modification, almost all the original extractives had disappeared and been replaced by modified compounds originating from degradation of hemicellulose and lignin. According to Manninen et al. [34], aldehydes and carboxylic acids and their esters dominate in pine wood samples thermally modified with steam.
In the samples modified at 180 °C, the cell wall was damaged and delaminated (Fig. 1). Those damages could be seen in smaller extent in the 150 °C sample. Fengel and Wegener [35] reported fissures between S1 and S2 layers at 180 °C and stated that the softening of the wood cell wall continues with elevation of the temperature. Hardwoods are more susceptible to heat modification because of higher reactivity of xylan (pentosan) compared to softwood hemicelluloses (mostly hexosans) [36]. Among softwoods, Boonstra et al. [37] reported a higher sensitivity of woods with an abrupt transition between earlywood and latewood. Scots pine falls into this category, and radial cracks along the tracheids in the earlywood, as well as distortion of cellular structure have been reported [37].
Porosity of thermally treated wood increases due to component removal. Depending on the wood species and modification process, increase or decrease in pore size may be seen. Hietala et al. [38] found an increase in pore size of Scots pine modified with ThermoWood process, while Zauer et al. [39] reported a reduction in the pore size of spruce wood, and to a lesser extent maple, after modification under a nitrogen atmosphere. The author accounted the smaller size to thermal stresses. However, though differences in wood species have to be taken into consideration, steam atmosphere is reported to reduce the stresses in wood because the material is in a hygroscopic equilibrium with its surroundings [7]. This could lead to larger pore size inside the modified material. Regardless of the pore size, increased porosity leaves wood more brittle, which has a major effect on the mechanical properties. Thermally modified wood is reported to have reduced tensile, flexural and impact strength [5], but the extent depends on the modification method and severity. Steam atmosphere accelerates the degradation of wood components, but as mentioned above, may also lead to better mechanical properties because of reduced or absent drying stresses. For the samples used in this study, impact strength was shown to decrease from 12 of reference to 8 kJ/m2 (at 180 °C) [14], so cutting with a razor blade could potentially damage the cell wall. However, the control sample in Fig. 1a does not seem damaged, so it is assumed that some of the damages on the sample treated at 180 °C, and to a smaller extent 150 °C, are due to thermal modification.
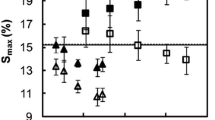
Figures 2 and 3 show the sorption properties of untreated and modified samples, as well as their extracted equivalents. Generally, thermal modification leads to a drop of EMC at any RH after thermal modification. The drop is accounted to volatilisation of amorphous polymers that leads to reduction of hydroxyl groups and a permanent reduction in the adsorption capacity of thermally modified wood. This can be quantified by measuring the amount of available hydroxyl groups through accessibility. In Fig. 4, the reduction of accessibility can be clearly observed. However, accessibility does not entirely explain the reduction of hygroscopicity, as poor correlation between the amount of accessible hydroxyl groups and the EMC has been found [27]. Lignin cross-linking and increasing cellulose crystallinity contribute to reduced EMC by hindering the access of water molecules [5, 40]. As mentioned before, hornified fibres are stiffer and therefore their swelling is limited, and the irreversible closure of pores further facilitates a lower EMC, but based on these results it is not possible to conclude the extent of this phenomenon. Hornification may still be one of the factors contributing to reduced EMC. Formation of physical barriers due to migration and following accumulation of extractives, recondensation of degradation products, as well as plastic flow of lignin, can also be considered to contribute to reduced accessibility, as pores and other points of access inside the wood can be blocked [40, 41]. The extracted samples seem to be less sensitive to thermal modification, in terms of reduction of accessibility (Fig. 4)—the absolute amount of accessible hydroxyl groups is higher for the extracted samples, which indicates there truly are some degradation products blocking the pores. With non-polar extractives taken out, D2O can penetrate deeper into the cell walls and occupy more hydroxyl groups. Additionally, the sorption rate (Fig. 5) is slightly higher in the extracted samples in the low and high end of relative humidity, indicating higher accessibility into the wood ultrastructure. However, it is worth considering, that it is possible not all OH-sorbing sites are subjected to O–D exchange, as they may be unavailable to the molecules, and/or longer measuring periods may be required [42]. Thermal modification also lowers the sorption rate slightly, though it is well known that wood modified at high temperatures is brittle [14]. Brittleness and smaller particle size should result in faster sorption rate, however, the change was somewhat small.
Considering the hysteresis of the thermally modified samples, the results show a decreasing trend (Fig. 3 ) in line with previous studies [19, 43, 44]. Mild thermal treatment increases hysteresis [45], but as the intensity of treatments increases, hysteresis has been shown to decrease. Hysteresis stems from the time lag between opening of nanopores upon water molecules entering the wood matrix, and the closing of those pores as the cell wall dries [26]. Hysteresis would, therefore, be larger in a material that is stiffer, such as in materials with high lignin content, as was found out by Hill et al. [44]. Cross-linking and potential hornification increase stiffness, but the hystereses of modified samples are smaller than those of untreated samples. Overall reduction of hygroscopicity can explain a part of the curves, but it could also be that the nanopore creation/destruction process may not be operative any longer as a result of small surface area, or blockage of pores [43]. Indeed, extracted samples in Fig. 3 exhibit higher hysteresis. Extraction slightly decreases the overall sorption differences between the treatments at the two temperatures. The reference samples’ hysteresis curve is not affected by the extraction, again indicating the active role of some degradation products during the thermal modification. Gonzalez-Peña et al. [30] performed similar experiments with extracted and non-extracted samples. The temperatures used were higher and the conditions were dry (nitrogen flow), but same results were obtained: higher sorption value and higher sorption rate for extracted samples.
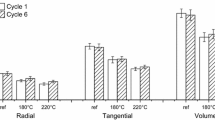
SAXS measurements were used to study ultrastructural changes in samples. Measurements were done on unextracted samples in two different overlapping q-ranges (q = 0.009–0.14 Å−1 and q = 0.024–0.37 Å−1). The SAXS profiles show the typical features for wood samples where a power law behaviour of the intensity dominates at small q-values (scattering from voids etc., Fig. 6), whereas at larger q-values a shoulder feature is seen (Figs. 6, 7) [46,47,48]. The shoulder is often attributed to the correlation distance of the microfibrils within the cellulose fibres, and it is shifting to smaller q-values for thermally modified samples. The shoulder shifts from 0.12 Å−1 (reference) to 0.05 Å−1 (modified at 180 °C), meaning that the correlation distance increases from 5 nm to 13 nm. Similar increase has been observed by Virtanen et al. [47] with mechanical and enzymatic treatments of pulp. In a study by Penttilä et al. [46], the interfibrillar distance was shown to decrease by hot water extraction. Increase of the interfibrillar distance could result from degradation of fibril encrusting hemicellulose layer and partial peeling of the fibrils from the bundles, or from enlargement of pores between microfibrils [47]. This could support the interpretation of steam modification producing larger pores in absence of thermal stresses, as postulated above. The larger pores following the opening of opening of microfibril bundles would naturally lead to higher surface area and theoretically, a higher accessibility. However, the clear reduction of accessibility and the changes induced onto it by extraction prove the theory of pore blockage by extractives and/or degradation products of extractives.
Thermal modification of Scots pine under saturated steam leads to changes in the composition of the material, reducing the sorption due to preferential degradation of hydrophilic components. Higher accessibility and sorption rate would be anticipated due to increased porosity and surface area, with the opening of structure determined by SAXS supporting the same hypothesis. Extraction increased the sorption and accessibility, and therefore it is quite obvious that the extractives or other soluble degradation products play an important role in the hygroscopic behaviour or thermally modified material. It has already been shown that as the modification process is a closed system, recombination processes dominate and the degradation products may accumulate in the wood [49]. However, how much the accessibility differs from traditionally modified wood, e.g. ThermoWood, remains for further analysis. According to these results, it is postulated that the degradation products block access into the modified wood material, thereby affecting the sorption properties. The composition of these products should be investigated more closely to reveal their impact on the characteristics and stability of modified wood in use.
Conclusions
The properties of Scots pine thermally modified in a high-pressure reactor were analysed with respect to chemical and ultrastructural changes. The chemical analysis revealed a gradual loss of hemicellulose fractions, and an increase of glucose and lignin because of preferential degradation of amorphous carbohydrates under heat, and possible formation of lignin-like compounds. Interfibrillar distance determined by SAXS increased, which indicated opening of microfibril bundles and/or enlargement of pores and increasing surface area. However, as the accessibility reduced with increasing modification severity, it was postulated that, in addition to changes in the hygroscopic behaviour, there was something blocking access of water molecules inside the modified structure. As the extracted samples exhibited higher accessibility and slightly higher sorption than their unextracted equivalents, it is concluded that non-polar degradation products possess an active role of blocking the pores and reducing hygroscopicity of thermally modified wood.
References
Hill CAS (2007) Wood modification: chemical, thermal and other processes, 2nd edn. Wiley, Chischester
Navi P, Sandberg D (2012) Thermo-hydro-mechanical wood processing, 1st edn. CRC Press, Lausanne
Sandberg D, Kutnar A (2016) Thermally modified timber: recent developments in Europe and North America. Wood Fiber Sci 48:28–39
Boonstra MJ, Tjeerdsma B (2006) Chemical analysis of heat treated softwoods. Holz Roh Werkst 64(3):204–211
Boonstra MJ, Van Acker J, Tjeerdsma B, Kegel EV (2007) Strength properties of thermally modified softwoods and its relation to polymeric structural wood constituents. Ann For Sci 64:679–690. doi:10.1051/forest:2007048
Esteves B, Pereira H (2009) Wood modification by heat treatment: a review. BioResources 4(1):370–404
Willems W (2009) A novel economic large-scale production technology for high-quality thermally modified wood. In: European conference on wood modification. Stockholm, pp 31–35
Sivonen H, Maunu SL, Sundholm F, Jämsä S, Viitaniemi P (2002) Magnetic resonance studies of thermally modified wood. Holzforschung 56(6):648–654
Nuopponen M, Vuorinen T, Jämsä S, Viitaniemi P (2005) Thermal modifications in softwood studied by FT-IR and UV resonance raman spectroscopies. J Wood Chem Technol 24(1):13–26. doi:10.1081/WCT-120035941
Bourgois J, Guyonnet R (1988) Characterization and analysis of torrefied wood. Wood Sci Technol 22:143–155
Windeisen E, Strobel C, Wegener G (2007) Chemical changes during the production of thermo-treated beech wood. Wood Sci Technol 41(6):523–536. doi:10.1007/s00226-007-0146-5
Alén R, Kotilainen R, Zaman A (2002) Thermochemical behavior of Norway spruce (Picea abies) at 180–225 °C. Wood Sci Technol 36:163–171. doi:10.1007/s00226-001-0133-1
Brito JO, Silva FG, Leão MM, Almeida G (2008) Chemical composition changes in eucalyptus and pinus woods submitted to heat treatment. Bioresour Technol 99:8545–8548. doi:10.1016/j.biortech.2008.03.069
Rautkari L, Honkanen J, Hill CAS, Ridley-Ellis D, Hughes M (2014) Mechanical and physical properties of thermally modified Scots pine wood in high pressure reactor under saturated steam at 120, 150 and 180 °C. Eur J Wood Wood Prod 72(1):33–41. doi:10.1007/s00107-013-0749-5
Tjeerdsma BF, Boonstra MJ, Militz H (1998) Thermal modification of non-durable wood species. 2. Improved wood properties of thermally treated wood. International Research Group on Wood Preservation, Document no. IRG/WP, 98-40124. http://www.irg-wp.com/irgdocs/details.php?928f2680-9e22-45f9-b0a1-0ddd090af5f0. Accessed 8 Dec 2015
Militz H (2002) Thermal treatment of wood: European processes and their background, Document no. IRG/WP40241
Wikberg H, Maunu SL (2004) Characterisation of thermally modified hard- and softwoods by 13C CPMAS NMR. Carbohydr Polym 58(4):461–466
Bhuiyan MTR, Hirai N (2005) Study of crystalline behavior of heat-treated wood cellulose during treatments in water. J Wood Sci 51:42–47
Hill CAS, Ramsay J, Keating B, Laine K, Rautkari L, Hughes M, Constant B (2012) The water vapour sorption properties of thermally modified and densified wood. J Mater Sci 47:3191–3197. doi:10.1007/s10853-011-6154-8
Bhuiyan MTR, Hirai N, Sobue N (2000) Changes of crystallinity in wood cellulose by heat treatment under dried and moist conditions. J Wood Sci 46(6):431–436
Dwianto W, Tanaka F, Inoue M, Norimoto M (1996) Crystallinity changes of wood by heat or steam treatment. Wood Res Bull Wood Res Inst Kyoto Univ 83:47–49
Guo J, Song K, Salmén L, Yin Y (2015) Changes of wood cell walls in response to hygro-mechanical steam treatment. Carbohydr Polym 115:207–214
Suchy M, Virtanen J, Kontturi E, Vuorinen T (2010) Impact of drying on wood ultrastructure observed by deuterium exchange and photoacoustic FT-IR spectroscopy. Biomacromolecules 11:515–520
Borrega M, Kärenlampi PP (2010) Hygroscopicity of heat-treated Norway spruce (Picea abies) wood. Eur J Wood Wood Prod 68(2):233–235. doi:10.1007/s00107-009-0371-8
Borrega M, Kärenlampi PP (2008) Mechanical behavior of heat-treated spruce (Picea abies) wood at constant moisture content and ambient humidity. Holz Roh Werkst 66(1):63–69. doi:10.1007/s00107-007-0207-3
Hill CAS, Norton AJ, Newman G (2010) The water vapour sorption properties of Sitka spruce determined using a dynamic vapour sorption apparatus. Wood Sci Technol 44:497–514. doi:10.1007/s00226-010-0305-y
Rautkari L, Hill CAS, Curling S, Jalaludin Z, Ormondroyd G (2013) What is the role of the accessibility of wood hydroxyl groups in controlling moisture content? J Mater Sci 48(18):6352–6356. doi:10.1007/s10853-013-7434-2
Xie Y, Hill CAS, Jalaludin Z, Curling SF, Anandijwala RD, Norton AJ, Newman G (2011) The dynamic vapour sorption behaviour of natural fibres and kinetic analysis ysing the parallel exponential kinetics model. J Mater Sci 46(2):479–489. doi:10.1007/s10853-010-4935-0
Kamdem DP, Pizzi A, Jermannaud A (2002) Durability of heat-treated wood. Holz Roh Werkst 60(1):1–6
Gonzalez-Peña MM, Breese MC, Hill CAS (2004) Hygroscopicity in heat-treated wood: effect of extractives. In: Proceedings of ICECFOP1: 1st international conference on environmentally compatible forest products, pp 105–119
Rousset P, Lapierre C, Pollet B, Quirino W, Perre P (2009) Effect of severe thermal treatment on spruce and beech wood lignins. Ann For Sci 66(1):110. doi:10.1051/forest/2008078
Nuopponen M, Vuorinen T, Jämsä S, Viitaniemi P (2003) The effects of a heat treatment on the behaviour of extractives in softwood studied by FTIR spectroscopic methods. Wood Sci Technol 37(2):109–115
Esteves B, Graça J, Pereira H (2008) Extractive composition and summative chemical analysis of thermally treated eucalypt wood. Holzforschung 62(3):344–351
Manninen A, Pasanen P, Holopainen J (2002) Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmos Environ 36:1763–1768
Fengel D, Wegener G (1984) Wood: chemistry, ultrastructure, reactions. Walter de Gruyter Co, Berlin
Prins MJ, Ptasinski KJ, Janssen FJJG (2006) Torrefaction of wood Part 1. Weight loss kinetics. J Anal Appl Pyrolysis 77:28–34
Boonstra MJ, Rijsdijk JF, Sander C, Kegel E, Tjeerdsma B, Militz H, van Acker J, Stevens M (2006) Microstructural and physical aspects of heat treated wood. Part 1. Softwoods Maderas Cienc Tecnol 8(3):193–208
Hietala S, Maunu SL, Sundholm F, Jämsä S, Viitaniemi P (2002) Structure of thermally modified wood studied by liquid state NMR measurements. Holzforschung 56(5):522–528
Zauer M, Hempel S, Pfriem A, Mechtcherine V, Wagenführ A (2014) Investigations of the pore-size distribution of wood in the dry and wet state by means of mercury intrusion porosimetry. Wood Sci Technol 48(6):1229–1240. doi:10.1007/s00226-014-0671-y
Pizzi A, Stephanou A, Boonstra MJ, Pendlebury AJ (1994) A new concept on the chemical modification of wood by organic anhydrides. Holzforschung 48:91–94
Ismadji S, Sudaryanto Y, Hartono SB, Setiawan LEK, Ayucitra A (2005) Activated carbon from char obtained from vacuum pyrolysis of teak sawdust: pore structure development and characterization. Bioresour Technol 96:1364–1369
Pönni R, Rautkari L, Hill CAS, Vuorinen T (2014) Accessibility of hydroxyl groups in birch kraft pulps quantified by deuterium exchange in D2O vapor. Cellulose 21:1217–1226
Kymäläinen M, Rautkari L, Hill CAS (2015) Sorption behaviour of torrefied wood and charcoal determined by dynamic vapour sorption. J Mater Sci 50:7673–7680. doi:10.1007/s10853-015-9332-2
Hill CAS, Norton A, Newman G (2009) The water vapor sorption behavior of natural fibers. J Appl Polym Sci 112:1524–1537
Jalaludin Z, Hill CAS, Xie Y, Samsi HW, Husain H, Awang K, Curling SF (2010) Analysis of the water vapour sorption isotherms of thermally modified acacia and sesendok. Wood Materi Sci Eng 5(3–4):194–203
Penttilä PA, Kilpeläinen P, Tolonen L, Suuronen J-P, Sixta H, Willför S, Serimaa R (2013) Effects of pressurized hot water extraction on the nanoscale structure of birch sawdust. Cellulose 20:2335–2347
Virtanen T, Penttilä PA, Maloney TC, Grönqvist S, Kamppuri T, Vehviläinen M, Serimaa R, Maunu SL (2015) Impact of mechanical and enzymatic pretreatments on softwood fibre wall structure studied with NMR spectroscopy and X-ray scattering. Cellulose 22:1565–1576
Leppänen K, Andersson S, Torkkeli M, Knaapila M, Kotelnikova N, Serimaa R (2009) Structure of cellulose and microcrystalline cellulose from various wood species, cotton and flax studied by X-ray scattering. Cellulose 16:999–1015
Altgen M, Willems W, Militz H (2015) Wood degradation affected by process conditions during thermal modification of European beech in a high-pressure reactor system. Eur J Wood Prod 74(5):653–662
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding this work.
Rights and permissions
About this article
Cite this article
Kymäläinen, M., Ben Mlouka, S., Belt, T. et al. Chemical, water vapour sorption and ultrastructural analysis of Scots pine wood thermally modified in high-pressure reactor under saturated steam. J Mater Sci 53, 3027–3037 (2018). https://doi.org/10.1007/s10853-017-1714-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1714-1