Abstract
This study aimed to investigate the phenolic composition and antioxidant capacity of Inula viscosa L. aerial parts as influenced by their geographic origin and the type of extractant used. We established the extraction yield and phenolic composition of I. viscosa plants supplied from Mediterranean, Aegean, and Black Sea regions and evaluated their antioxidant capacity. Hierarchical clustering agglomerative (CA) and principal components analysis (PCA) was performed for further evaluation of similarities and differences among the I. viscosa extracts. Based on PCA and CA, the plants were specified in three distinct groups; one group presented higher bioactive composition and more potent antioxidative properties. The extractant type was one of the parameters affecting the clustering of the plants on the PCA biplot and CA dendrogram. Amongst the screened plants, Plant 1 was discriminated by its higher extraction efficiency, bioactive compounds, and antioxidant capacity compared to other plants. Ethanol was the most effective extractant studied when compared with ethyl acetate and hexane in terms of extraction yield, phenolic composition, and antioxidant capacity. Due to our findings, the phenolic composition was successfully used as a biochemical indicator to specify natural I. viscosa plants. The results highlighted that I. viscosa plant could be an excellent natural source of antioxidants to be evaluated in food and pharmaceutical industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accumulation of reactive oxygen species during oxidative metabolic processes is a vital factor in diseases such as diabetes, aging, inflammation, cardiovascular, neurodegenerative, cancer, and brain dysfunction [1]. Thus, investigations of antioxidants to decrease disease incidence due to oxidative damage are fundamental [2]. Synthetic antioxidants are also used in the food and cosmetic industry to increase the stability of products [3]. However, research and developments have been pushed into novel, safe, efficient natural antioxidants from fruits, vegetables, and herbs because of synthetic antioxidants' recent side effects [2].
Herbs are the focus of the world as a source of new antioxidant compounds since they are safer than synthetic antioxidants [4]. Many natural extracts from herbs containing phenolic and flavonoid compounds have excellent biological properties and are used as alternative therapies [5]. Among the extensive diversity of Mediterranean folkloric herbs, Inula viscosa belonging to the Asteraceae family, has proven to be a source of natural products forming the basis for alternative medicine and natural therapies [5]. In the Mediterranean region, I. viscosa is being applied for years in traditional medicine for its anti-inflammatory, antipyretic, antiseptic, antiphlogistic activities and to treat various diseases, such as cancer, bronchitis, diabetes, and injuries [6, 7]. Several flavonoids were isolated from I. viscosa aerial parts and their resinous exudate. Some of these compounds' antiproliferative, antimicrobial, and apoptosis efficacy and the diversity in hydroxycinnamic acids, namely mono- and dicaffeoylquinic acids, allowed Inula leaves to be considered a potential source for food additives and preservatives [7,8,9,10].
The present study aimed to (i) perform a quantitative analysis of the extraction yield, antioxidant capacity, total phenolic, flavonoid, condensed tannin, and caffeoylquinic acid content in different solvent extracts of I. viscosa aerial parts collected from ten different geographical regions of Turkey (ii) correlate phenolic composition with the antioxidant activities since such a comparative study of different varieties of I. viscosa has not been reported (iii) discriminate between ten I. viscosa plants native to Turkey by chemometric methods using phenolic compositions and antioxidant activities. This information is essential since little data are currently available concerning the effect of different geographical conditions and extraction solvents on changes in antioxidant capacity and bioactive properties of I. viscosa.
Materials and methods
Materials
Folin–Ciocalteau phenol reagent and sodium carbonate were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). All solvents and reagents were analytical or chromatographic grade and received from Merck (Darmstadt, Germany).
Plants materials and preparation of crude extracts
Plants were collected from July to September 2019 during the plant's growing season from different cities of Turkey in the Mediterranean, Aegean, and Black Sea regions and dried at ambient conditions. The dry matter content of the dried plants was between 94.10 and 95.90%. The agricultural engineers identified the plants (Directorates of Provincial Agriculture and Forestry, Republic of Turkey Ministry of Agriculture and Forestry). All samples were codified as ‘P’ with a respective number for recognition (1–10) in this study. P1, P2, P3, P4, P5, P6, P7, P8, P9, and P10 correspond to plants supplied from cities of Antalya, Çanakkale, Zonguldak, Trabzon, Hatay, Mersin, Manisa, Kastamonu, Muğla, and Balıkesir, respectively. Figure 1 shows the sampling locations.
Geographic locations of the collection regions of Turkish I. viscosa. Maps generated by Scribble Maps [11]
Dried plants were ground to finer particles with a grinder and subjected to extraction by three different organic solvents with decreasing polarity index: ethanol, ethyl acetate, and hexane, respectively. For ethanol and ethyl acetate, extraction of the plants was done at a solid to liquid ratio of 10% (w/v) for 6 h at 25 °C in a rotary incubator (New Brunswick Scientific, Nova 40, Edison, NJ, USA). The optimum extraction time of 6 h was regulated by trying the time between 1 and 8 h. The residues were separated by centrifugation at 4 °C and 6000 rpm for 10 min. The supernatants were concentrated in a rotary vacuum evaporator (Heidolph Instrument GmbH & Co.KG. Schwabach, Germany) at 40 °C and then kept at 4 °C for subsequent analysis. For hexane, 10 g of the ground plant was extracted using 250 mL of n-hexane in a Soxhlet apparatus for 6 h as mentioned by Ghorbanzadeh and Rezaei [12]. n-hexane was then removed at 50 °C using a rotary evaporator (Heidolph Instrument GmbH & Co.KG. Schwabach, Germany). The extracts were stored at 4 °C. The extraction yield was calculated as follows:
Total phenolic content (TPC)
TPC of the extracts was determined using Folin–Ciocalteau colorimetric method suggested by Maisuthisakul et al. [13]. The extract (1 mg) was dissolved in 10 mL methanol, and then 0.2 mL of diluted extract was mixed with 1 mL of Folin–Ciocalteau reagent. After 3 min, 0.8 mL of 7.5% Na2CO3 was added to the mixture, and it was incubated in the dark for 30 min. The absorbance was read at 765 nm using Lambda 25 UV/Vis spectrophotometer (PerkinElmer). Different concentrations of gallic acid (12.5–400 mg/L) were used to plot a calibration curve. The results were indicated as milligram of gallic acid equivalent per gram of extract (mg GAE/g extract).
Total flavonoid content (TFC)
TFC was analyzed using the aluminum chloride colorimetric technique as suggested by Chang et al. [14]. 100 µL of 10% (w/v) aluminum chloride and 100 µL of 0.1 mM potassium acetate were added to 2 mL of extract solution (1 mg extract/mL methanol). The mixture was diluted to 4 mL and rest for 30 min at dark. The absorbance values were then read at 415 nm using a UV–visible spectrophotometer. Quercetin was prepared at different concentrations between 0.1 and 0.02 mg/mL to plot a standard curve. Total flavonoid content was reported as milligram of quercetin per gram of extract (mg quercetin/g extract).
Condensed tannin analysis (CTC)
CTC was determined by applying the procedure suggested by Price et al. [15]. 0.5 mL of the extract solution (0.02 g extract/mL methanol) was mixed vigorously with 3 mL vanillin (4%, in methanol) and 1.5 mL concentrated HCl. The samples were left at 20 °C for 20 min. Then, absorption (Asample) was read at 500 nm with a Lambda 25 UV/Vis spectrophotometer (PerkinElmer). The control for each extract solution was prepared by adding 3 mL methanol and 1.5 mL concentrated HCl to 0.5 mL extract. The controls were left at 20 °C for 20 min and then the absorbance (Acontrol) was measured at 500 nm. The absorbance of the control (no vanillin) is subtracted from the absorbance of the corresponding vanillin-containing sample. A standard curve was plotted using catechin at concentrations of 0.05–0.03 mg/mL. CTC was expressed as milligram catechin equivalent per gram of extract (mg CE/g extract).
Caffeoylquinic acid content (CQC)
Molybdate assay was applied to calculate CQC of plant extracts as suggested by Chan et al. [16]. Sodium molybdate (16.5 g), dipotassium hydrogen phosphate (8.0 g), and potassium dihydrogen phosphate (7.9 g) were dissolved in 1 L deionized water to prepare molybdate reagent. A 0.2 mL of each extract dissolved in methanol (1 mg/mL) was mixed with 10 mL molybdate reagent. Absorption was measured at 370 nm with a Lambda 25 UV/Vis spectrophotometer (PerkinElmer) against a blank prepared for each extract. The blanks were prepared by mixing 0.2 mL of each extract dissolved in methanol (1 mg/mL) and 10 mL potassium phosphate buffer solution. A standard curve was plotted using chlorogenic acid within the concentrations of 0.1–0.02 mg/mL. Caffeoylquinic acid content was presented as milligram chlorogenic acid equivalent per gram extract (mg CAE/g extract).
Antioxidant capacity
The free radical scavenging activity of plant extracts was detected based on a method proposed by Maisuthisakul et al. [13]. Different concentrations of extract solutions were prepared in methanol and were added to 3.5 ml of 0.2 mM methanolic solution of DPPH. These mixtures were held for 30 min in dark. The absorbance of each sample was read at 517 nm using Lambda 25 UV/Vis spectrophotometer (PerkinElmer). The control was performed using methanol instead of the extract. Scavenging activity (%) was determined as follows:
where Asample represents the A517 of the sample extracts, Acontrol represents the A517 of the control. The antioxidant capacity of the extracts was declared as IC50, which is the sufficient concentration at which DPPH radicals were scavenged by 50% and was determined by linear regression analysis between the extract concentration and the scavenging activity (%).
Statistical analysis
Analysis of variance (ANOVA) and Tukey's post hoc test was performed to establish whether the differences in extraction yield and bioactive properties of the plant extracts were statistically significant using SPSS 20 software (SPSS Inc., Chicago, IL, USA). Statistical significance was determined at p < 0.05. Pearson correlation analyses were performed with SPSS 20 software. Principle component analysis (PCA) and hierarchical cluster analysis (CA) were performed using Origin Lab software version 2019 (Northampton, MA, USA). All determinations were carried out in three replicates and experimental results were expressed as means ± standard deviations or average.
Results and conclusion
Extraction yields
Statistically significant differences were observed with respect to extraction solvent and plant growing region according to ANOVA results (p < 0.05). The highest extraction yields of I. viscosa plants were obtained in ethanol (34.53% for plant 1), whereas the lowest yield was in hexane (11.50% for plant 9) (p < 0.05). Higher extraction yields obtained may be related to the fact that most of the flavonoids, phenolic acids, and anthocyanins are soluble in ethanol or methanol as suggested by Prasad et al. [17].
Plant 1 gave the highest percentage yield amongst the investigated plant sources for all solvents. The extraction yields of Plant 1 were 34.53, 31.56, and 28.56% for ethanol, ethyl acetate, and hexane, respectively. Plant 9 gave the lowest extraction yields for all solvents compared to other plant sources (Fig. 2). The differences amongst the extraction yields of I. viscosa plants obtained from different geographical origins might result from the different chemical composition of plants, leading to various availability of extractable components as already outlined by Sultana et al. and Hsu et al. [18, 19]. Chahmi et al. [20] found relatively lower extraction yields of I. viscosa aerial part from three regions of Morocco within the range of 13.35–23.90% for ethanol and 10.50–21.30% (g of dry matter) for ethyl acetate extractions. Salim et al. [21] reported the ethanol extraction efficiency of the whole I. viscosa plant as 3.1% which is extensively lower than those of presented I. viscosa plants.
Extraction efficiency of I. viscosa aerial part collected from different regions in different kinds of solvents. Different capital letters represent significant differences (p < 0.05) between different solvent type in the same plant origin and different lowercase letters represent significant differences (p < 0.05) between the same solvent type in different plant origins of I. viscosa
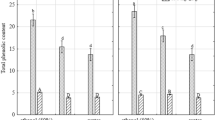
Total phenolic content
TPC of the extracts depended on the extractant type and region of the plant (p < 0.05). I. viscosa plant extracts exhibited TPC at various degrees due to the polarity of extractant used (ethanol PI = 5.2, ethyl acetate PI = 4.3, and hexane PI = 0.1) (Fig. 3). The order of effectiveness in the extraction of phenolic compounds was ethanol (281–634.2 mg GA/g plant extract) > ethyl acetate (92.8–567 mg GA/g plant extract) > hexane (63–403.2 mg GA/g plant extract) (Fig. 3). Iloki-Assanga et al. noticed that phenolic compounds' extraction depends on the type of used extractant, its polarity index, and the solubility of phenolic compounds in the used extractant and stated that polar solvents are more effective in the extraction of phenolic compounds [22]. Besides, TPC of the plant extracts varied strongly depending on the plant growing area (p < 0.05). Plant 1 comprised the highest TPC for all extractants (Fig. 3). TPC results were 2–3-fold higher than the previously reported TPC results for I. viscosa from different geographical origins, which were 177 mg GA/g extract [6], 299.1 ± 34.5 mg GA/g extract [7], 140 to 274 mg GAE/g extracts [20], and 103 mg GA/g extract [8].
Total phenolic content in different solvent extracts of I. viscosa aerial part collected from different regions. Different capital letters represent significant differences (p < 0.05) between different solvent type in the same plant origin and different lowercase letters represent significant differences (p < 0.05) between the same solvent type in different plant origins of I. viscosa. GAE, gallic acid equivalent
Total flavonoid content
The TFC of I. viscosa plants extracted using different organic solvents is presented in Fig. 4. There was a statistically significant correlation between solvent type and TFC of the plant extracts (p < 0.05). It was clear that higher content of flavonoids could be extracted with increasing polarity of the extractant. The TFC of extract ranged from 57.56 to 98.27 mg quercetin/g for ethanol, from 45.36 to 90.67 mg quercetin/g for the ethyl acetate, and from 42.98 mg quercetin/g to 74.19 mg quercetin/g for the hexane. Ethanol was statistically most efficient (p < 0.05) for extraction of flavonoids from I. viscosa plants, while hexane gave the lowest flavonoid content. In this manner, our results agree with Li et al. [23] and Sriti Eljazi et al. [24], who reported that an extractant with a greater polarity could yield higher total phenolic and flavonoid contents.
Total flavonoid content in different solvent extracts of I. viscosa aerial part collected from different regions. Different capital letters represent significant differences (p < 0.05) between different solvent type in the same plant origin and different lowercase letters represent significant differences (p < 0.05) between the same solvent type in different plant origins of I. viscosa. QE, quercetin equivalent
The ethanolic extract of Plant 1 offered the highest TFC (98.27 mg quercetin/g extract) followed by the ethanolic extract of Plant 2 (92.25 mg quercetin equivalent/g extract). The lowest TFC was obtained using I. viscosa plant collected from Plant 10 for all extraction solvents (57.56, 45.36, and 42.98 mg quercetin/g for ethanol, ethyl acetate, and hexane extract, respectively) (Fig. 4). The current work presented higher TFC in I. viscosa than those formerly documented by Chahmi et al. [19], 44 mg quercetin/g extract for both ethanol and ethyl acetate extracts. Fortunately, the I. viscosa plant studied exhibited a relatively higher TFC than previous studies dealing with different plant sources [25,26,27].
Condensed tannin content
CTC highlighted significant variations (p < 0.05) amongst the plant extracts depending on the geographical origin of the plant and extractant type (Fig. 5). CTC of the extracts was detected in the range of 70.76–15.44 mg catechin/g extract in ethanol, 58.12–8.12 mg catechin/g extract in ethyl acetate, and 47.05–6.81 mg catechin/g extract in hexane. CTC of the ethanol extracts was significantly (p < 0.05) higher than ethyl acetate and hexane extracts. The highest CTC was observed in Plant 1 (70.76–47.05 mg catechin/g extract) while Plant 10 (15.44–6.81 mg catechin/g extract) gave the lowest CTC for all solvents tested (Fig. 5). Rhimi et al. [5] evaluated the lower CTC of I. viscosa leaves collected from Tunisia ranged between 7.05 ± 1.6 and 27.15 ± 2.21 mg CE/g. Besides, Mahmoudi et al. [8] reported 84.32 mg catechin/g I. viscosa in the aqueous methanolic extract which is higher than our findings. Comparatively, CTC of the investigated I. viscosa plants is higher than different plant species grown in the Mediterranean area, ranging between 0 and 40 mg condensed tannin/g extract reported by Piluzza and Bullitta [26].
Condensed tannin content in different solvent extracts of I. viscosa aerial part collected from different regions. Different capital letters represent significant differences (p < 0.05) between different solvent type in the same plant origin and different lowercase letters represent significant differences (p < 0.05) between the same solvent type in different plant origins of I. viscosa. CE, catechin equivalent
Caffeoylquinic acid content
As seen in Fig. 6, plant growing area and the extraction solvent affected the CQC significantly (p < 0.05). The extraction efficiency of caffeoylquinic acid decreases with different solvents in the order: ethanol (53.14–116.2 mg CA/g extract) > ethyl acetate (45.29–97.69 mg CA/g extract > hexane (41.59–95.57 mg CA/g extract). These differences in CQC may result from the higher solubility of mono and dicaffeoylquinic acids in the lower alcohols or alcohol–water mixtures. Dicaffeoylquinic acids are soluble in ethyl acetate, butyl acetate, and acetone by Wianowska and Gil [28]. At the geographical level, the highest CQC (116.2 mg chlorogenic acid/g extract) was observed in Plant 1, while the lowest was observed in Plant 10 (53.14 mg chlorogenic acid/g extract).
Caffeoylquinic acid content in different solvent extracts of I. viscosa aerial part collected from different regions. Different capital letters represent significant differences (p < 0.05) between different solvent type in the same plant origin and different lowercase letters represent significant differences (p < 0.05) between the same solvent type in different plant origins of I. viscosa. CE, chlorogenic acid equivalents
Antioxidant capacity
The extractant and growing area were (p < 0.05) efficient on the antioxidant activities of the plant extracts according to ANOVA results (Fig. 7). Ethanol was found the most efficient extractant for higher antioxidative capability (IC50 = 1.37–27.21 µg/mL), whereas lower antioxidant activities were obtained from samples extracted with ethyl acetate (IC50 = 6.09–34.87 µg /mL) and hexane (IC50 = 16.65–38.01 µg/mL), respectively. Statistically significant differences in IC50 values of the plant extracts obtained using different solvents may result from various chemical characteristics and polarities of phenolic compounds present in the plants. Rotta et al. [29] declared that solvent characteristics are crucial parameters that influence the extraction capacity of phenolic compounds. These compounds were thought to perform a prominent role in the antioxidant capacity of the plant.
Radical scavenging activity (IC50) of I. viscosa aerial part extracts collected from different regions. Different capital letters represent significant differences (p < 0.05) between different solvent type in the same plant origin and different lowercase letters represent significant differences (p < 0.05) between the same solvent type in different plant origins of I. viscosa
The ethanol extract of Plant 1 gave the highest DPPH radical activity (IC50 = 1.37 μg/mL). The lowest antioxidant capacity was observed for Plant 10, being 27.21, 34.87, and 38.01 µg/mL for ethanol, ethyl acetate, and hexane extracts, respectively. Indeed, the extracts of all investigated I. viscosa plants presented low IC50 values, which are stronger markers of antioxidative capability. Most of the investigated I. viscosa plants supplied from different regions of Turkey showed remarkably stronger antioxidant capacity than those reported for the same species from Algeria (IC50 = 14.1 μg/mL) [7] and Morocco (0.18–1.86 g/L) [20]. The high efficiency of scavenging activity of the I. viscosa extracts may be due to its high and effective phenolic composition.
Pearson's correlation analysis between extraction yield, phenolic composition, and antioxidant capacity
Pearson's correlation coefficients between the extraction yield, phenolic composition, and antioxidant capacity showed a strong and significant relationship (p < 0.01) with respect to each other at different extractants (Table 1). The results showed a negatively strong correlation between extraction yield, TPC, TFC, CTC, CQC, and IC50 value for all solvents investigated. The negative interactions resulted from the reverse relation between IC50 value and antioxidant capacity. Pearson's correlation analysis assayed a high and negative correlation between TPC and antioxidant capacity at p < 0.01 level (ethanol r = − 0.942, ethyl acetate r = − 0.904, hexane r = − 0.639). Likewise, a strong correlation was also available between TFC and IC50 value (ethanol r = − 0.895, ethyl acetate r = − 0.879, hexane R = − 0.813). Many researchers have also described a positive correlation between TPC, TFC, and antioxidant capacity of different plant extracts [27, 30]. In contrast, no substantial correlation was obtained between TFC and antioxidant capacity of the plant samples in some studies [25, 26].
CTC was one of the significant bioactive compounds of I. viscosa for its antioxidant ability (ethanol r = − 0.946, ethyl acetate r = − 0.898, hexane r = − 0.815). Koleckar et al. [31] reported that tannins have good antioxidant potential due to their high molecular weight and high hydroxylation of aromatic rings. CQC was also effective on the antioxidant capacity of I. viscosa plant with a high Pearson's correlation coefficient (ethanol r = − 0.896, ethyl acetate r = − 0.857, hexane r = − 0.793). The high correlation between CQC and IC50 is parallel to the results of Danino et al. [32], who demonstrated that 1,3-dicaffeoylquinic acid exhibits antioxidant properties, probably through the involvement of a direct scavenging effect on several free radicals. The high R values between TPC, TFC, CTC, CQC, and IC50 values showed that phenolic compounds are the major contributors to the antioxidant capacity of I. viscosa extracts. CTC and CQC seem to be the most effective groups of polyphenols in I. viscosa, indicating high and significant correlations with IC50 value. Besides, ethanol presented the highest R values in the relation between bioactive content and IC50 (Table 1).
Hierarchical cluster analysis
Cluster analysis was performed using all observed variables (extraction yield, TPC, TFC, IC50, CTC, CQC) depending on the extractant type and the results are shown as a dendrogram in Fig. 8. Group I showed fewer similarities compared to Groups II and III due to the long Euclidean distance to that group (Fig. 8). The first group included ethanolic extracts of Plants 1, 2, 4, and 5, and these plants showed greater similarity. However, Plant 1 differed from Plant 2, 4, and 5 according to the linkage distance within the group. Ethanol extract of Plant 1 presented higher extraction yield (34.53%, g extract/g plant) as well as higher TPC (634 mg gallic acid/g extract), TFC (98.27 mg quercetin /g extract), CTC (70.76 mg catechin/g extract), and CQC (116.2 mg chlorogenic acid equivalents/g extract) resulting in a strong antioxidant capacity (IC50 = 1.37 µg extract/mL). Additionally, the ethyl acetate extract of Plant 1 belongs to that cluster. Group II contains the extracts which contain moderate levels of extraction yield, bioactive content, and antioxidant capacity. Group III was composed of ethyl acetate and hexane extracts of Plant 7, 9, and 10, which were the samples containing the lowest bioactive contents (Figs. 2 and 7).
Principle component analysis
PCA was performed to analyze the relationships between the different variables, determine the optimum number of extracted principal components, and decrease the investigated variables (Extraction yield, TPC, IC50, TFC, CTC, and CQC) in a smaller number of variables (principal component).
The first two principal components (PC1 and PC2) represented 83.32% and 9.21% of the total data variance, providing a good approximation of the variation present in the data. PC1 showed a high correlation with TPC, IC50, TFC, CTC, and CQC, indicating that phenolic compounds and the antioxidant property of the I. viscosa plant were highly correlated. In the PCA plot (Fig. 9), the acute angle between the regression vectors of TPC, TFC, CTC, and CQC indicates the strong positive relationship with each other, while the opposite positioning of IC50 regression vector shows the inverse relationship of IC50 with TPC, TFC, CTC, and CQC. Additionally, PC2 is correlated with extraction yield showing the variation between samples with regard to extraction yield. Extraction yield has a moderate correlation with TPC, TFC, CTC, and CQC (Fig. 9). The results were in accordance with the Pearson correlation analysis (Table 1).
According to the PCA biplot, ethanol extract of Plant 1 with the richest phenolic composition and strongest DPPH radical scavenging activity was situated furthest to the right along PC1 (Fig. 9). The plants with relatively low levels of phenolic content and weak scavenging activities against DPPH radicals were on the opposite side of PC1. The plants 7, 9, and 10 which have low TPC, TFC, CTC, and CQC with a high IC50 value were on the left side of the PC1. These three plants were grouped in Group III in the hierarchical analysis dendrogram (Fig. 8). Almost all the ethanol extracts located in the positive part of the PC1 were recognized with their relatively high TPC, TFC, CTC, and CQC values. Comparing the extraction yield, bioactive compounds, antioxidant capacity, CA, and PCA, it can be concluded that Plant 1 presented an isolated behavior compared with other plants due to its high bioactive content and radical scavenging activity.
Due to obtained results, variations in the extraction yield, bioactive composition, and antioxidative activity of I. viscosa plants selected from different geographical regions are not directly dependent on the distance between areas. It might be related to their natural environments, also suggested by Dolkar et al. [33], Kabtni et al. [34], and Zargoosh et al. [35]. In literature, it is explained that the concentration of plant secondary metabolites are influenced by environmental variations such as temperature, drought, salinity, seasonality, altitude, light, ultraviolet radiation, metal ions, wounding and nutrient deficiencies, growing conditions, and metabolic pathways of related metabolites [34, 36].
Conclusion
Significant variation was observed between I. viscosa plants collected from different geographical regions in Turkey in terms of extraction yields, phenolic composition, and antioxidant capacity. Plant 1 showed the highest extraction yield, phenolic composition, and antioxidant capacity. Further assessment of I. viscosa plants indicated that ethanol was the most efficient solvent for extracting polyphenol compounds compared to ethyl acetate and hexane. Pearson's correlation tests pointed out that the phenolic composition existing in I. viscosa aerial part shows an essential role in the antioxidant capacity. The kind of extractant was also identified as one of the factors specifying the plants on the PCA scatterplot and CA dendrogram. The prospective studies will consist of optimization of extraction and antioxidant studies in model systems to support the potential development of new plant-based natural antioxidants and extend their use in the food and pharmaceutical industries.
References
Anokwuru C, Sigidi M, Boukandou M, Tshisikhawe P, Traore A, Potgieter N (2018) Molecules 23:1303
Yang R, Guan Y, Wang WX, Chen HJ, He ZC, Jia AQ (2018) PLoS ONE 13:e0195508
Ulewicz-Magulska B, Wesolowski M (2019) Plant Foods Hum Nut 74:61–67
Kumar S, Yadav A, Yadav M, Yadav JP (2017) BMC Res Notes 10:60
Rhimi W, Ben Salem I, Iatta R, Chaabane H, Saidi M, Boulila A, Cafarchia C (2018) Ind Crops Prod 113:196–201
Gokbulut A, Ozhan O, Satilmis B, Batcioglu K, Gunal S, Sarer E (2013) Nat Prod Commun 8:475–478
Brahmi-Chendouh N, Piccolella S, Crescente G, Pacifico F, Boulekbache L, Hamri-Zeghichi S, Akkal S, Madani K, Pacifico S (2019) J Food Drug Anal 27:692–702
Mahmoudi H, Hosni K, Zaouali W, Amri I, Zargouni H, Ben Hamida N, Kaddour R, Hamrouni L, Ben Nasri M, Ouerghi Z (2016) J Food S 36:77–88
Talib WH, Zarga MH, Mahasneh AM (2012) Molecules 17:3291–3303
Seca AML, Grigore A, Pinto DCGA, Silva AMS (2014) J Ethnopharmacol 154:286–310
Scribble Maps. https://www.scribblemaps.com. Accessed on 20 May 2021.
Ghorbanzadeh R, Rezaei K (2017) J Am Oil Chem Soc 94:1491–1501
Maisuthisakul P, Suttajit M, Pongsawatmanit R (2007) Food chem 100:1409–1418
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) J Food Drug Anal 10:3
Price ML, Van Scoyoc S, Butler LG (1978) J Agric Food Chem 26:1214–1218
Chan EWC, Lim YY, Ling SK, Tan SP, Lim K, Khoo MG (2009) LWT Food Sci Technol 42:1026–1030
Prasad NK, Yang B, Zhao MM, Wang BS, Chen F, Jiang YM (2009) Int J Food Sci Technol 44:960–966
Sultana B, Anwar F, Ashraf M (2009) Molecules 14:2167–2180
Hsu B, Coupar IM, Ng K (2006) Food Chem 98:317–328
Chahmi N, Anissi J, Jennan S, Farah A, Sendide K, El Hassouni M (2015) Asian Pac J Trop Biomed 5:228–233
Salim H, Rimawi WH, Mjahed A (2017) J Chem and Biochem 5:12
Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, Gil-Salido AA, Fernandez-Angulo D, Rubio-Pino JL, Haines DD (2015) BMC Res Notes 8:1–14
Li H, Zhang D, Tan LH, Yu B, Zhao SP, Cao WG (2017) Afr J Bot 109:1–8
Sriti Eljazi J, Selmi S, Zarroug Y, Wesleti I, Aouini B, Jallouli S, Limam F (2018) Int J Food Prop 21:2309–2319
Kumar S, Sandhir R, Ojha S (2014) BMC Res Notes 7:560
Piluzza G, Bullitta S (2011) Pharma Biol 49:240–247
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Plants (Basel) 8:96
Wianowska D, Gil M (2019) Phytochem Rev 18:273–302
Rotta EM, Haminiuk CWI, Maldaner L, Visentainer JV (2017) Int J Food Sci Technol 52:954–963
Kainama H, Fatmawati S, Santoso M, Papilaya PM, Ersam T (2020) Pharm Chem J 53:1151–1157
Koleckar V, Kubikova K, Rehakova Z, Kuca K, Jun D, Jahodar L, Opletal L (2008) Mini Rev Med Chem 8:436–447
Danino O, Gottlieb HE, Grossman S, Bergman M (2009) Food Res Inter 42:1273–1280
Dolkar P, Dolkar D, Angmo S, Kumar B, Stobdan T (2017) J Ber Res 7:109–116
Kabtni S, Sdouga D, Bettaib Rebey I, Save M, Trifi-Farah N, Fauconnier ML, Marghali S (2020) Sci Rep 10:8293
Zargoosh Z, Ghavam M, Bacchetta G, Tavili A (2019) Sci Rep 9:16021
Verma N, Shukla S (2015) J App Res Med Arom Plants 2:105–113
Acknowledgements
This study was supported financially by the Scientific Research Projects Governing Unit of the University of Gaziantep is [Grant Number MF.YLT.20.01] gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported financially by the Scientific Research Projects Governing Unit of the University of Gaziantep, Turkey.
Compliance with ethics requirements
The article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keskin Çavdar, H., Yıldırım, Z.İ. & Fadıloğlu, S. Evaluation of the effect of geographical origin and extraction solvents on bioactive and antioxidative properties of Inula viscosa L. grown in Turkey by chemometric approach. Eur Food Res Technol 248, 253–261 (2022). https://doi.org/10.1007/s00217-021-03877-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03877-w