The radical scavenging assays of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic (ABTS) acid for various extracts and fractions of Garcinia lasoar Pam stem bark had been examined. The methanol extract showed high total phenolic (272.98 mg GAE (gallic acid equivalent)/100 g) and total flavonoid (223.16 mg QE (quercetin equivalent)/100 g) contents with potential antioxidant activity (DPPH: 0.72 μg/mL; ABTS: 0.24 μg/mL). The ethyl acetate fraction of methanol extract has higher activity than the dichloromethane fraction with IC50 values of 1.90 and 0.90 μg/mL in DPPH and ABTS assays, respectively. The Pearson correlation coefficients were calculated in order to identify the relationship between antioxidant activity and the total phenolic and total flavonoid contents. The antioxidant activity showed high positive relationship in both assays (r = 0.762, p < 0.05). The highest positive correlation between total flavonoid content and ABTS radical scavenging activity for methanol extract and then ethyl acetate had correlation between total flavonoid content. These results suggest that G. lasoar stem bark extracts can be used as a natural source of antioxidants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1. INTRODUCTION
Oxidative stress is a negative effect caused by free radicals in the organism, which can cause various diseases such as arteriosclerosis, diabetes, tumors, heart disease and aging [1, 2]. However, healthy humans can detoxify or eliminate these free radicals by antioxidant enzymes such as superoxide dismutase, catalase, and peroxide, and also by food derived antioxidants [3]. Natural sources of antioxidant such as vegetables, fruits and medicinal plants which are relatively cheaper and have fewer side effects are interesting objects for the investigation of new antioxidants such as flavonoids, stilbenes, xanthones and coumarins [4, 5]. At present, plants of the Guttiferae family (Clusiaceae) including Garcinia hombroniana, G. mangostana, G. brasiliensis, G. lateriflora var. javanica, G. Combogia. G.virgate, G. speciosa, G. celebica G. ferrea, G. bentami, G. subelliptica, G. cylindrocarpa are well-nown sources of a variety of biologically active natural compounds, e.g., xanthones, terpenoids, benzophenones, flavonoids. and depsidones [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Indonesia was reported to have 77 species of Garcinia family. Some of these species are consumable and have been cultivated, such as G. atroviridis, G. dulcis, G. mangostana, G. Nigrolineata, and G. parviflora, while others grow wild in the forests and are only used as logging [19]. Maluku Islands is known to be rich in herbs, spices, and endemic plants. Maluku is geographically located in the Wallacea region, which is unique and has large potential for growing extensive flora, in both primary and secondary forests. The genus Garcinia in Maluku has been reported to include 17 species, four of which are endemic [20].
The plant of G. lasoar, which is locally known as “manggustan utang”, is growing up in primary forests. The local people in Maluku, especially on Ambon island, have used this plant as treatment for tuberculosis, malaria, diabetes and for increasing immunity. To the best of the authors′ knowledge, no phytochemical investigations of this plant has been reported so far. It is also necessary to perform additional research related to the activities of G. lasoar as a folk medicine.
In this work, we have examined the antioxidant activity of G. lasoar stem bark in various extracts by using DPPH and ABTS assays. The total phenolic content (TPC) and total flavonoid content (TFC) of the extract were also determined. This paper provides a basic information on this Garcinia species that can be used as a natural antioxidant.
2. MATERIALS AND METHODS
2.1. Plant Material
The stem bark of G. lasoar was collected from Hattu Forest on Ambon island, Indonesia. This plant was identified and a voucher specimen (No. 51) was deposited at Biology Laboratory of the Biology Departement, Pattimura University, Indonesia.
2.2. Chemicals and Reagents
Petroleum ether, n-hexane, dichloromethane, ethyl acetate, methanol, ethanol, sodium carbonate, trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphanic acid) diammonium salt, DPPH (1,1-diphenyl-2-picrylhydrazyl), and DMSO, gallic acid, Folin – Ciocalteau (FC) reagent, and quercetin, all chemicals used for the analysis were of analytical grade.
2.3. Preparation of Extracts and Fractions
The stem bark of G. lasoar was dried at room temperature and reduced to coarse powder using a disk mill SMJMA, FFC-15, Shandong Jimo. Each dried stem bark powder (100 g) was mixed separately with 500 mL etanol 70%, ethyl acetate, dichloromethane and n-hexane. The solvent was evaporated under reduced pressure (Rotavapor R-210, Buchi, Switzerland) to obtain a solid mass extract of ethanol (EE; 12.8% w/w), ethyl acetate (EAE; 13.3% w/w), dichloromethane (DE; 3.8% w/w), and n-hexane (n-HE; 2.5 % w/w). Aliquots (20 g) of solid powder were extracted with 250 mL petroleum ether (PEE) in soxhlet extractor for 72 h at 55oC. The extract was filtered off and evaporated to yield extract (4.5% w/w). The aqueous extract (AE; 20.2 % w/w) was obtained by infusion in hot water, prepared just before use. The air-dried stem bark of G. lasoar (3.0 kg) was also extracted with methanol (ME) (3 × 7 L) by maceration at room temperature for three days. The extract was concentrated in vacuo to yield 30.0 g of brown crude extract, and then separated by vacuum liquid chromatography (VLC) on silica gel (300 g) with increasing solvent polarity to get n-hexane, dichloromethane (DF), ethyl acetate (EAF) and methanol fractions. EAF (46.66% w/w) and DF (23.33% w/w) fractions was further subjected to DPPH and ABTS assays.
2.4. DPPH and ABTS Free Radical Scavenging Assay
The DPPH radical scavenging activity was tested by method of Fitriana, et al. [21]. The ABTS radical scavenging assay was carried out according to the method of Hidayati, et al. [22]. Trolox was used as a positive control.
2.5. Phytochemical Analysis
Determination of TPC and TFC. Total phenolic and flavonoid contents were determined by Folin-Ciocalteu and aluminium chloride colometric methods, respectively [23, 24], followed by quantification on the basis of standard curve in terms of gallic acid (GAE) and quercetin (QE) equivalent, respectively.
2.6. Statistical Analysis
Determination of total phenolic and flavonoid contents and antioxidants activity by ABTS and DPPH assay was conducted in triplicate. The value for each sample was calculated as Mean ± SD. Correlation coefficients were calculated using SPSS v22. Differences at p < 0.05 were concidered to be significant.
3. RESULTS AND DISCUSSION
3.1. Free Radical Scavenging Activity of G. lasoar Extracts and Fractions in DPPH and ABTS Assays
In the present study, aqueous infusion and three different solvents were chosen to extract compounds of different polarities. Petroleum ether, n-hexane and dichloromethane were used to obtain nonpolar fractions. The remaining crude extract was then treated with ethyl acetate for extracting semipolar fractions and polar fractions were extracted by using methanol and ethanol. The aqueous extract was obtained by traditional empirical methods of green chemistry.
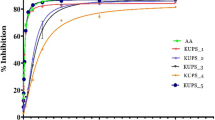
The antioxidant activity of seven extracts of G. lasoar stem bark and two fractions of methanol extract as tested using ABTS and DPPH assays is shown in Fig. 1. Concentrations of sample required to scavenge 50% of DPPH and ABTS radicals (IC50) are listed in Table 1. In tests at the ABTS concentration of 99.0 μg/mL, ME showed the maximum inhibition (96.97 1.39%; IC50 3.59 μg/mL) while PEE produced the lowest inhibition (20.42 1.20%) with IC50 value of >100 μg/mL. The results for ABTS showed trolox (IC50 value of 0.88 μg/mL) to exhibit lower activity of radical scavenging as compared to ME. The ME, AE and EE extracts have higher activity of DPPH radical scavenging than trolox.
The ME extract was the strongest inhibitor (98.58 ± 1.49%; IC50 = 0.24 μg/mL), while PEE was the weakest one (14.80 ± 1.28%; IC50 100) in DPPH concentration of 319.45 μg/mL in the radical scavenging activity test. In the same concentration, fractions from ME, EAF have higher activity than that of DF with IC50 value of 0.90 and 1.20 μg/mL in DPPH and ABTS assay, respectively.
Trolox used as positive control showed higher activities against DPPH (IC50 value of 2.16 μg/mL) than all extracts and fractions. The result for ABTS showed trolox (IC50 value of 4.11 μg/mL) to have lower activity of radical scavenging than ME.
3.2. Total Phenolic and Flavonoid Content
The extraction of bioactive compounds from plant materials is the first step toward potential utilization of phytochemical extracts, in the preparation of functional food, dietary supplements or nutraceuticals, cosmetics and pharmaceutical products. The extraction yield of phenolics as well as their antioxidant efficacy not only depend upon plant part/type, genetic make up of species, agroclimatic conditions, harverst time, post-harverst processing, and it is strongly affected by the chemical nature of extraction media employed [25, 26].
Successful prediction of botanical compounds isolated from plant material much depends on the type of solvent selected for the extraction [27]. In previous studies, polar and nonpolar solvents such as methanol, ethanol, acetone, propanol, ethyl acetate and water have been commonly used for the extraction of phenolic compounds [28,29,30]. The recovery of phenolics from plant materials is influenced by the solubility of these phenolic compounds in the solvent used for the extraction process. Importance of solvent variation for accumulation of total phenolic and flavonoid contents as antioxidants has been recognized.
The phenolic contents among the different varieties of G. lasoar stem bark were expressed in quercetin equivalent using the standard curve equation y = 0.00384x + 0.101, R2= 0.976. ME gave the highest amount of phenolic compounds (272.98 ± 0.61 mg GAE/100 g extract). This result suggests that the nature of these polyphenols is polar [31, 32]. Total phenolic content of EE was higher than that of AE with 270.16 ± 1.61 and 212.63 ± 1.05 mg GAE/100 g extract, respectively. The lowest values were obtained in the EA extract (77.54 ± 1.61 mg GAE/100 g) (Fig. 2A). Extracts of nonpolar solvents (PEE, n-HE, DE) were not detected.
Flavonoids are a group of polyphenolic compounds, which exhibit several biological effects such as anti-inflammatory, anti-hepatotoxic, anti-ulcer, anti-allergic, anti-viral and anti-cancer [33]. They are capable of effectively scavenging reactive O2 species because of their phenolic hydroxyl groups, so they are potent antioxidants [34]. Their effects on human nutrition and health are also considerable. The total flavonoid content in different of G. lasoar stem bark extracts was determined by aluminum chloride method. This compound forms stable acid complexes with the carbonyl group at C-4 and hydroxyls at C-3 in flavonols and C-5 in both flavonols and flavones. Besides, it form labile acid complexes with hydroxyls in the ortho position in A or B rings of flavonoids [35]. The flavonoid contents in different varieties of G. lasoar stem bark were expressed in gallic acic equivalent using the standard curve equation y = 0.009x + 0.0161, R2 = 0.983. The flavonoid content in solvents of different polarity was as follow: ME > EE > AE > EAE (Fig. 2B). Extracts of the nonpolar solvents (PEE, n-HE, DE) were not detected.
3.3. Relationship between TPC, TFC and Antioxidant Activity
The highest and positive correlation between total phenolic content and ABTS scavenging activity (r = 0.977, p < 0.001) is observed for EE (Table 2). There was moderate correlation between total phenolic content and ABTS scavenging (r = 0.444, p < 0.001) for ME, followed by AE (r = 0.312, p < 0.001). Negative correlation between total phenolic content and ABTS scavenging activity was given by EAE (r = –0.908). The highest and positive correlation between total flavonoid content and ABTS scavenging activity (r = 0.998, p < 0.001) was observed for ME, followed by AE (r = 0.865, p < 0.001). There was positive correlation between total flavonoid content and ABTS scavenging (r = 0.524, p < 0.001) for EAE. Negative correlation between total flavonoid content and ABTS scavenging activity was given by EE (r = –0.908).
The highest and positive correlation between total phenolic content and DPPH scavenging activity (r = 0.862, p < 0.001) was observed for EAE. There was positive correlation between total phenolic and DPPH scavenging (r = 0.023, p < 0.001) for ME. Negative correlation between total phenolic content and DPPH scavenging activity was given by EE (r = –0.481) and AE (r = –0.149). The highest and positive correlation between total flavonoid content and DPPH scavenging activity (r = 0.756, p < 0.001) was observed for AE, followed by EE (r = 0.655, p < 0.001). The EAE extract showed positive correlation between total flavonoid and DPPH scavenging activity (r = 0.024, p .001). Negative correlation between total flavonoid content and DPPH scavenging activity was given by ME (r = –0.854). The highest and positive correlation between total flavonoid content and DPPH scavenging activity (r = 0.756, p < 0.001) was observed for AE, followed by EE (r = 0.655, p < 0.001).
The high and positive correlation between total phenolic content and ABTS scavenging activity (r = 0.943, p < 0.001) was observed for DF. Negative correlation between total phenolic content and ABTS scavenging activity was given by EAF (r = –0.843). The high and positive correlation between total flavonoid content and ABTS scavenging activity (r = 0.943, p < 0.001) was observed for EAF. Negative correlation between total flavonoid and ABTS scavenging activity was given by DF (r = –0.874). The positive correlation between total phenolic content and DPPH scavenging activity (r = 0.294, p < 0.001) was observed for EAF. Negative correlation between total phenolic content and DPPH scavenging activity was given by DF (r = –0.843). Negative correlation between total flavonoid and DPPH scavenging activity was given by EAF (r = –0.673) followed by DF (r = –0.368).
The ME extract had high positive correlation between total flavonoid and ABTS scavenging activities. There was negative corrrelation between total flavonoid content and DPPH scavenging activities for ME. Furthermore, positive correlation between total phenolic and both of DPPH and ABTS scavenging activities. These data indicate that higher total flavonoid in ME would give higher ABTS scavenging activity.
The EE extract had high positive correlation of total phenolic content – ABTS and total flavonoid content – DPPH scavenging activities. It can be concluded that ABTS and DPPH scavenging activities of EE extracts can be predicted indirectly by using total phenolic and total flavonoid contents, while the total flavonoid content in AE extract had positive high correlation with both of ABTS and DPPH scavenging activities. Besides that, AE had positive correlation between total phenolic content and ABTS scavenging activity. It was demostrated for both ABTS and DPPH scavenging activities that AE extract can be assessed indirectly by using its total phenolic and flavonoid contents.
The EAE extract had high positive correlation between total phenolic content and DPPH scavenging activities. There was negative correlation between total phenolic content of EEA extract and ABTS scavenging activities. The EAE extract had positive correlation total flavonoid content with ABTS and DPPH scavenging activities. It can be concluded that DPPH and ABTS scavenging activities of EEA extract are contributed by its total phenolic and flavonoid contents.
The EAF fraction had high positive correlation between total flavonoid and ABTS scavenging activities. It can be concluded that ABTS scavenging activities of EAF can be predicted indirectly by using total flavonoid contents. While the total phenolic in DF had positive high correlation with of ABTS and positive moderate correlation between DPPH scavenging activities. It was demostrated that both of ABTS and DPPH scavenging activities of DF can be estimated indirectly by using its total phenolic contents. There were high negative correlations between total flavonoid and ABTS and DPPH scavenging activities. This shows that total flavonoid content in DF lower contributed to ABTS and DPPH scavenging activities. It was predicted that flavonoid in DF has no –OH in ortho C3′,4′; –OH in C3, oxo function in C4, double bond at C2 and C3, which would influence the scavenging activity.
In general, total phenolic and flavonoid contributed in antioxidant activity which is function as chain breakers, free radical scavengers and electron donors. It is assumed that as concentration of phenolic or the degree of hydroxylation of the phenolic compounds increases DPPH, also increase radical scavenging activity and antioxidant activity [36]. In this research, flavonoid is related with antioxidant activity, but the different showed that ME had negative correlation between total flavonoid and DPPH. The present study showed ME have high radical scavenging activities. The best results is supported by previous reports that some of phenolic compounds beside flavonoid like xanthones, benzophenones, coumarins, stilbenes, and depsidones would give higher antioxidant activities [37,38,39,40,41,42]. The other side, this can be explained by the fact that not only phenolic compounds but also some other compounds may also have DPPH free radical [43]. Previous studies have also indicated some of triterpenoid compounds have antioxidant activity such as dysoxyhaine A-D, chilianthin A-C, myriceric B and uncarinic acid E [44, 45].
DPPH and ABTS methods have the same mechanism of reaction based on the electron transfer [46, 47]. The finding from results of the present study shows that ABTS scavenging activity correlates with DPPH scavenging activity. The Pearson correlation coefficients of various G. lasoar stem bark extracts (r = 0.762) also indicated that there was correlation between DPPH and ABTS activities.
Among the two assays used for determining antioxidant activity in the present study, ABTS gave best results followed by DPPH. ABTS is solluble in both aqueous and organic solvents and exhibit reducing properties of 2,2-azinobis(-3-ethylbenzoline sulphonate) radical, from wich the antioxidant activity can be precised due to the hydrophylic and lipophilic nature of a compound. DPPH, possesing ability to dissolve only in organic solvents, in particular ethanol, can be predicted as having imperative restriction while interpreting the role of hydrophylic antioxidants. Previous studies also indicated preference of using ABTS assay in assessing antioxidant potential of extracts [31, 48].
Linear regression analysis revealed that total phenolic content contributes 11.9 – 18.8% of radical scavenging property (r2 = 0.119 for ABTS and 0.188 for DPPH). Likewise, total flavonoid content contributed 21.1 – 24.0% of radical scavenging property (r2 = 0.211 for ABTS and 0.240 for DPPH) (Table 3). On the whole, these correlations confirm that phenolic compounds are the main contituents contributing to the antioxidant activity of these extratcs. It is also possible that a synergistic effect among the bioactives led to increase in overal antioxidant activity of the extracts.
In the present study, aqueous extract used as empirical etnobotanical mediicines by local people in Maluku island for treatment of several diseases showed high antioxidant activity. These results sugest that G. lasoar stem bark extracts can be used as a natural source of antioxidants. Therefore, further research is needed for isolation and identification of phenolic compounds in polar to semipolar G. lasoar extracts.
References
P. A. Morrissey and N. M. O. Brien, Int. Dairy J., 8, 463 – 472 (1998).
F. V. Santa-Cecília, F. C. Vilela, C. Q. Da Rocha, et al., J. Ethnopharmacol., 133, 467 – 473 (2011).
N. J. Temple, Nutrition Res., 20(3), 449 – 459 (2000).
M. Leopoldini, N. Russo, and M. Toscano, Food Chem., 125, 288 – 306 (2011).
J. P. Chaverri, N. C. Rodríguez, M. O. Ibarra, and J. M. Perez-Rojas, Food Chem. Toxicol., 46, 3227 – 3239 (2008).
G. J. Bennett and H. H. Lee, Phytochemistry, 28, 967 – 998(1989).
J. Merza, M. C. Aumond, D. Rondeau, et al., Phytochemistry, 65, 2915 – 2920 (2004).
Nilar and L. J. Harrison, Phytochemistry, 60, 541 – 548(2002).
L. M. M. Vieira, A. Kijjoa, A. M. S. Silva, et al., Phytochemistry, 65, 393 – 398 (2004).
H. A. Jung, B. N. Su, W. J. Keller, et al., J. Agric. Food Chem., 54, 2077 – 2082 (2006).
L. Yu, M. Zhao, B. Yang, et al., Food Chem., 104, 176 – 181 (2007).
E. Elfita, M. Muharni, M. Latief, et al., Phytochemistry, 70, 907 – 912 (2009).
K. W. Lin, A. M. Huang, S. C. Yang, et al., Food Chem., 135, 851 – 859 (2012).
N. Jamila, M. Khairuddean, N. S. Yaacob, et al., Bioorg. Chem., 54 60 – 67 (2014).
D. A. Bui, M. K. Vu, H. D. Nguyen, et al., Phytochem. Lett., 10, 123 – 126 (2014).
T. Q. Bui, A. T. Bui, K. T. Nguyen, et al., Tetrahedron Lett., 57, 2524 – 2529 (2016).
E. R. Sukandar, P. Siripong, S. Khumkratok, et al., Fitoterapia, 111, 73 – 77 (2016).
E. Muriithi, G. Bojase-Moleta, and R. R. T. Majinda, Phytochem. Lett., 18, 29 – 34 (2016).
Sulassih, Sobir Sobir, and E. Santosa, Sabrao J. Breed. Genet., 45, 478 – 490 (2013).
T. Uji, Biodiversitas, 8, 157 – 167 (2007).
W. Fitriana, T. Ersam, S. Koniyoshi, and Sri Fatmawati, Indones. J. Chem., 16, 297 – 301 (2016).
R. Re, N. Pellegrini, A. Proteggente, et al., Free Radic. Biol. Med., 26, 1231 – 1237 (1999).
R. S. G. Singh, P. S. Negi, C. Radha, J. Funct. Foods, 5, 1883 – 1891 (2013).
J. Zhishen, T. Mengcheng, and W. Jianming, Food Chem., 64, 555 – 559 (1999).
J. Dai and R. J. Mumper, Molecules, 15, 7313 – 7352 (2010).
C.Wendakoon, C. Peter, and D. Gagnon, J. Med. Act. Plants, 1, 60 – 68 (2012).
N. Kalidindi, N. V. Thimmaiah, N. V. Jagadeesh, et al., J. Food Drug Anal., 23, 795 – 802 (2015).
D. L. Luthria and. S. Mukhopadhyay, J. Agric. Food Chem., 54, 41 – 47 (2006).
M. Alothman, R. Bhat, and A. A. Karim, Food Chem., 115, 785 – 788 (2009).
M. Singh, A. Jha, A. Kumar, et al., J. Food Sci. Technol. 51, 2070 – 2077 (2014).
D. O. Kim, K. W. Lee, H. J. Lee, C. Y. Lee, J. Agric. Food Chem., 50, 3713 – 3717 (2002).
G. Spigno, L. Tramelli, and D. M. De Faveri, J. Food Eng., 81, 200 – 208 (2007).
M. Umamaheswari and T. K. Chatterjee, African J. Tradit. Complement. Altern. Med., 5, 61 – 73 (2008).
X. Xia, J. Cao, Y. Zheng, et al., Ind. Crops Prod., 58, 91 – 98 (2014).
C. C. Chang, M. H. Yang, and H. M. Wen, J. Food Drug Anal., 10, 178 – 182 (2002).
C. Sanchez-Moreno, Food Sci. Technol. Int., 8, 121–137 (2002).
V. Rukachaisirikul, S. Saelim, P. Karnsomchoke, and S. Phongpaichit, J. Nat. Prod., 1222 – 1225 (2005).
P. Chomcheon, S. Wiyakrutta, N. Sriubolmas, and N. Ngamrojanavanich, Phytochemistry, 70 407 – 413 (2009).
H. Fouotsa, A. M. Lannang, J. P. Dzoyem, et al., Planta Med., 81, 594 – 599 (2015).
S. Matsjeh, H. D. Pranowo, and C. Anwar, Indon. J. Chem., 11, 180 – 185 (2011).
T. Ersam, S. Fatmawati, and D.N. Fauzia, Indon. J. Chem., 16, 151 – 155 (2016).
Y. Bai, D. Li, T. Zhou, et al., J. Funct. Foods, 20, 453 – 462 (2016).
N. Sultana and N. H. Lee, Phytother. Res., 21, 1171 – 1176 (2007).
J. Hee, H. Rae, H. Kyong, et al., Bioorg. Chem., 66, 97 – 101 (2016).
Y. Zou, W. Liu, J. Zhang, and D. Xiang, Fitoterapia, 121, 159 – 163 (2017).
V. Bondet, W. B. Williams, and C. Berset, LWT - Food Sci. Technol., 30, 609 – 615 (1997).
W. Huang, H. Zhang, W. Liu, and C. Li, J. Agric. Food Chem., 53, 1841 – 156 (2005).
S. Rawat, I. D. Bhatt, and R. S. Rawal, J. Food Compos. Anal., 24 574 – 579 (2011).
Acknowledgements
This work was financially supported by the Ministry of Research, Technology and Higher Education of Indonesia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kainama, H., Fatmawati, S., Santoso, M. et al. The Relationship of Free Radical Scavenging and Total Phenolic and Flavonoid Contents of Garcinia lasoar PAM. Pharm Chem J 53, 1151–1157 (2020). https://doi.org/10.1007/s11094-020-02139-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02139-5