Abstract
Response surface methodology employing a five-level, four-variable central composite rotatable design was applied to study the effects of extraction time, extraction temperature, pH and water/solid ratio on the extraction yield of pomegranate seed oil using an aqueous extraction approach. In addition, quality indices, fatty acid composition and antioxidant activity of the obtained oil were studied and compared with those of typical hexane-, cold press- and hot press-extracted oil. Aqueous extraction resulted in the maximum oil recovery of 19.3% (w/w), obtained under the following critical values: water/solid ratio (2.2:1.0, mL/g), pH 5.0, extraction temperature = 63 °C and extraction time = 375 min. This yield is lower than that obtained via hexane extraction (26.8%, w/w) and higher than the yields from cold press (7.0%, w/w) and hot press (8.6%, w/w) extraction. A comparison of the characteristics of the oils based on extraction method revealed that the unsaturated fatty acid content was highest for the oil obtained by aqueous extraction. In addition, higher levels of iodine and peroxide and lower levels of acid, p-anisidine and unsaponifiable matter were observed. The oil obtained with aqueous extraction also exhibited higher antioxidant activity than oils obtained by hexane or hot press extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pomegranate seed, a by-product of the pomegranate juice industry, contains numerous valuable components, including vitamin E, sterols and punicic acid [1]. A number of studies have investigated various aspects of pomegranate seed oil. For example, Qu et al. [2] studied the effects of drying before extraction and of processing conditions on the properties of the antioxidants extracted from the peel and seeds of pomegranate marc. The results showed that the drying process had no significant effect on the yield, content or activity of the extracted antioxidants. Also, increasing the water/sample ratio resulted in a higher yield and the contents of the extracted antioxidants were also higher. Tong et al. [3] attempted to determine the estrogen content of pomegranate seed oil by liquid chromatography-mass spectrometry but no estrogen was detected. In another study, Yamasaki et al. [4] showed that dietary pomegranate seed oil promoted immunoglobulin production by mouse splenocytes. Meerts et al. [5] evaluated the toxicology and safety of pomegranate seed oil by in vitro and in vivo tests. The results showed that in the absence and presence of metabolic activation up to precipitating concentrations of 5000 μg/plate (Ames test) or 333 μg/mL (chromosome aberration test) no mutagenicity of pomegranate seed oil was observed. The effects of pomegranate seed oil on lipoperoxidation and the activity of antioxidant enzymes in the liver and brain of rats were studied by de Melo et al. [6]. It was found that the pomegranate seed oil has a dose–response influence on an important antioxidant defense in the liver. Park et al. [7] showed that pomegranate extract protected the skin against UVB-induced damage. Asadpour et al. [8] reported that pomegranate seed oil exerted a protective effect in the kidneys of rats against gentamicin-induced nephrotoxicity. Harzallah et al. [9] studied the effects of pomegranate flower, peel and seed oil on insulin resistance and inflammation in mouse models of high-fat and high-sucrose diet-induced obesity. They found that pomegranate flower, peel, and seed oil demonstrated anti-inflammatory properties, and pomegranate seed oil improved insulin sensitivity. Sharma et al. [10] noted that pomegranate and its constituents could play an important role against certain types of cancer. Bihamta et al. [11] found that pomegranate seed oil protected cardiomyocytes against oxidative stress-induced damage and could be considered a natural cardioprotective agent for the prevention of cardiovascular disease. Miranda et al. [12] reported that dietary supplementation of 0.5% punicic acid obtained from pomegranate seed oil did not lead to a reduction in fat accumulation in adipose tissue, liver, or skeletal muscle or an increase in glycemic control in rats.
Conventional methods for producing edible oils from oilseeds typically involve the use of screw press or organic solvent extraction. The use of water as an extraction medium is a feasible alternative to traditional processing technologies. Unlike screw press and organic solvent extraction, an aqueous method can extract oil and protein simultaneously with minimal impact on the environment [13]. In fact, compared to solvent extraction, an aqueous extraction medium is much safer and more environmentally friendly and economical.
In a previous study, ultrasound-assisted aqueous enzymatic extraction of oil from pomegranate seeds was performed using cellulase and Peclyv V (a pectinase preparation from Lyven, Colombelles, France), resulting in 15.33 g oil/100 g dry seeds under the optimal operating conditions of 2-h extraction time, 2% (w/w) enzyme concentration, 6:1 (mL/g) liquid/solid ratio and extraction temperature of 55 °C [14]. Abbasi et al. [15] evaluated the effects of process variables on the extraction yield of oil from pomegranate seeds using hexane and petroleum benzene and concluded that different methods of extraction with organic solvents (Soxhlet, microwave irradiation, ultrasonic irradiation and normal stirring) significantly impact the oil extraction yields. Balvardi et al. [16] used a protease and a Cellulase for the extraction of oil from Iranian wild almond and reported 77.8% recovery for oil using an aqueous enzymatic extraction procedure under the optimal extraction conditions suggested by response surface methodology (RSM), pH 5.0; extraction temperature 50 °C and extraction time 4 h, when both enzymes were used at 1.0% (v/w) concentration. It has been reported that the quality indices of wild almond oil obtained by aqueous extraction were somewhat similar to those of oil extracted by cold press and much superior to those of oil obtained by Soxhlet extraction [17].
RSM is used for modeling and analysis of the processes using a collection of mathematical and statistical techniques. RSM predicts the best performance conditions and optimizes the responses of interest that are affected by numerous variables. However, it has some limitations with respect to industrial systems. For example, it fits the data to a second-order polynomial, which does not encompass all systems [18].
In the present study, an aqueous extraction method was developed to obtain oil from pomegranate seeds. The main objective of this work was the optimization of a process for the extraction of pomegranate seed oil using a five-level, four-variable central composite rotatable design from RSM to study the effects of extraction time, extraction temperature, pH and water/solid ratio on the yield of pomegranate seed oil. In addition, the quality indices and fatty acid composition of pomegranate seed oil from the aqueous extraction method were compared with those of hot press extracted oil (HPEO), cold press extracted oil (CPEO) and hexane-extracted oil (HEO).
Materials and methods
Materials
Pomegranate seeds were purchased from a juice producing company in Saveh (Markazi Province of Iran). The initial moisture content of pomegranate seeds used in this work was 3.5% (w/w). Extra-pure (~95%) hexane, used as an organic solvent, was purchased from Mojallali Chemical Company (Tehran, Iran). 2,2-Diphenyl-1-picrylhydrazyl (Sigma–Aldrich, St. Louis, MO, USA), NaOH (Scharlau, Barcelona, Spain) and HCl (Mojallali, Tehran, Iran) were also used in the study. All other reagents were of analytical grade.
Aqueous extraction procedure
First, seeds were pulverized using a grinder and passed through a 40-mesh sieve. Then, 30 g of the ground materials were mixed with distilled water in a plastic container (250 mL) to achieve water/solid ratios of 1.0, 1.5, 2, 2.5 and 3.0 (mL/g). NaOH and HCl at 0.1 N were used to set the pH of the obtained mixtures to 3.0, 5.0, 7.0, 9.0 and 11.0. Then, the mixtures were incubated at 10, 25, 40, 55 and 70 °C for 30, 120, 210, 300 and 390 min using a shaker incubator with a constant shaking rate of 100 rpm, and the extracted oil was separated from the aqueous phase by centrifugation (5000×g for 10 min) (8KS, Sigma, Osterode am Harz, Germany) [19]. After centrifugation, the free oil was carefully collected from the other phases using a pipette and weighed. The oil extraction yield was determined using Eq. (1):
The oil trapped in the emulsion phase was not measured in the current study and therefore, the reported yields represent only the oil obtained in the upper phase.
Design of the experiments to optimize extraction conditions
A central composite design (CCD) by RSM was used to study the effects of four independent variables (pH, water/solid ratio, time and temperature) at five levels on the extraction yield. The ranges and the center points for the four independent variables were based on the results of preliminary experiments (Table 1). The CCD in the experimental design consists of 24 factorial points and seven replicates of the central point (Table 1). The behavior of the system was explained by the second degree polynomial Eq. (2) [20]:
where Y is the response function, β 0 the intercept, β i , β ii and β ij the coefficients of the linear, quadratic and interactive terms, respectively, and X i and X j the coded independent variables. The fitted polynomial equation is expressed as surface and contour plots to visualize the relationship between the responses and the experimental levels of each factor. Design-Expert® V7 (Stat-Ease Inc., Minneapolis, MN, USA) was used to determine the analysis of variance (ANOVA) and coefficient of determination (R 2) to estimate the fitness of the model.
Organic solvent extraction
For organic solvent extraction, 10 g of ground pomegranate seeds (40-mesh) was extracted using 250 mL of n-hexane in a Soxhlet apparatus (model B-810, BÜCHI Labortechnik AG, Flawil, Switzerland) at 68 °C for 6 h. The solvent (n-hexane) was then removed at 50 °C under reduced pressure using a rotary evaporator (Laborota 4003, Heidolph Instruments GmbH & Co. KG, Kelheim, Germany), and the oil was then dried to constant mass in an oven at 85 °C. The obtained oils were weighed to determine the extraction yield and stored at 4 °C under a nitrogen atmosphere [19].
Mechanical extraction of oil
Extraction by press was carried out at 26 ± 2 °C for cold press and ~50 °C for hot press [21]. The pomegranate seeds were divided into 500-g batches and pressed at a feed rate of 6.25 kg/h using a pilot-scale expeller (model Y2-80M2-4-WS06028, Iran Cold Pressing Co., Tehran, Iran). Crude press oils were collected and centrifuged (8KS, Sigma, Osterode am Harz, Germany) to remove the solids. The oils were then weighed to determine the extraction yield and stored at 4 °C under nitrogen atmosphere until use in the next stages.
Fatty acid analysis
The fatty acid composition of the extracted oils was analyzed using a gas chromatograph (Perkin Elmer, Clarus 500, Bellefonte, PA, USA) equipped with a flame ionization detector and a polar capillary column (SP 2560, Supelco, Bellefonte, PA, USA) 100 m in length, with an internal diameter of 0.25 mm and film thickness of 0.2 µm. Before the injection, a 50-µg sample was mixed vigorously with 2.0 mL methanolic potassium hydroxide (5.6 g KOH in 100 mL dried pure methanol). The suspension was then kept at 50 °C for 1 h in a thermostated oven. Next, 1.0 mL distilled water was added and mixed vigorously for 2 min. The fatty acid methyl esters (FAME) produced in this procedure were then extracted using 1.0 mL hexane, transferred into a clean vial, dried using sodium sulphate powder [15] and injected (0.5 μL) into a PerkinElmer Clarus 500 gas chromatograph (PerkinElmer, Waltham, MA, USA) equipped with a Supelco SP-2560 column (100 m × 0.25 mm I.D. × 0.2 μm film thickness). With regard to the operating conditions, both the injector and the detector temperatures were set at 250 °C. The oven temperature was programmed to start at 90 °C, where it was held for 5 min, and it was then increased to 230 °C at a rate of 2 °C/min, where it was held for 15 min. Nitrogen gas of 99.99% purity was used as the carrier gas, and a split ratio of 1–20 (v/v) was applied on the injection system. The identities of the obtained FAME were determined by comparing the retention times of the components with those of a mixture of FAME standards.
Quality attributes of the extracted oils
Iodine value (IV), refractive index (RI), unsaponifiable matter (UM) and saponification value (SV) were determined using AOAC [22] standard analytical methods. IV was expressed as the grams of iodine absorbed per 100 g of oil sample. Additionally, the acid value (AV), peroxide value (PV) and p-anisidine value (PAV) were determined following the standard IUPAC methods [23]. The TOTOX value was obtained as 2 × PV + PAV [16].
Determination of antioxidant activity with the DPPH radical-scavenging assay
The anti-radical scavenging activities of the oil samples were measured according to the method described by Lv et al. [24], with slight modification. Briefly, the samples were diluted in ethyl acetate (2.5–40 µg/mL), and 2.0 mL of this solution was added to 2 mL of DPPH solution (300 µM in ethyl acetate). The mixture was then shaken vigorously and left in the dark for 20 min. Finally, the absorbance of the mixture was measured against ethyl acetate (blank) at 517 nm using a UV–visible spectrophotometer (Spectrum SP-UV 500DB; Spectrum Instruments, Victoria, Australia).
Statistical analysis
All experiments performed under the CCD were analyzed using Design-Expert® version 7 software (Stat-Ease, Inc., Minneapolis, MN, USA). Data were analyzed via Duncan’s test using SPSS version 15 software (SPSS Inc., Chicago, IL, USA). Data are presented as means ± standard deviations obtained by triplicate experiments, and a probability value of p < 0.05 is considered significant for the differences among the mean values.
Results and discussion
Optimization of aqueous oil extraction from pomegranate seeds by RSM
The conditions for aqueous extraction of oil from pomegranate seeds were optimized by RSM using CCD. Table 1 shows the design matrix and the responses obtained for the extraction yield. A mathematical equation was used to calculate data for the extraction yield of oil (Y) obtained from pomegranate seed, as provided in Eq. (3):
where A, B, C and D correspond to the coded values of the four independent variables (pH, extraction temperature, extraction time and water/solid ratio). The oil extraction yield is in the range of 9.16–18.89% (w/w). The analysis of variance for the model of stability is shown in Table 2. The p value of the model was less than 0.0001 indicating that the model was significant. Regression analysis showed that the coefficient of determination (R 2 = 0.9720) was satisfactory for validating the significance of the model. All the independent variables (A, B, C and D), three interaction terms (AB, BC and BD), and four quadratic terms (A 2, B 2, C 2 and D 2) had a significant effect on Y (p < 0.05). Figures 1 and 2 show the response surfaces generated by the proposed models. These results express the interactions between the two independent variables while the other two variables were both maintained at the central point.
The effects of extraction temperature and pH at fixed extraction time (210 min) and water/solid ratio (2.0) (a), extraction time and pH at fixed extraction temperature (55 °C) and water/solid ratio (2.0 mL/g) (b), water/solid ratio and pH at fixed extraction temperature (55 °C) and extraction time (210 min) (c) on the oil recovery from pomegranate seeds via the aqueous extraction method developed in the current study (shaking rate = 100 rpm)
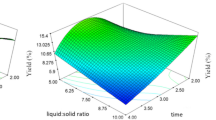
The effects of extraction time and extraction temperature at fixed pH (7.0) and water/solid ratio (2.0 mL/g) (a), water/solid ratio and extraction temperature at fixed pH (7.0) and extraction time (210 min) (b) and water/solid ratio and extraction time at fixed pH (5.0) and extraction temperature (55 °C) (c) on the oil recovery from pomegranate seeds via the aqueous extraction method developed in the current study (shaking rate = 100 rpm)
The 3-D response plot in Fig. 1a, which provides the extraction yield of oil as a function of pH and extraction temperature at a fixed extraction time (210 min) and water/solid ratio (2:1, v/w), indicates that the oil extraction yield increased with increasing pH from 3.0 to 5.0, but rapidly decreased with an increase in pH beyond 5.0. Adjusting the pH affects the withdrawal of the oil from the oilseed by changing protein solubility, which depends on the isoelectric point of the protein in the oilseed grain and can vary depending on the nature of different oilseeds [25, 26]. Another reason for the differences in the oil extraction efficiencies at various pH levels is the effect of pH on the oleosins in the membrane of fatty tissues that can affect fat tissue stability and, consequently, the accumulation of fat globules [27, 28]. Changes in the pH affect the solubilities of the proteins surrounding the oil droplets resulting in a difference in the release of oil droplets from the particles. The stability and solubility levels of the oleosins that surround the oil droplets are also affected by changes in the pH. The optimal pH for the aqueous extraction of oils from different seeds can vary due to the different proteins in their structures [29, 30]. The extraction yield of oil initially increased (somewhat rapidly) with an increase in the extraction temperature from 10 to 63 °C, and then gradually decreased with a further increase in temperature from 63 to 70 °C. This may result from changes in the viscosity of the oil at different temperatures. Oil viscosity decreases with an increase in the temperature, and therefore the withdrawal of oil from plant tissues becomes easier; however, excessive temperatures can cause the coagulation of proteins, and oil can be trapped in the coagulated protein. The effect of temperature on the oleosins in the membrane of fatty tissues may be another explanation for the increased extraction yield with an increase in temperature to 63 °C or less. According to Rosenthal et al. [31], the ability of oleosins to sustain oil droplets within cells decreases with increasing temperature. Figure 1b shows the 3-D response surface plot at varying extraction times and pH at a fixed extraction temperature (40 °C) and a constant water/solid ratio (2:1, v/w). The extraction yield increased considerably between 30–375 min of extraction time but reached a plateau for extraction times beyond 375 min, where maximum yield was maintained. The solubilization of cell wall components increased with increased extraction time, and eventually reached a maximum. In addition, the extraction yield increased rapidly with an increase in pH from 3.0 to 5.0, but then rapidly declined beyond pH 5.0. Figure 1c shows the 3-D response surface plot at varying water/solid ratios and pH levels at a fixed extraction time of 210 min and extraction temperature of 40 °C. The maximum extraction yield of oil was achieved when the water/solid ratio and pH were 2.2 mL/g and 5.0, respectively. At a low water/sample ratio, the extraction of droplets from the cell structure is more difficult, and efficiency is reduced due to the high concentration of solids. On the other hand, at a higher water/sample ratio, the amount of oil remaining in the aqueous phase increases with increased volume of the aqueous phase. Changes in the water/sample ratio can also influence protein solubility and extraction yield by changing the concentration of dissolved ions in water [32]. The 3-D response surface plot in Fig. 2a was developed for the extraction of oil at varying extraction times and temperatures at a fixed pH of 7.0 and a water/solid ratio of 1:2 mL/g. The maximum oil extraction yield was achieved with extraction time and temperature of 375 min and 63 °C, respectively. Figure 2b shows the 3-D response surface plot developed for the extraction of oil at varying extraction temperatures and water/solid ratios at a fixed extraction time of 210 min and pH 7.0. The maximum extraction yield was achieved with extraction temperature of 63 °C and water/solid ratio of 2.2:1 mL/g. The 3-D response surface plot based on extraction time and water/solid ratio is shown in Fig. 2c, with the extraction temperature and pH maintained at 40 °C and 7.0, respectively. The extraction yield increased with an increase in the water/solid ratio from 1:1 to 2.2:1 mL/g, and then declined as the water/solid ratio was further increased to 3:1 mL/g. In addition, the yield increased rapidly as the extraction time was increased from 30 to 300 min, after which no further increase in yield was observed.
According to the model, the predicted maximum oil yield was 20.3% (w/w) using the following critical values: water/solid ratio of 2.2 mL/g, pH of 5.0, extraction temperature of 63 °C and extraction time of 375 min. The validation tests were carried out in triplicate under optimal conditions to determine the adequacy of the quadratic model where a mean value of 19.3% (w/w) was found for the extraction yield under the conditions predicted by the model, which did not differ significantly from the theoretical predicted value. Therefore, the extraction conditions suggested by RSM were considered reliable and practical. A photographic image of the phase separation in the aqueous extraction of pomegranate seed oil under the optimal conditions of the CCD is shown in Fig. 3. The free oil is located at the top, and the cream (oil-in-water emulsion) and skim (aqueous phase) are located below the oil phase, while the residual phase is at the bottom of the tube. Thus, it can be concluded (from CCD) that the optimal extraction conditions for pomegranate seed oil include an extraction temperature of 63 °C, pH of 5.0, extraction time of 375 min and water/solid ratio of 2.2 mL/g. However, these conditions can affect the quality of the extracted proteins, which requires further investigation.
Among the four parameters studied, the extraction temperature had the greatest effect on oil yield, followed by pH, extraction time, and water/solid ratio, according to the regression coefficient significance of the quadratic polynomial model (Table 2) and the gradient of slope in the 3-D response surface plots (Figs. 1, 2).
Fatty acid composition
The fatty acid profile of pomegranate seed oil extracted using the aqueous procedure under the optimal conditions of the CCD was compared with those of the oils extracted by hexane, cold press and hot press. As shown in Table 3, seven main components, three saturated fatty acids (SFAs), two monounsaturated fatty acids (MUFAs) and two poly-unsaturated fatty acids (PUFAs) were identified. The relative concentrations of fatty acids in all samples are as follows: punicic acid > oleic acid > linoleic acid > palmitic acid > gadoleic acid > stearic acid > arachidic acid, which are in agreement with those reported by Abbasi et al. [15]. Palmitic acid was the main SFA in the oils studied here ranging from 3.04% in aqueous-extracted oil (AEO) to 4.00% in CPEO. Two other SFAs (stearic and arachidic acids) were within 2.14–2.61 and 0.5–0.58%, respectively. All of the oil samples exhibited high amounts of total unsaturated fatty acids. The most prevalent MUFA among the oils from all extraction methods was oleic acid, which ranged from 6.14% (for AEO) to 8.01% (for HEO).
Considering the fatty acid compositions of the oils extracted by cold press, hot press, and hexane, the concentration of stearic acid was slightly lower than that in the CPEO and HEO. On the other hand, the concentration of linoleic acid in CPEO was slightly higher than those in HPEO and HEO. However, the fatty acid composition of AEOs differed somewhat from that of oils obtained with the other three extraction methods. For example, the amount of punicic acid was higher (81.40 ± 0.17%) in AEO than in oils from other methods (Table 3). This was verified by the level of PUFA in AEO (87.29%), which was greater than that in the CPEO (84.50%), HEO (84.04%) or HPEO (83.77%). As a consequence, the ratio of unsaturated to saturated FAs in AEO in the current study (16.47) was higher than that in HPEO (13.87), CPEO (13.49) and HEO (13.19). In agreement with the results of this study, Khoddami et al. [33] reported that the ratio of unsaturated to saturated FAs in the oils of pomegranate seeds extracted by cold press from the Torshe Malas variety and two other pomegranate seed oils (one from Iran and one from Turkey) were 12.43, 12.50 and 13.07, respectively.
Quality indices of the extracted oils
Quality indices of pomegranate seed oil obtained with the aqueous extraction process under the optimal conditions of CCD were compared with those of oils obtained by cold press, hot press and hexane (Table 4). No significant differences were found in the refractive indices and saponification values of these oils. However, the iodine value (g I2/100 g oil) was higher in AEO (260 ± 0) than CPEO (243 ± 1), HPEO (242 ± 2) or HEO (242 ± 1), which was expected based on the fatty acid composition data (Table 3). In this study, the peroxide value of the oil derived from aqueous extraction was higher than that for other oils (Table 4). In agreement with the findings of the current study, Hanmoungjai et al. [34] reported that the peroxide value for AEO from rice bran was higher than that for HEO.
The acid value for AEO was lower than those obtained for HPEO and HEO, but higher than that for CPEO. This may be explained in part by the fact that the free fatty acids are stripped away by water during aqueous oil extraction. In agreement with the findings in this study, a study carried out on Moringa oleifera seed oil showed that the acid value for HEO (2.48 ± 0.11) was higher than that for AEO (1.13 ± 0.08) [35]. Li et al. [36] reported that the acid value for AEO (0.54 ± 0.02) was lower than that for HPEO (1.36 ± 0.03). Based on the results of the current study, the p-anisidine value for AEO (10.00 ± 0.04) was higher than that for HPEO or CPEO (Table 4), but lower than that for HEO (16.19 ± 0.03). Such differences can be attributed to the exposure of oil to the surrounding water during the extraction process, which warrants further investigation.
The amount of unsaponifiable matter (%, w/w) was higher in HEO (3.27 ± 0.15) than in AEO (1.81 ± 0.04), HPEO (1.39 ± 0.08) or CPEO (1.27 ± 0.04). This may be due to the ability of hexane to extract materials solubilized in the oil [37]. In the present study, the TOTOX value as a measure of the total amount of oil oxidation was determined for each of the oils, with results showing that the TOTOX value for CPEO, at 11.61 ± 0.04, was the lowest among all oils studied.
The extraction yield for AEO under optimal conditions suggested by RSM was 19.3 ± 0.4 (%, w/w), which was higher than those for CPEO (7.0 ± 0.0%, w/w) or HPEO (8.6 ± 0.0%, w/w). However, the AEO extraction yield was only about 72% of that for HEO (26.8 ± 0.0%, w/w). When considering the environmental and health-related issues associated with the use of hexane as an extraction solvent, the oil obtained by the aqueous method can be considered a safer product.
Antioxidant activities of the oils extracted by different methods
The differences in the antioxidant capacities of the oils extracted via different methods are shown in Fig. 4. The results show that CPEO and AEO have higher antioxidant activities than HPEO or HEO. The high antioxidant capacity of the oil obtained via aqueous extraction may be due to the higher levels of unsaturated fatty acids in this oil. According to Li et al. [36], tocopherols were found at higher levels in peanut oil from aqueous extraction than cold or hot press. The amount of oil required to quench 50% of total DPPH free radicals (i.e., that of the initial concentration of the DPPH solution) is presented as the IC50 value of the respective oils (Fig. 5). AEO exhibited the lowest IC50 value (10.5 µg/mL) and HPEO the highest.
The DPPH radical scavenging activity of oils obtained from pomegranate seeds via different extraction methods. Pomegranate seed oil was obtained by the aqueous process under the optimal conditions of CCD [water/solid ratio (mL/g) = 2.2, pH 5.0, extraction temperature = 63 °C and extraction time = 375 min]. The data are expressed as the mean ± SD (n = 3)
In a study on the aqueous-enzymatic extraction of pumpkin seed oil with microwave pretreatment, Jiao et al. [37] reported higher antioxidant capacity for oil obtained by aqueous extraction than by hexane extraction. With results similar to the findings of the current study, Long et al. [38] reported on the aqueous-enzymatic extraction of flaxseed oil using ultrasonic pretreatment of the seeds, in which the oil extracted using an aqueous method had higher antioxidant capacity than oil extracted using a hexane method. This difference can be explained in part by the higher levels of unsaturated fatty acids in the oils obtained by aqueous extraction.
Conclusion
According to the results of this study, extraction temperature, extraction time, pH and water/solid ratio affect the aqueous extraction of oil from pomegranate seeds. A change in the extraction temperature influences numerous parameters, including the viscosity of the oil and the extraction kinetics. However, excessively high temperatures can negatively impact the yield obtained via aqueous oil extraction, due to protein coagulation. The pH primarily influences the solubilities of the surrounding proteins in the seeds based on their isoelectric points. The extraction time is another parameter that is important within a given range. In this case, an increase in the extraction time allowed greater solubilization of the cell wall components. The water/solid ratio must also be optimized in aqueous oil extraction. A minimum amount of water is required to suspend the seed powder and additional water is required to improve the mass transfer of oil during agitation. Therefore, water increases the extraction yield. In this study, the optimal conditions predicted by RSM for the aqueous extraction of oil included a water/solid ratio of 2.20 mL/g, temperature of 63 °C, extraction time of 375 min and pH of 5.0. The yield of aqueous oil extraction under these conditions was lower than that obtained by hexane but higher than that using cold press or hot press methods. With regard to the fatty acid content of the oils, punicic acid—the most important fatty acid in pomegranate seed oil—was observed in higher concentrations in oil obtained by aqueous extraction, indicating a higher nutritional value for oil extracted with this method. Comparing the quality attributes of the extracted oils, our results show that the oil obtained by cold press had lower PV, AV, PAV and TOTOX than oils extracted by hot press, hexane or aqueous methods, and the oil obtained by aqueous extraction was of higher quality than the oil obtained by hot press extraction. In general, aqueous extraction of oil is safer than hexane extraction, and achieves higher yields than hot or cold press methods.
References
Tian Y, Xu Z, Zheng B, Lo YM (2013) Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason Sonochem 20:202–208
Qu W, Pan Z, Ma H (2010) Extraction modeling and activities of antioxidants from pomegranate marc. J Food Eng 99:16–23
Tong P, Kasuga Y, Khoo C (2006) Liquid chromatographic-mass spectrometric method for detection of estrogen in commercial oils and in fruit seed oils. J Food Compos Anal 19:150–156
Yamasaki M, Kitagawa T, Koyanagi N, Chujo H, Maeda H, Kohno-Murase J, Imamura J, Tachibana H, Yamada K (2006) Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition 22:54–59
Meerts I, Verspeek-Rip C, Buskens C, Keizer H, Bassaganya-Riera J, Jouni Z, Van Huygevoort A, Van Otterdijk F, Van de Waart E (2009) Toxicological evaluation of pomegranate seed oil. Food Chem Toxicol 47:1085–1092
De Melo ILP, de Carvalho EBT, e Silva AMdO, Mancini-Filho J (2010) Effects of pomegranate seed oil on lipoperoxidation and activity of antioxidant enzymes in liver and brain of rats. Free Radic Bio Med 49:S189
Park HM, Moon E, Kim AJ, Kim MH, Lee S, Lee JB, Park YK, Jung HS, Kim YB, Kim SY (2010) Extract of Punica granatum inhibits skin photoaging induced by UVB irradiation. Int J Dermatol 49:276–282
Asadpour E, Boroushaki MT, Sadeghnia H (2010) Protective effect of pomegranate seed oil against gentamicin induced nephrotoxicity in rat. Toxicol Lett 196:S232
Harzallah A, Hammami M, Kępczyńska MA, Hislop DC, Arch JR, Cawthorne MA, Zaibi MS (2016) Comparison of potential preventive effects of pomegranate flower, peel and seed oil on insulin resistance and inflammation in high-fat and high-sucrose diet-induced obesity mice model. Arch Physiol Biochem 122:75–87
Sharma P, McClees SF, Afaq F (2017) Pomegranate for prevention and treatment of cancer: an update. Molecules 22:177
Bihamta M, Hosseini A, Ghorbani A, Boroushaki MT (2017) Protective effect of pomegranate seed oil against H2O2-induced oxidative stress in cardiomyocytes. Avicenna J 7:46–53
Miranda J, Aguirre L, Fernández-Quintela A, Macarulla MT, Martínez-Castaño MG, Ayo J, Bilbao E, Portillo MP (2013) Effects of pomegranate seed oil on glucose and lipid metabolism-related organs in rats fed an obesogenic diet. J Agr Food Chem 61:5089–5096
Zhang S, Zu Y-G, Fu Y-J, Luo M, Liu W, Li J, Efferth T (2010) Supercritical carbon dioxide extraction of seed oil from yellow horn (Xanthoceras sorbifolia Bunge.) and its anti-oxidant activity. Bioresourc Technol 101:2537–2544
Goula AM, Papatheodorou A, Karasavva S, Kaderides K Ultrasound-assisted aqueous enzymatic extraction of oil from pomegranate seeds. Waste Biomass Valoriz:1–11. doi:10.1007/s12649-016-9740-9
Abbasi H, Rezaei K, Rashidi L (2008) Extraction of essential oils from the seeds of pomegranate using organic solvents and supercritical CO2. J Am Oil Chem Soc 85:83–89
Balvardi M, Rezaei K, Mendiola JA, Ibáñez E (2015) Optimization of the aqueous enzymatic extraction of oil from Iranian wild almond. J Am Oil Chem Soc 92:985–992
Moghadas HC, Rezaei K (2017) Laboratory-scale optimization of roasting conditions followed by aqueous extraction of oil from wild almond. J Am Oil Chem Soc 92:985–992
Bashir MJ, Amr SSA, Aziz SQ, Aun NC, Sethupathi S (2015) Wastewater treatment processes optimization using response surface methodology (RSM) compared with conventional methods: review and comparative study. Middle-East J Sci Res 23:244–252
Zhang Q-A, Fan X-H, Zhang Z-Q, Zhang B-S, Zhang Z-Q, Jia X-Y (2009) Optimization of SC-CO2 extraction of oil from almond pretreated with autoclaving. LWT Food Sci Technol 42:1530–1537
Lu C-L, Li Y-M, Fu G-Q, Yang L, Jiang J-G, Zhu L, Lin F-L, Chen J, Lin Q-S (2011) Extraction optimisation of daphnoretin from root bark of Wikstroemia indica (L.) CA and its anti-tumour activity tests. Food Chem 124:1500–1506
De Paula RCM, Soaresb AG, Freitasa SP (2015) Volatile compounds in passion fruit seed oil (Passiflora setacea BRS Pérola do Cerrado and Passiflora alata BRS Doce Mel). Chem Eng Trans 44:103–108
AOAC International (2002) Official methods of analysis of AOAC international. AOAC International G, USA
Paquot C (2013) Standard methods for the analysis of oils, fats and derivatives, IUPAC commission on oils, fats and derivatives. Pergamon, London
Lv J, Yang X, Ma H, Hu X, Wei Y, Zhou W, Li L (2015) The oxidative stability of microalgae oil (Schizochytrium aggregatum) and its antioxidant activity after simulated gastrointestinal digestion: relationship with constituents. Eur J Lipid Sci Technol 117:1928–1939
Tabtabaei S, Diosady LL (2013) Aqueous and enzymatic extraction processes for the production of food-grade proteins and industrial oil from dehulled yellow mustard flour. Food Res Int 52:547–556
Wu J, Johnson L, Jung S (2009) Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soybean flakes. Bioresourc Technol 100:527–533
Bair C, Snyder H (1980) Electron microscopy of soybean lipid bodies. J Am Oil Chem Soc 57:279–282
Tzen J, Huang A (1992) Surface structure and properties of plant seed oil bodies. J Cell Biol 117:327–335
Campbell KA, Glatz CE, Johnson LA, Jung S, De Moura JMN, Kapchie V, Murphy P (2011) Advances in aqueous extraction processing of soybeans. J Am Oil Chem Soc 88(4):449–465
Olsen HS (1988) Aqueous enzymatic extraction of oil from seeds. Food Sci Technol Ind Dev 1:30–37
Rosenthal A, Pyle D, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb Tech 19:402–420
Picuric-Jovanovic K, Vrbaski Z, Milovanovic M (1997) Aqueous-enzymatic extraction of plum kernel oil. Eur J Lipid Sci Tech 99:433–435
Khoddami A, Man YBC, Roberts TH (2014) Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur J Lipid Sci Tech 116:553–562
Hanmoungjai P, Pyle D, Niranjan K (2001) Enzymatic process for extracting oil and protein from rice bran. J Am Oil Chemi Soc 78:817–821
Abdulkarim S, Long K, Lai O, Muhammad S, Ghazali H (2005) Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem 93:253–263
Li P, Gasmalla MAA, Zhang W, Liu J, Bing R, Yang R (2016) Effects of roasting temperatures and grinding type on the yields of oil and protein obtained by aqueous extraction processing. J Food Eng 173:15–24
Jiao J, Li Z-G, Gai Q-Y, Li X-J, Wei F-Y, Fu Y-J, Ma W (2014) Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chem 147:17–24
Long J-j Fu, Y-g Y-j, Zu, Li J, Wang W, C-b Gu, Luo M (2011) Ultrasound-assisted extraction of flaxseed oil using immobilized enzymes. Bioresourc Technol 102:9991–9996
Acknowledgements
The authors would like to acknowledge the Research Council of the College of Agriculture and Natural Resources of the University of Tehran. In addition, the authors gratefully acknowledge the help provided by Saeedeh Salahi, the laboratory assistant at the Department of Food Science, Engineering and Technology, University of Tehran.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ghorbanzadeh, R., Rezaei, K. Optimization of an Aqueous Extraction Process for Pomegranate Seed Oil. J Am Oil Chem Soc 94, 1491–1501 (2017). https://doi.org/10.1007/s11746-017-3045-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-3045-4