Abstract
Nadia Chaudhri worked with us as a graduate student in the Center for Neuroscience at the University of Pittsburgh from 1999 until she earned her PhD in 2005, a time that coincided with the discovery in our lab of the dual reinforcing actions of nicotine, a concept that she played an important role in shaping. The research that was described in her doctoral thesis is among the foundational pillars of the now well-accepted notion that nicotine acts as both a primary reinforcer and an amplifier of other reinforcer stimuli. This reinforcement-enhancing action of nicotine is robust and likely to be a powerful driver of nicotine use. Below, we discuss the evidence that these two actions of nicotine — primary reinforcement and reinforcement enhancement — are distinct and dissociable, a finding that Nadia was closely associated with. We go on to address two other topics that greatly interested Nadia during that time, the generalizability of the reinforcement-enhancing action of nicotine to multiple classes of reinforcing stimuli and potential sex differences in the dual reinforcing actions of nicotine. The research has greatly expanded since Nadia’s involvement, but the core ideas that she helped to develop remain central to the concept of the dual reinforcing actions of nicotine and its importance for understanding the drivers of nicotine use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We had the privilege of working with Nadia Chaudhri when she was a graduate student in the Center for Neuroscience at the University of Pittsburgh, from 1999 until she earned her PhD in 2005. At the time Nadia joined our laboratory, it was becoming apparent that in studies of intravenous nicotine self-administration in rats, in which nicotine was delivered with accompanying visual cues, nicotine seemed to increase the reinforcing value of the cues, and this drove much of the self-administration behavior. Indeed, among the first studies that she was involved in, it was demonstrated that in rats responding for a visual cue that was typically used in nicotine self-administration studies (cue light on for 1 s and chamber light off for 60 s; VS), non-contingent administration of nicotine promoted high levels of responding, comparable to those produced by contingent (i.e., self-administered) nicotine (Donny et al. 2003). Nadia’s subsequent work with us, much of which comprised her doctoral thesis (Chaudhri 2005), went on to show that this reinforcement-enhancing action of nicotine is observed across a range of nicotine doses and is dependent upon the strength of the non-nicotine reinforcer, with greater enhancement of more reinforcing stimuli. These remarkable findings remain foundational pillars of the dual reinforcement model of nicotine action, which is central to current thought regarding the rewarding properties of nicotine and nicotine dependence.
Below, we discuss the dual reinforcing actions of nicotine, focusing on the reinforcement-enhancing effect. In particular, we address the evidence that these are distinct, dissociable actions of nicotine, a topic that was a great interest to Nadia. Furthermore, we explore the relevance of this to pharmacotherapies used to treat nicotine dependence. In addition, we discuss two other topics that greatly interested Nadia during her time working with us, the generalizability of the reinforcement-enhancing action of nicotine across multiple classes of reinforcing stimuli and potential sex differences in the dual reinforcing actions of nicotine.
Dual reinforcement actions of nicotine
It is now widely accepted that nicotine acts as both a primary reinforcer and an enhancer for other reinforcing stimuli (Caggiula et al. 2009; Chaudhri et al. 2006a, b; Rupprecht et al. 2015). Although the bulk of the evidence for this comes from studies in rodents, similar effects have been demonstrated in people (Perkins et al. 2017). In self-administration experiments in rats, it is clear that whereas nicotine is a relatively weak primary reinforcer, the reinforcement-enhancing effect of nicotine can be quite robust. It should be noted that the reinforcement-enhancing effect of nicotine can be demonstrated with a variety of experimental approaches, including some that do not strictly rely on enhancement of primary reinforcement, and thus might better be referred to as ‘incentive amplification’ (Palmatier et al. 2014). Nonetheless, we will use the terminology of reinforcement enhancement, as it remains dominant in the literature. These two actions of nicotine, primary reinforcer and reinforcer enhancer, undoubtably relate to the high incidence of nicotine use disorder and they must also be taken into account when considering smoking cessation pharmacology.
Approaches to study the reinforcement actions of nicotine in experimental animals
Self-administration
Self-administration of a drug is the gold standard for measuring its reinforcing actions, and this is often viewed as measuring the primary reinforcing actions of the drug. However, that would be the case if the self-administration of the drug was clearly isolated from any other action of the drug, such as enhancing the response elicited by a concurrently available reinforcing stimulus. As drug self-administration procedures typically involve other environmental stimuli (e.g., a cue light) that might be mildly reinforcing or become so through repeated pairing with the reinforcing drug, it is possible that the drug may interact with these other stimuli and that the resulting self-administration behavior is the product of this complex interaction. As will be detailed below, the importance of this complex interaction between drug and other stimuli is particularly critical for nicotine.
Most nicotine self-administration is a complex mixture of the primary reinforcing action of nicotine, which tends to be weak, and the reinforcement-enhancing action of nicotine, which tends to be more robust (Caggiula et al. 2009; Chaudhri et al. 2006a, b; Rupprecht et al. 2015). In most nicotine self-administration studies, these two actions of nicotine occur together but are often interpreted as if they reflect the primary reinforcing action of nicotine, despite observations suggesting that much of the reinforced behavior is driven by the reinforcement-enhancement action (Caggiula et al. 2009; Chaudhri et al. 2006a, b; Rupprecht et al. 2015). Several different approaches can be taken to tease apart these two actions of nicotine.
Non-contingent nicotine along with operant responding for another reinforcer
One way to isolate the reinforcement-enhancing effects of nicotine from its primary reinforcing actions is via experimenter administered nicotine in animals responding for a non-nicotine stimulus. This has typically involved rats that are responding for a tone and/or light stimulus after receiving systemic injection of nicotine (Barret and Bevins 2013; Barrett et al. 2017, 2018; Constantin and Clarke 2018; Guy et al. 2014; Guy and Fletcher 2013; Satanove et al. 2021; Swalve et al. 2015). Most studies of this sort have used subcutaneous injection of nicotine administered just prior to the operant session and, as discussed below, this has provided a wealth of data regarding the reinforcement-enhancing action of nicotine. However, the use of subcutaneous injection of nicotine makes it difficult to compare doses of nicotine required for the primary reinforcing action of nicotine, since those studies rely on intravenous self-administration of the drug. Ways around this issue have included experimental designs in which rats receive intravenous injections of nicotine based on self-administration dosing either using average data from nicotine self-administration experiments or a stricter yoked design (Chaudhri et al. 2006a, b; Chaudhri et al. 2007; Liu et al. 2007). Another approach, which has distinct advantages, is a dual operant procedure in which rats respond on one operant to receive intravenous infusions of nicotine and a different operant to receive a different non-nicotine stimulus (Palmatier et al. 2006, 2007).
Self-administration of nicotine and other reinforcing stimulus via a separate and concurrently available operant responses
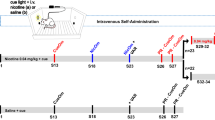
The approach of using two distinct operant responses to concurrently assess the primary and reinforcement-enhancing actions of nicotine is particularly powerful. In this approach, for example, the rat might respond on one lever to earn VS presentations and a different lever to receive intravenous infusions of nicotine (Palmatier et al. 2006, 2007). This allows for the study of how self-administered doses of nicotine interact with responding for reinforcing stimuli presented independent of nicotine. Interestingly, when rats are permitted to respond separately for nicotine and a non-nicotine stimulus such as VS presentations, rats choose to take less nicotine and more of the non-nicotine stimulus than they would if nicotine and VS were tied together with the same operant response, at least at certain doses of nicotine (Palmatier et al. 2007) (Fig. 1). Using this approach and comparing it with rats responding only for intravenous infusions of nicotine or only for the non-nicotine stimulus, it is possible to address whether nicotine self-administration can enhance responding for the non-nicotine stimulus and also whether the non-nicotine stimulus can influence the responding for nicotine. Results from such studies are clear; nicotine enhances responding for concurrently available reinforcing stimuli, whereas responding for the other reinforcer generally does not influence nicotine self-administration, though this has been investigated in only a limited manner.
Responding for concurrently available nicotine and non-pharmacological stimulus. Four groups of male rats were allowed to respond on two levers during 1-h self-administration sessions. For the four groups (n = 8/group), the two levers, schematized at the left portion of the figure, were nicotine (NIC, 60 µg/kg/infusion) plus VS (1-s illumination of a white cue light located directly above the lever, followed by 1-min deactivation of the camber light) and inactive; nicotine on one lever and VS on the other, nicotine on one lever and the other inactive; VS on one lever and inactive on the other. For details of the methods, see Palmatier et al. (Palmatier et al. 2007). What is available on each of the levers in each of the groups is schematized on the left side of the figure. The bar graph shows the average of the last 3 days of responding on an FR2 schedule, representing days 20–22 of the experiment; the data are adapted from Palmatier et al. (Palmatier et al. 2007). Responding for NIC + VS was significantly higher than responding for NIC in other groups, p < 0.05. Responding for VS alone was significantly lower than responding when VS was accompanied by nicotine, and responding for VS when it was separately but concurrently available with nicotine was significantly higher than if it were coupled with nicotine on the same lever, p < 0.05. # represents responding on the inactive lever, which was low and similar in all groups (Palmatier et al. 2007)
Effects of non-contingent nicotine using other approaches to address reward
Another approach that has been used to study the reinforcement-enhancing action of nicotine is the impact of nicotine on responding for intracranial self-stimulation (ICSS). Numerous reports have shown that nicotine lowers the threshold for ICSS, indicating an enhancement of the reinforcing properties of ICSS (Harris et al. 2018; Harrison et al. 2002; Kenny and Markou 2006; LeSage et al. 2016; Negus and Miller 2014; Paterson et al. 2008). Place preference can be conditioned to rewarding stimuli (conditioned place preference, CPP) and subcutaneous injection of nicotine prior to a test session can increase the strength of a conditioned place preference (Buffalari et al. 2014). Thus, CPP is another approach that has been used to examine the reinforcement enhancement action of nicotine.
Lessons learned using the two operant approach and other approaches
The dose response curves for the primary and reinforcement enhancement actions of nicotine may differ
Nicotine acts on a family of nicotinic cholinergic receptors comprised of five protein subunits with the specific subunit composition impacting the properties of the receptor (Dani and Bertrand 2007;Gotti and Clementi 2004). If the receptor subtype(s) involved in mediating these two reinforcing actions of nicotine differ, then the two actions might have different sensitivity to nicotine and different dose–response curves. This would require that the reinforcement enhancement effect is studied using doses and routes of nicotine that are self-administered, such as intravenous injections of nicotine, as that is the only way to study the primary reinforcing action in direct comparison to the reinforcement-enhancing action. Chaudhri et al. (Chaudhri et al. 2007) were the first to take this approach by directly comparing lever pressing in male rats that were self-administering nicotine alone (just primary reinforcement) or along with a compound visual stimulus (cue light one for 1 s, chamber light off for 60 s; VS) (primary reinforcement of nicotine plus primary reinforcement of VS plus reinforcement enhancement) or were responding for just VS with their nicotine injections yoked to the rats self-administering nicotine. Two key observations from that study were (1) across a range of nicotine doses (10 µg/kg/inf- 90 µg/kg/inf; doses expressed as nicotine free base), responding on the active lever that delivered VS was the same whether or not the nicotine was also contingent upon responding on that lever or was yoked to other rats and (2) without the VS, responding was not observed at the lower doses. This was noted using both FR and PR schedules of reinforcement. These were among the first data to suggest that the primary reinforcing, and reinforcement-enhancing actions of nicotine might be pharmacologically distinct, since the reinforcement-enhancing action seemed to be more sensitive to nicotine dose than the primary reinforcing action. However, this difference in nicotine dose supporting the two actions of nicotine is not robustly supported by the literature. Liu et al. (Liu et al. 2007) tested a range of intravenous nicotine doses delivered in a manner similar to rats self-administering nicotine on responding for a visual stimulus, and observed that the threshold for the reinforcement enhancing effect was between 7.5 and 15 µg/kg/infusion (total dose ~ 0.1 mg/kg), which is roughly similar to what is noted in self-administration studies conducted without an intrinsically-reinforcing cue (e.g., Schassburger et al. 2016; Smith et al. 2013)). However, these studies are not directly comparable, since they used Sprague–Dawley rats from different suppliers, used different operant responses (levers versus nose poke holes), and the nicotine self-administration used a cue light that was not intrinsically reinforcing but which might have become mildly reinforcing through repeated pairing with nicotine, emphasizing the difficulty of truly dissociating these two actions of nicotine. Still, it might be that the threshold dose of nicotine required for the reinforcement-enhancing action of nicotine is less than that required for primary reinforcement. Using the approach of having rats respond on different operants for nicotine and a non-nicotine stimulus would be the ideal way to address this issue, but unfortunately those detailed studies have not been done. And, for doses of nicotine below the threshold for self-administration, the reinforcement enhancing effect would need to be tested by non-contingent nicotine administration. Palmatier et al. (Palmatier et al. 2007) did compare a few doses of nicotine in such a paradigm, but doses low enough to determine threshold were not tested; at the lowest dose tested in that study, 30 µg/kg/inf, both actions were observed.
Both the primary reinforcement effect and the reinforcement-enhancing effect of nicotine require nicotinic receptors, but possibly different subtypes
If nicotinic receptors with different subunit compositions mediate the primary reinforcing and reinforcement enhancement actions of nicotine, then these two actions may be differentially sensitive to different nicotinic receptor antagonists. Again, the approach of having rats respond on separate levers for nicotine and VS (or other non-nicotine reinforcer) along with systemic injection of selective nicotinic receptor antagonists would be an ideal way to study this. However, this has only been examined using mecamylamine (MEC), a non-subtype selective nicotinic antagonist (Palmatier et al. 2007). Interestingly, acute MEC (or saline substitution for nicotine) blocked nicotine-enhanced responding for VS, whereas it took several days of treatment with MEC (or saline substitution) to reduce responding on the nicotine lever. While this does not address the receptor subtypes involved in these responses, it does highlight that these two responses can be dissociated. One interpretation of this finding is that the impact of MEC on responding for nicotine requires learning (i.e., extinction) whereas a similar, experience-dependent process is not required to change responding for the VS.
Other studies have examined nicotinic receptor antagonists with more subtype selectivity, but none with the goal of trying to distinguish between nicotine’s primary reinforcing action versus its reinforcement-enhancing action. Dihydro-β-erythroidine (DHβE), an antagonist of receptor subtypes with high affinity for nicotine (primarily α4β2 and α4β4), effectively blocks reinforcement enhancement (Barrett et al. 2018; Guy and Fletcher 2013; Liu et al. 2007). DHβE also blocked the effect of nicotine to lower ICSS threshold (Kenny and Markou 2006). In contrast, an α7-selective antagonist, methyllycaconitine (MLA), had no effect in these tests of the reinforcement-enhancing action of nicotine (Barrett et al. 2018; Guy and Fletcher 2013; Liu et al. 2007) or on nicotine self-administration (Grottick et al. 2000), though conflicting data exist (Markou and Paterson 2001). Mice in which the β2 subunit has been genetically knocked out fail to develop nicotine self-administration (Orejarena et al. 2012; Picciotto et al. 1998; Pons et al. 2008), though it is unclear to what extent reinforcement enhancement was involved in these effects on self-administration. β2 knockout mice also fail to develop nicotine-induced conditioned responses to food (Brunzell et al. 2006), consistent with reinforcement-enhancing action of nicotine requiring the β2 subunit.
Nicotinic receptors in the ventral tegmental area (VTA) are necessary for intravenous nicotine self-administration when paired with cues, as this is blocked by infusion of DHβE directly into the VTA in rats (Corrigall et al. 1994). Furthermore, in β2 subunit knockout mice, the absence of intravenous nicotine self-administration is restored by selectively re-expressing this subunit in the VTA (Orejarena et al. 2012); similar findings were noted with intra-VTA nicotine self-administration (Maskos et al. 2005). Importantly, rats and mice will respond to self-administer nicotine directly into VTA (Besson et al. 2006; Ikemoto et al. 2006) and, like with intravenous nicotine self-administration, much of this responding is accounted for by reinforcement enhancement (Farquhar et al. 2012), suggesting that β2 subunits in the VTA are critical for the reinforcement-enhancing action of nicotine.
The likelihood that the α4 subunit combined with β2 subunits (i.e., α4β2) are critical for one or both of the reinforcing actions of nicotine is supported by a lack of intracerebral nicotine self-administration in α4 knockout mice (Exley et al. 2011). However, there are also conflicting data showing that α4 subunits are not critical for nicotine self-administration (Cahir et al. 2011); α4 knockout mice displayed nicotine CPP and intravenous nicotine self-administration, similar to wild-type littermates. Using the α4-S248F mutant mouse model, in which α4 subunit containing receptors are refractory to MEC (Teper et al. 2007), Madsen et al. (2015) showed that nicotine self-administration was not blocked by MEC in the mutant mice, in contrast to wildtype mice, suggesting the important involvement of α4 subunit-containing nAChR. These mutant α4 subunit-containing receptors are also more sensitive to nicotine and the α4-S248F mice display nicotine CPP and self-administration at lower doses than wildtype littermate (Cahir et al. 2011; Tapper et al. 2004). On the other hand, potential involvement of α6 subunit-containing nAChR is supported by α6-selective antagonists blocking nicotine self-administration (Beckmann et al. 2015; Madsen, et al. 2015; Neugebauer et al. 2006; Wooters et al. 2011). Furthermore, infusion of an α6β2-selective conotoxin into the VTA (Gotti et al. 2010) or nucleus accumbens (Brunzell et al. 2010) blocks nicotine self-administration. However, α6 knockout mice did develop nicotine self-administration (Exley, et al. 2011), in contrast to what was observed in α4 knockout mice. One interpretation of these data is that nAChR containing both α4 and α6 units in addition to β2 are required for the reinforcing actions of nicotine, though the issue of how these receptor subtypes may be involved in primary reinforcement versus reinforcement enhancement remains unaddressed.
AT-1001, largely selective for α3β4-containing receptors, blocks nicotine + VS self-administration (Cippitelli et al. 2015; Toll et al. 2012), but with the design of these experiments, it is not clear whether AT-1001 blocked the primary reinforcing action of nicotine, the reinforcement-enhancing action, or both. Given that in vitro, AT-1001 may also have agonist properties at α3β4 and antagonist properties at α4β2 at higher concentrations, it may be that these actions contribute to the observed effect on nicotine self-administration. Even so, another drug that is a relatively selective α3β4 antagonist, 18-methoxycoronaridine, decreased nicotine self-administration (Glick et al. 2002); although the methods for this study do not mention if cues were provided along with nicotine delivery, the relatively high number of nicotine infusions earned by the rats suggests that nicotine delivery was paired with cues. Studies in which rats have access to nicotine and a different reinforcer (e.g., VS) delivered by separate operant responses and then treated with an α3β4 antagonist would be very useful in determining whether such drugs inhibit nicotine’s primary reinforcing action, reinforcement-enhancing action, or both.
In summary, while it is clear that nicotinic receptors are necessary for both the primary reinforcing and reinforcement-enhancing actions of nicotine, the specific subtypes of receptors necessary for these responses is unclear. Nonetheless, the temporal difference in MEC blocking the two actions of nicotine provides evidence that the actions are dissociable.
The primary reinforcing and reinforcement enhancement actions of nicotine can be pharmacologically distinguished using drugs that target different neural systems
There are a number of studies addressing the neuropharmacology of nicotine’s reinforcement enhancement effect (e.g., (Guy, et al. 2014; Guy and Fletcher 2014;Satanove et al. 2021)), but few studies have been designed to directly compare the pharmacological profiles of nicotine’s primary reinforcing effect and the reinforcement enhancement effect. Still, the limited data available document that these two effects of nicotine can be pharmacologically dissociated. The approach of using separate operant responses for nicotine and a non-nicotine reinforcer (e.g., VS) is a powerful way to study this.
One particularly compelling example is the effect of antagonists of the metabotropic glutamate receptor 5 (mGluR5) (Palmatier et al. 2008). This class of drugs had been reported to reduce nicotine self-administration (Liechti and Markou 2007; Paterson and Markou 2005; Paterson et al. 2003; Tessari et al. 2004; Tronci et al. 2010). Antagonists of mGluR5 also block nicotine enhancement of ICSS (Harrison et al. 2002). When mGluR5 antagonists were administered to rats that were responding for intravenous nicotine plus VS, responding was markedly decreased. If rats were allowed to respond on separate levers for nicotine and VS, responses on both levers were decreased by mGluR5 antagonists, but because the rats were self-administering so little nicotine it was unclear whether responding for the VS was blocked or it was simply reduced because nicotine was not being administered. To address this issue, a separate group of rats was allowed to respond for VS while being given infusions of nicotine to mimic the amount of nicotine that would be self-administered (Palmatier et al. 2008). In those animals, responding for the VS was enhanced and not attenuated by mGluR5 antagonists. However, there is also a report that an mGluR5 antagonist does interfere with enhancement of responding for a VS-like stimulus by subcutaneous injection of nicotine (Tronci et al. 2010); the reason underlying the difference between these results and those of Palmatier et al. (Palmatier et al. 2008) is unclear, but may relate to differences in nicotine dose or route of administration. Still, based on the data of Palmatier et al. (Palmatier et al. 2008), mGluR5 antagonists may selectively reduce the primary reinforcing effect of nicotine without interfering with the reinforcement-enhancing action of nicotine.
Varenicline is another interesting example. This drug, used as a smoking cessation aid, is generally accepted as a partial agonist of a4β2 receptors (Coe et al. 2005; Rollema et al. 2007) though with a more complicated pharmacology (Grady et al. 2010; Ortiz et al. 2012). Varenicline has been shown to mimic the reinforcement-enhancing action of nicotine (Barrett et al. 2018; Levin et al. 2012) and it is self-administered when coupled with other reinforcing stimuli (e.g., cue lights) (Rollema et al. 2007; Schassburger et al. 2015). However, when tested using separate levers to administer varenicline and VS, it fails to show a primary reinforcing action (Schassburger et al. 2015). Thus, varenicline mimics the reinforcement-enhancing action of nicotine while failing to support primary reinforcement. (This might also be taken as evidence that the receptor subtypes mediating these two actions of nicotine are different.) Garcia-Rivas et al. (Garcia-Rivas et al. 2019) also suggested that varenicline targets reinforcement enhancement as opposed to the primary reinforcing effects of nicotine. Thus, the available data suggest that varenicline mimics the reinforcement-enhancing action of nicotine while lacking activity related to the primary reinforcing action of nicotine.
Since varenicline appears to selectively target the reinforcement enhancement action of nicotine, does bupropion, another major smoking cessation pharmacotherapy, have a similar profile? Bupropion increases responding for VS (Barrett et al. 2017; Coddington et al. 2010; Palmatier et al. 2009) and conditioned stimuli (Guy et al. 2014) and decreases ICSS threshold (Cryan et al. 2003), indicating that bupropion, like nicotine, has a reinforcement-enhancing action. However, the effects of bupropion on intravenous nicotine self-administration are inconsistent across studies, likely having to do with differences in doses of nicotine and bupropion, schedules of reinforcement, and additional stimuli (Bruijnzeel and Markou 2003; Glick et al. 2002; Liu et al. 2008; Rauhut et al. 2003; Shoaib et al. 2003; Stairs and Dworkin 2008). In an experiment in which rats responded on different levers for concurrently available nicotine and VS, subcutaneous injection of bupropion increased responding for VS without altering nicotine responding Coddington et al. 2010). Taken together, these results suggest that bupropion may be an effective smoking cessation therapy at least in part by substituting for the reinforcement-enhancing action of nicotine, though the behavioral and pharmacological actions of bupropion related to nicotine are quite complex (Paterson 2009).
These 3 drugs provide an interesting perspective regarding smoking cessation pharmacotherapy. Two drugs that show some efficacy in helping smokers quit, varenicline and bupropion, appear to target the reinforcement-enhancing action of nicotine. In stark contrast, a class of drugs that appears to selectively target the primary reinforcing action of nicotine, mGluR5 antagonists, have not been shown to have efficacy as smoking cessation aids (Barnes et al. 2018; Chiamulera et al. 2017).
Other drugs may also selectively impact these two actions of nicotine
Antagonists of D1 dopamine receptors block nicotine self-administration (Corrigall and Coen 1991a, b; DiPalma et al. 2019; Hall et al. 2015; Stairs et al. 2010) and also the reinforcement-enhancing action of nicotine when it is studied in isolation (Barrett et al. 2017; Guy and Fletcher 2014; Palmatier et al. 2014; Satanove et al. 2021). Unfortunately, no studies to date have examined these drugs in a paradigm that is selective for the primary reinforcing effects of nicotine, and this class of drugs has not yet been tested in rats responding concurrently on different operants for nicotine and a non-nicotine stimulus. Nonetheless, given the well-documented role of dopamine in reinforcement and reward, we would hypothesize that D1 receptors are necessary for both the primary reinforcing and reinforcement enhancing actions of nicotine.
Opioid antagonists, such as naloxone and naltrexone, have been shown to block or attenuate the reinforcement-enhancing action of nicotine (Guy et al. 2014; Kirshenbaum et al. 2016; Satanove et al. 2021). On the other hand, these opiate antagonists, or other mu-selective antagonists, have been reported to either block intravenous nicotine self-administration (Ismayilova and Shoaib 2010; Liu and Jernigan 2011) or have minimal effect (Corrigall and Coen 1991a, b;DeNoble and Mele 2006;Liu et al. 2009), though the reason for these difference remain unclear. Again, testing these drugs in a paradigm with concurrently available responses for nicotine and a non-nicotine stimulus would provide a clear indication of whether these drugs truly selectively target the reinforcement-enhancing action of nicotine; the existing evidence cited above suggests that this might be the case. Interestingly, these drugs have been suggested to be useful smoking cessation aids (Byars et al. 2005; Epstein and King 2004; Fridberg et al. 2014; King et al. 2013, 2006, 2012; Krishnan-Sarin et al. 2003), which is consistent with other smoking cessation pharmacotherapies targeting the reinforcement-enhancing action of nicotine. However, data on long-term smoking quit rates remains unconvincing (David et al. 2014; Norman and D'Souza 2017).
How well does the reinforcement-enhancing action of nicotine generalize to classes of non-nicotine stimuli?
In her doctoral thesis, Nadia highlighted two areas for future research to extend her work on the dual reinforcing actions of nicotine (Chaudhri 2005). One was: “how well does the reinforcement-enhancing action of nicotine generalize to classes of non-nicotine stimuli?” In the years since Nadia defended her thesis, this question has received considerable attention, and the answer is that in rats it appears to generalize to a wide variety of reinforcing non-nicotine stimuli. As already noted, nicotine enhances ICSS, and as described in greater detail below, nicotine enhances CPP driven by a variety of rewards (including sucrose, social interaction, reinforcing drugs) and operant responding for many different reinforcing stimuli (including drugs, flavored solutions, and neutral stimuli that have become reinforcing through repeated association with reinforcing stimuli). However, before we discuss those topics, we want to address another important generalization: generalization to humans.
The reinforcement-enhancement action of nicotine has been documented in human subjects. In a series of studies, Perkins and colleagues have documented that nicotine enhances the reinforcing properties of other stimuli (Perkins and Karelitz 2013a, b; Perkins and Karelitz 2013a, b; Perkins and Karelitz 2014; Perkins et al. 2017, 2019, 2015). For example, Perkins and Karelitz (Perkins and Karelitz 2013a, b) observed that smoking approximately 8 puffs of a cigarette increased responding for a subject’s preferred music; a smaller number of cigarette puffs or a subjects non-preferred music failed to produce enhancement of responding. In another interesting study, Kirshenbaum and Hughes (Kirshenbaum and Hughes 2021) observed that nicotine delivered via e-cigarettes increased PR responding for video gaming, as well as enjoyment of the video game, in young adults. Also, in a clever adaptation of the rodent CPP paradigm to humans using virtual reality, nicotine has been shown to increase the development of a CPP to a rewarding stimulus (chocolate treats) (Palmisano and Astur 2020).
One class of non-nicotine stimuli that has repeatedly been shown to be enhanced by nicotine are sweet and flavored solutions (Barret and Bevins 2013; Palmatier et al. 2013, 2020; Tannous et al. 2021). Palmatier et al. (Palmatier et al. 2012) showed that in rats responding for a sucrose solution by pressing a lever, the magnitude of the enhancement effect increased with increasing concentrations, consistent with the notion that the reinforcement-enhancing action of nicotine is systematically related to the salience of the non-nicotine reinforcer. Non-contingent nicotine also enhanced operant responding for flavored solutions (Palmatier et al. 2013, 2020) and sucrose pellets (Rupprecht et al. 2016). The relevance of these observations to the widespread use of flavored e-liquid solutions that account for the vast majority of e-cigarette vaping cannot be ignored (Palmatier et al. 2020; Patten and De Biasi 2020; Rupprecht et al. 2016).
Nicotine has been shown to increase social interaction reward in rats (Achterberg and Vanderschuren 2020; Cheeta et al. 2001; Thiel et al. 2009), in both CPP tests and operant responding for social interaction, at least under certain conditions. Similarly, as noted above, nicotine lowers the threshold stimulation for ICSS, suggesting that this reinforcement-enhancing action of nicotine may generalize broadly to reinforcing stimuli.
It is also important to note that nicotine enhances responding for stimuli that are reinforcing as a result of being previously paired with other reinforcing stimuli. Furthermore, in Pavlovian conditioning paradigms, nicotine increased approach to contexts (Thiel et al. 2009) and discrete stimuli associated with rewards (Olausson et al. 2003). This reinforcement enhancement action of nicotine might be more appropriately referred to as “incentive amplifying” (Palmatier et al. 2014).
Another class of non-nicotine stimuli that is enhanced by nicotine are substances of abuse, and this is highly relevant to the co-use of tobacco products and other drugs of abuse. Nicotine increases alcohol self-administration in rats (Barrett et al. 2020; Bito-Onon et al. 2011; Clark et al. 2001; Hauser et al. 2014; Le et al. 2000, 2003; Lopez-Moreno et al. 2004; Montanari et al. 2021), consistent with nicotine also increasing alcohol consumption (Blomqvist et al. 1996; Le, et al. 2000; Olausson et al. 2001; Potthoff et al. 1983; Smith et al. 1999), though this has not been observed in all studies (Sharpe and Samson 2002). Dr. Nadia Chaudhri’s lab contributed to this research by showing that nicotine, by acting on nicotinic cholinergic receptors, enhanced responding for alcohol-paired cues (Maddux and Chaudhri 2017), a finding that has been confirmed and extended (Loney et al. 2019). Interestingly, Le et al. (Le et al. 2010) reported that when rats were allowed to concurrently self-administer intravenous nicotine and oral ethanol, the amount of nicotine or ethanol that was self-administered did not differ from the amount of the substance rats would self-administer if it was the only substance available. While this would seem to conflict with the notion that nicotine enhances ethanol intake, the observation that rats tended to take almost all of the ethanol during the beginning of the self-administration session whereas the majority of nicotine was taken later; thus, the way the rats chose to take the two substances would preclude the action of nicotine to enhance ethanol self-administration. This issue was addressed in a subsequent study (Le et al. 2014). In this later study, Le et al. found that when available in 5 min alternating periods, nicotine self-administration increased alcohol self-administration, but not the converse. Overall, the studies examining the effect of nicotine on alcohol consumption provides evidence that nicotine increases the reinforcing effects of alcohol. These preclinical findings are consistent with the observations in humans that exposure to nicotine via smoking or transdermal patch can increase alcohol intake (Acheson et al. 2006; Barrett et al. 2006).
Nicotine has also been shown to promote cocaine self-administration in rats (Bechtholt and Mark 2002) and conditioned place preference in rats (Buffalari et al. 2014) and mice (Levine et al. 2011), consistent with reported interactions between cigarette smoking and cocaine use in people (Brewer et al. 2013; Reid et al. 1998; Shoptaw et al. 1996). Manzardo et al. (Manzardo et al. 2002) examined the self-administration of concurrently available nicotine and cocaine in rats to assess the relative reinforcing strength of the two drugs. While rats clearly preferred cocaine to nicotine, the data also showed that nicotine self-administration did not enhance cocaine self-administration. While what may account for the difference between this study and the clear enhancement of cocaine self-administration by subcutaneous injection of nicotine reported by Bechtholt et al. (Bechtholt and Mark 2002) is unclear, it may relate differences in cocaine dose or schedule of reinforcement.
It has also been reported that nicotine enhances the self-administration of the opiate agonists remifentanil and morphine (Honeycutt et al. 2022; Loney et al. 2021). This is consistent with increased use of opiates in cigarette smokers (Rajabi et al. 2019; Romberg et al. 2019; Skurtveit et al. 2010; Zale et al. 2015).
Recent studies have examined the impact of nicotine on self-administration of THC and a synthetic cannabinoid CB1 agonist, WIN55212-2 and found that subcutaneous injection of nicotine prior to the self-administration session increased responding for these cannabinoids (Stringfield et al. 2022). Furthermore, in a double operant response paradigm, nicotine self-administration increased WIN55212-2 self-administration whereas nicotine self-administration was not impacted by WIN55212-2. These data suggest that co-use of nicotine and cannabinoids promotes cannabinoid use in excess of what would be taken alone, and further highlight the role that the reinforcement-enhancing action of nicotine may have in promoting drug co-use.
In summary, nicotine appears to increase the reinforcing properties of a wide variety of drugs of abuse, ranging from alcohol to cocaine to opiates and cannabinoids. This likely contributes to the high incidence of co-use of nicotine (e.g., tobacco products) and these other drugs. It is also likely that this reinforcement enhancement action of nicotine must be taken into account in the treatment of substance abuse.
Are there sex differences in the dual reinforcing actions of nicotine?
A second unanswered question that Nadia highlighted in her doctoral thesis as a future direction was potential sex differences in the dual reinforcing actions of nicotine. This question, which Nadia began to study (Chaudhri et al. 2005) but did not include in her doctoral thesis (Chaudhri 2005), has continued to receive attention. Chaudhri et al. (Chaudhri et al. 2005) reported that responding for nicotine + VS was higher in females than males, at least at some doses (60 and 150 µg/kg/inf, but not at 30 µg/kg/inf), and responding for the VS alone was also greater in females than males. In a larger study focused on the reinforcement enhancing action of nicotine, McNealy et al. (McNealy et al. 2022) tested a range of sc nicotine doses on lever responding for a complex VS, and observed no sex differences, with a threshold dose of 0.1 mg/kg (with no greater effect at 0.3 mg/kg). Flores et al. (Flores et al. 2019) conducted a meta-analysis of studies examining sex differences in intravenous nicotine self-administration in rats and reported that females self-administered nicotine more than males, and cue was a contributing factor. Thus, at least in nicotine self-administration studies, it appears that females may respond more than males for nicotine plus reinforcing cues, suggesting that cues and the reinforcement-enhancing action of nicotine may play a larger role in females. Several studies by Barrett and Bevins and colleagues (Barrett et al. 2017, 2018, 2020) examined the impact of subcutaneous injections of nicotine on responding for VS or ethanol in male and female rats across a range of schedules. On a PR schedule of reinforcement, females responded more than males for the VS or ethanol, and the enhancement effect was significantly larger in females than males, though the effective doses appeared similar in males and females. However, using a wide range of FR schedules of reinforcement to conduct a full reinforcement demand analysis, sex differences were not apparent in rats receiving either saline or nicotine, although an enhancement effect of nicotine was observed as both an increase in intensity of demand (Qo; how much of the reinforcer the rat will consume when it is ‘free’) and essential value, which reflects how sensitive consumption is to increased cost. Thus, this issue of potential sex differences in the dual reinforcing actions of nicotine, and how they may interact, is still an unsettled issue and deserves further study. Nonetheless, sex differences in the use of other reinforcing drugs, such as cocaine, with females more prone to substance abuse, (Becker and Hu 2008; Carroll and Anker 2010; Lynch 2006) point to the likelihood that sex differences in the reinforcing actions of nicotine exist. Studies comparing males and females using a dual operant current access approach may be particularly enlightening in teasing apart sex differences in responding for nicotine, reinforcing cues, and their interaction. The potentially complex interaction is further highlighted by studies in human subjects showing that sex differences in the reinforcement-enhancing action of nicotine are dependent upon the reinforcing stimulus being tested (Perkins and Karelitz 2016).
Summary and conclusions
Nadia Chaudhri began her research career as a doctoral student examining the reinforcing actions of nicotine by studying intravenous nicotine self-administration in rats. Her observations contributed to the development of the notion that nicotine is a primary reinforcer while also being a more robust enhancer of other reinforcers. This dual reinforcement model of nicotine action, first proposed by Donny et al. in 2003 (Donny et al. 2003) and laid out in Nadia’s 2006 review (Chaudhri et al. 2006a, b) and in her doctoral thesis (Chaudhri 2005), is now well established. While these two actions of nicotine are often observed together and can be difficult to disentangle in nicotine self-administration studies, they are distinct and dissociable. The reinforcement-enhancing action of nicotine was initially demonstrated using a visual stimulus often used in nicotine self-administration studies, but it generalizes to multiple classes of reinforcers, including other reinforcing drugs. Interestingly, these two reinforcing actions of nicotine can be pharmacologically dissociated and drugs that are useful smoking cessation aids seem to target the reinforcement-enhancing action of nicotine. The reinforcement-enhancing action of nicotine is likely central to understanding vaping of flavored nicotine solutions and the prevalence of co-use of tobacco and other drugs of abuse. While much has been learned about the dual reinforcing actions of nicotine since Nadia’s early work on this topic, there is still much that awaits discovery and clarification, including one of particular interest to Nadia, potential sex differences in these two actions of nicotine.
References
Acheson A, Mahler SV, Chi H, de Wit H (2006) Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology 186:54–63
Achterberg EJM, Vanderschuren L (2020) Treatment with low doses of nicotine but not alcohol affects social play reward in rats. Int J Play 9:39–57
Barnes SA, Sheffler DJ, Semenova S, Cosford NDP, Bespalov A (2018) Metabotropic glutamate receptor 5 as a target for the treatment of depression and smoking: robust preclinical data but inconclusive clinical efficacy. Biol Psychiat 83:955–962
Barret ST, Bevins RA (2013) Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacol Biochem Behav 114:9–15
Barrett SP, Tichauer M, Leyton M, Pihl RO (2006) Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend 81:197–204
Barrett ST, Geary TN, Steiner AN, Bevins RA (2017) Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology 234:187–198
Barrett ST, Geary TN, Steiner AN, Bevins RA (2018) A behavioral economic analysis of the value-enhancing effects of nicotine and varenicline and the role of nicotinic acetylcholine receptors in male and female rats. Behav Pharmacol 29:493–502
Barrett ST, Thompson BM, Emory JR, Larsen CE, Pittenger ST, Harris EN, Bevins RA (2020) Sex differences in the reward-enhancing effects of nicotine on ethanol reinforcement: a reinforcer demand analysis. Nicotine Tob Res 22:238–247
Bechtholt AJ, Mark GP (2002) Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology 162:178–185
Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47
Beckmann JS, Meyer AC, Pivavarchyk M, Horton DB, Zheng GR, Smith AM, Wooters TE, McIntosh JM et al (2015) r-bPiDI, an alpha 6 beta 2*Nicotinic receptor antagonist, decreases nicotine-evoked dopamine release and nicotine reinforcement. Neurochem Res 40:2121–2130
Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S (2006) Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology 187:189–199
Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE (2011) Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol 16:440–449
Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B (1996) Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol 314:257–267
Brewer AJ, Mahoney JJ, Nerumalla CS, Newton TF, De La Garza R (2013) The influence of smoking cigarettes on the high and desire for cocaine among active cocaine users. Pharmacol Biochem Behav 106:132–136
Bruijnzeel AW, Markou A (2003) Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50:20–28
Brunzell D, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto M (2006) Beta 2-subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology 184:328–338
Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM (2010) Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology 35:665–673
Buffalari DM, Marfo NYA, Smith TT, Levin ME, Weaver MT, Thiels E, Sved AF, Donny EC (2014) Nicotine enhances the expression of a sucrose or cocaine conditioned place preference in adult male rats. Pharmacol Biochem Behav 124:320–325
Byars JA, Frost-Pineda K, Jacobs WS, Gold MS (2005) Naltrexone augments the effects of nicotine replacement therapy in female smokers. J Addict Dis 24:49–60
Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009) The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv 55:91–109
Cahir E, Pillidge K, Drago J, Lawrence AJ (2011) The necessity of alpha 4* nicotinic receptors in nicotine-driven behaviors: dissociation between reinforcing and motor effects of nicotine. Neuropsychopharmacology 36:1505–1517
Carroll ME, Anker JJ (2010) Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav 58:44–56
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF et al (2005) Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180:258–266
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X et al (2006a) Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology 189:27–36
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006b) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology 184:353–366
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X et al (2007) Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology 190:353–362
Chaudhri N (2005) Complex interactions between nicotine and nonpharmacological stimuli reveal a novel role for nicotine in reinforcement, University of Pittsburgh, 2005
Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE (2001) In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology 25:601–607
Chiamulera C, Marzo CM, Balfour DJK (2017) Metabotropic glutamate receptor 5 as a potential target for smoking cessation. Psychopharmacology 234:1357–1370
Cippitelli A, Wu JH, Gaiolini KA, Mercatelli D, Schoch J, Gorman M, Ramirez A, Ciccocioppo R et al (2015) AT-1001: a high-affinity alpha 3 beta 4 nAChR ligand with novel nicotine-suppressive pharmacology. Br J Pharmacol 172:1834–1845
Clark A, Lindgren S, Brooks SP, Watson WP, Little HJ (2001) Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology 41:108–117
Coddington SB, Kraus EL, Palmatier MI, Caggiula AR, sved AF, Donny ED (2010) Effects of buprobion on the primary reinforcement and reinforcement enhancing effects of nicotine. Society for Nicotine and Tobacco Research annual meeting, February 24–27, 2010:POS4–18
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang JH, Sands SB, Davis TI et al (2005) Varenicline: an alpha 4 beta 2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477
Constantin A, Clarke PBS (2018) Reinforcement enhancement by nicotine in adult rats: behavioral selectivity and relation to mode of delivery and blood nicotine levels. Psychopharmacology 235:641–650
Corrigall WA, Coen KM (1991a) Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology 104:167–170
Corrigall WA, Coen KM (1991b) Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology 104:171–176
Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653:278–284
Cryan JF, Bruijnzeel AW, Skjei KL, Markou A (2003) Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 168:347–358
Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47:699–729
David SP, Chu IM, Lancaster T, Stead LF, Evins AE, Prochaska JJ (2014) Systematic review and meta-analysis of opioid antagonists for smoking cessation. Bmj Open 4
DeNoble VJ, Mele PC (2006) Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology 184:266–272
DiPalma D, Rezvani AH, Willette B, Wells C, Slade S, Hall BJ, Levin ED (2019) Persistent attenuation of nicotine self-administration in rats by co-administration of chronic nicotine infusion with the dopamine D-1 receptor antagonist SCH-23390 or the serotonin 5-HT2C agonist lorcaserin. Pharmacol Biochem Behav 176:16–22
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology 169:68–76
Epstein AM, King AC (2004) Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav 77:29–37
Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S et al (2011) Distinct contributions of nicotinic acetylcholine receptor subunit alpha 4 and subunit alpha 6 to the reinforcing effects of nicotine. Proc Natl Acad Sci USA 108:7577–7582
Farquhar MJ, Latimer MP, Winn P (2012) Nicotine self-administered directly into the VTA by rats is weakly reinforcing but has strong reinforcement enhancing properties. Psychopharmacology 220:43–54
Flores RJ, Uribe KP, Swalve N, O’Dell LE (2019) Sex differences in nicotine intravenous self-administration: a meta-analytic review. Physiol Behav 203:42–50
Fridberg DJ, Cao DC, Grant JE, King AC (2014) Naltrexone improves quit rates, attenuates smoking urge, and reduces alcohol use in heavy drinking smokers attempting to quit smoking. Alcoholism-Clinical and Experimental Research 38:2622–2629
Garcia-Rivas V, Fiancette JF, Cannella N, Carbo-Gas M, Renault P, Tostain J, Deroche-Gamonet V (2019) Varenicline targets the reinforcing-enhancing effect of nicotine on its associated salient cue during nicotine self-administration in the rat. Frontiers in Behavioral Neuroscience 13
Glick SD, Maisonneuve IM, Kitchen BA (2002) Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol 448:185–191
Gotti C, Clementi F (2004) Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 74:363–396
Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R et al (2010) Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha 6 beta 2*receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci 30:5311–5325
Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P et al (2010) Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology 58:1054–1066
Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA (2000) Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther 294:1112–1119
Guy EG, Fletcher PJ (2013) Nicotine-induced enhancement of responding for conditioned reinforcement in rats: role of prior nicotine exposure and alpha 4 beta 2 nicotinic receptors. Psychopharmacology 225:429–440
Guy EG, Fletcher PJ (2014) Responding for a conditioned reinforcer, and its enhancement by nicotine, is blocked by dopamine receptor antagonists and a 5-HT2C receptor agonist but not by a 5-HT2A receptor antagonist. Pharmacol Biochem Behav 125:40–47
Guy EG, Fisher DC, Higgins GA, Fletcher PJ (2014) Examination of the effects of varenicline, bupropion, lorcaserin, or naltrexone on responding for conditioned reinforcement in nicotine-exposed rats. Behav Pharmacol 25:775–783
Hall BJ, Slade S, Allenby C, Kutlu MG, Levin ED (2015) Neuro-anatomic mapping of dopamine D-1 receptor involvement in nicotine self-administration in rats. Neuropharmacology 99:689–695
Harris AC, Muelken P, Smethells JR, Yershova K, Stepanov I, Olson TT, Kellar KJ, LeSage MG (2018) Effects of nicotine-containing and “nicotine-free” e-cigarette refill liquids on intracranial self-stimulation in rats. Drug Alcohol Depend 185:1–9
Harrison AA, Gasparini F, Markou A (2002) Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology 160:56–66
Hauser SR, Deehan GA, Toalston JE, Bell RL, McBride WJ, Rodd ZA (2014) Enhanced alcohol-seeking behavior by nicotine in the posterior ventral tegmental area of female alcohol-preferring (P) rats: modulation by serotonin-3 and nicotinic cholinergic receptors. Psychopharmacology 231:3745–3755
Honeycutt SC, Paladino MS, Camadine RD, Mukherjee A, Loney GC (2022) Acute nicotine treatment enhances compulsive-like remifentanil self-administration that persists despite contextual punishment. Addiction biology 27
Ikemoto S, Qin M, Liu ZH (2006) Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci 26:723–730
Ismayilova N, Shoaib M (2010) Alteration of intravenous nicotine self-administration by opioid receptor agonist and antagonists in rats. Psychopharmacology 210:211–220
Kenny PJ, Markou A (2006) Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 31:1203–1211
King A, de Wit H, Riley RC, Cao DC, Niaura R, Hatsukami D (2006) Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res 8:671–682
King AC, Cao DC, O’Malley SS, Kranzler HR, Cai XC, deWit H, Matthews AK, Stachoviak RJ (2012) Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol 32:630–636
King A, Cao DC, Zhang LJ, Rueger SY (2013) Effects of the opioid receptor antagonist naltrexone on smoking and related behaviors in smokers preparing to quit: a randomized controlled trial. Addiction 108:1836–1844
Kirshenbaum AP, Suhaka JA, Phillips JL, Pinto MVD (2016) Nicotine enhancement and reinforcer devaluation: interaction with opioid receptors. Pharmacol Biochem Behav 150:1–7
Kirshenbaum AP, Hughes JR (2021) Reinforcement enhancement by nicotine: a novel abuse-liability assessment of E-cigarettes in young adults. Experimental and clinical psychopharmacology
Krishnan-Sarin S, Meandzija B, O’Malley S (2003) Naltrexone and nicotine patch in smoking cessation: a preliminary study. Nicotine Tob Res 5:851–857
Le AD, Corrigall WA, Harding JWS, Juzytsch W, Li TK (2000) Involvement of nicotinic receptors in alcohol self-administration. Alcoholism-Clin Exp Res 24:155–163
Le AD, Wang A, Harding S, Juzytsch W, Shaham Y (2003) Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology 168:216–221
Le AD, Lo S, Harding S, Juzytsch W, Marinelli PW, Funk D (2010) Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology 208:475–486
Le AD, Funk D, Lo S, Coen K (2014) Operant self-administration of alcohol and nicotine in a preclinical model of co-abuse. Psychopharmacology 231:4019–4029
LeSage MG, Staley M, Muelken P, Smethells JR, Stepanov I, Vogel RI, Pentel PR, Harris AC (2016) Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats. Drug Alcohol Depend 168:76–88
Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC (2012) Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine Tob Res 14:299–305
Levine A, Huang YY, Drisaldi B, Griffin EA, Pollak DD, Xu SQ, Yin DQ, Schaffran C, et al. (2011) Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Science Translational Medicine 3
Liechti ME, Markou A (2007) Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol 554:164–174
Liu X, Jernigan C (2011) Activation of the opioid mu 1, but not delta or kappa, receptors is required for nicotine reinforcement in a rat model of drug self-administration. Prog Neuropsychopharmacol Biol Psychiatry 35:146–153
Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF (2007) Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology 194:463–473
Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF (2008) Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology 196:365–375
Liu X, Palmatier MI, Caggiula AR, Sved AF, Donny EC, Gharib M, Booth S (2009) Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats. Psychopharmacology 202:589–598
Loney GC, Angelyn H, Cleary LM, Meyer PJ (2019) Nicotine produces a high-approach, low-avoidance phenotype in response to alcohol-associated cues in male rats. Alcoholism-Clin Exp Res 43:1284–1295
Loney GC, King CP, Meyer PJ (2021) Systemic nicotine enhances opioid self-administration and modulates the formation of opioid-associated memories partly through actions within the insular cortex. Scientific Reports 11
Lopez-Moreno JA, Trigo-Diaz JM, de Fonseca FR, Cuevas GG, de Heras RG, Galan IC, Navarro M (2004) Nicotine in alcohol deprivation increases alcohol operant self-administration during reinstatement. Neuropharmacology 47:1036–1044
Lynch WJ (2006) Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 14:34–41
Maddux JMN, Chaudhri N (2017) Nicotine-induced enhancement of Pavlovian alcohol-seeking behavior in rats. Psychopharmacology 234:727–738
Madsen HB, Koghar HS, Pooters T, Massalas JS, Drago J, Lawrence AJ (2015) Role of alpha 4-and alpha 6-containing nicotinic receptors in the acquisition and maintenance of nicotine self-administration. Addict Biol 20:500–512
Manzardo AM, Stein L, Belluzzi JD (2002) Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res 924:10–19
Markou A, Paterson NE (2001) The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res 3:361–373
Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P et al (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436:103–107
McNealy KR, Houser SD, Barrett ST, Bevins RA (2022) Investigating sex differences and the effect of drug exposure order in the sensory reward-enhancing effects of nicotine and D-amphetamine alone and in combination. Neuropharmacology 202
Montanari C, Secci ME, Driskell A, McDonald KO, Schratz CL, Gilpin NW (2021) Chronic nicotine increases alcohol self-administration in adult male Wistar rats. Psychopharmacology 238:201–213
Negus SS, Miller LL (2014) Intracranial self-stimulation (ICSS) is a behavioral procedure in which operant responding is maintained by pulses of electrical brain stimulation. Pharmacol Rev 66:869–917
Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT (2006) Effect of a novel nicotinic receptor antagonist, N, N ’-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology 184:426–434
Norman H, D’Souza MS (2017) Endogenous opioid system: a promising target for future smoking cessation medications. Psychopharmacology 234:1371–1394
Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B (2001) Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol 417:117–123
Olausson P, Jentsch JD, Taylor JR (2003) Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology 28:1264–1271
Orejarena MJ, Herrera-Solis A, Pons S, Maskos U, Maldonado R, Robledo P (2012) Selective re-expression of beta 2 nicotinic acetylcholine receptor subunits in the ventral tegmental area of the mouse restores intravenous nicotine self-administration. Neuropharmacology 63:235–241
Ortiz NC, O’Neill HC, Marks MJ, Grady SR (2012) Varenicline blocks beta 2*-nAChR-mediated response and activates beta 4*-nAChR-mediated responses in mice in vivo. Nicotine Tob Res 14:711–719
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S et al (2006) Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology 184:391–400
Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF (2007) The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology 32:1098–1108
Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF (2008) Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology 33:2139–2147
Palmatier MI, Levin ME, Mays KL, Donny EC, Caggiula AR, Sved AF (2009) Bupropion and nicotine enhance responding for nondrug reinforcers via dissociable pharmacological mechanisms in rats. Psychopharmacology 207:381–390
Palmatier MI, O’Brien LC, Hall MJ (2012) The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology 219:1119–1131
Palmatier MI, Lantz JE, O’Brien LC, Metz SP (2013) Effects of nicotine on olfactogustatory incentives: preference, palatability, and operant choice tests. Nicotine Tob Res 15:1545–1554
Palmatier MI, Kellicut MR, Sheppard AB, Brown RW, Robinson DL (2014) The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav 126:50–62
Palmatier MI, Smith AL, Odineal EM, Williams EA, Sheppard AB, Bradley CA (2020) Nicotine self-administration with tobacco flavor additives in male rats. Nicotine Tob Res 22:224–231
Palmisano AN, Astur RS (2020) Nicotine facilitation of conditioned place preference to food reward in humans. Subst Use Misuse 55:2156–2164
Paterson NE (2009) Behavioural and pharmacological mechanisms of bupropion’s anti-smoking effects: recent preclinical and clinical insights. Eur J Pharmacol 603:1–11
Paterson NE, Markou A (2005) The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology 179:255–261
Paterson NE, Semenova S, Gasparini F, Markou A (2003) The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology 167:257–264
Paterson NE, Balfour DJK, Markou A (2008) Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res 10:995–1008
Patten T, De Biasi M (2020) History repeats itself: role of characterizing flavors on nicotine use and abuse. Neuropharmacology 177
Perkins KA, Karelitz JL (2013a) Influence of reinforcer magnitude and nicotine amount on smoking’s acute reinforcement enhancing effects. Drug Alcohol Depend 133:167–171
Perkins KA, Karelitz JL (2013b) Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology 228:479–486
Perkins KA, Karelitz JL (2014) Sensory reinforcement-enhancing effects of nicotine via smoking. Exp Clin Psychopharmacol 22:511–516
Perkins KA, Karelitz JL (2016) Potential sex differences in the pattern of sensory reinforcers enhanced by nicotine. Exp Clin Psychopharmacol 24:156–161
Perkins KA, Karelitz JL, Michael VC (2015) Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug Alcohol Depend 153:104–108
Perkins KA, Karelitz JL, Boldry MC (2019) Reinforcement enhancing effects of nicotine via patch and nasal spray. Nicotine Tob Res 21:778–783
Perkins KA, Karelitz JL, Boldry MC (2017) Nicotine acutely enhances reinforcement from non-drug rewards in humans. Frontiers in Psychiatry 8
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta 2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U et al (2008) Crucial role of alpha 4 and alpha 6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci 28:12318–12327
Potthoff AD, Ellison G, Nelson L (1983) Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav 18:489–493
Rajabi A, Dehghani M, Shojaei A, Forjam M, Motevalian SA (2019) Association between tobacco smoking and opioid use: a meta-analysis. Addict Behav 92:225–235
Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT (2003) Effect of bupropion on nicotine self-administration in rats. Psychopharmacology 169:1–9
Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP (1998) An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend 49:95–104
Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS et al (2007) Pharmacological profile of the alpha(4)beta(2) nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994
Romberg AR, Lo EJM, Barton AA, Xiao HJ, Vallone DM, Hair EC (2019) Cigarette smoking, prescription opioid use and misuse among young adults: an exploratory analysis. Preventive medicine 129
Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC (2015) Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci 24:19–53
Rupprecht LE, Smith TT, Schassburger RL, Donny EC, Sved AF (2016) Effects of nicotine on rewards varying in palatability and caloric value: implications for E-cigarette flavoring Tobacco Regulatory. Science 2:343–351
Satanove DJ, Rahman S, Chan TMV, Ren S, Clarke PBS (2021) Nicotine-induced enhancement of a sensory reinforcer in adult rats: antagonist pretreatment effects. Psychopharmacology 238:475–486
Schassburger RL, Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Donny EC, Sved AF (2015) Differentiating the primary reinforcing and reinforcement-enhancing effects of varenicline. Psychopharmacology 232:975–983
Schassburger RL, Pitzer EM, Smith TT, Rupprecht LE, Thiels E, Donny EC, Sved AF (2016) Adolescent rats self-administer less nicotine than adults at low doses. Nicotine Tob Res
Sharpe AL, Samson HH (2002) Repeated nicotine injections decrease operant ethanol self-administration. Alcohol (Fayetteville, NY 28:1–7
Shoaib M, Sidhpura N, Shafait S (2003) Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology 165:405–412
Shoptaw S, Jarvik ME, Ling W, Rawson RA (1996) Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict Behav 21:409–412
Skurtveit S, Furu K, Selmer R, Handal M, Tverdal A (2010) Nicotine Dependence predicts repeated use of prescribed opioids. Prospective population-based cohort study. Ann Epidemiol 20:890–897
Smith BR, Horan JT, Gaskin S, Amit Z (1999) Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology 142:408–412
Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC (2013) Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res 15:1918–1925
Stairs DJ, Dworkin SI (2008) Rate-dependent effects of bupropion on nicotine self-administration and food-maintained responding in rats. Pharmacol Biochem Behav 90:701–711
Stairs DJ, Neugebauer NM, Bardo MT (2010) Nicotine and cocaine self-administration using a multiple schedule of intravenous drug and sucrose reinforcement in rats. Behav Pharmacol 21:182–193
Stringfield SJ, Sanders BE, Suppo JA, Sved AF, Torregrossa MM (2022) Nicotine enhances intravenous self-administration of cannabinoids and saline in adult rats. https://www.biorxiv.org/content/10.1101/2022.10.06.510908v1
Swalve N, Barrett ST, Bevins RA, Li M (2015) Examining the reinforcement-enhancement effects of phencyclidine and its interactions with nicotine on lever-pressing for a visual stimulus. Behav Brain Res 291:253–259
Tannous S, Darlot F, Cador M, Caille S (2021) Flavor additives facilitate oral self-administration of nicotine solution in mice. Psychopharmacology 238:2235–2247
Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ et al (2004) Nicotine activation of alpha 4*receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032
Teper Y, Whyte D, Cahir E, Lester HA, Grady SR, Marks MJ, Cohen BN, Fonck C et al (2007) Nicotine-induced dystonic arousal complex in a mouse line harboring a human autosomal-dominant nocturnal frontal lobe epilepsy mutation. J Neurosci 27:10128–10142
Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA (2004) Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol 499:121–133
Thiel KJ, Sanabria F, Neisewander JL (2009) Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology 204:391–402
Toll L, Zaveri NT, Polgar WE, Jiang FM, Khroyan TV, Zhou W, Xie XM, Stauber GB et al (2012) AT-1001: a high affinity and selective alpha 3 beta 4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology 37:1367–1376
Tronci V, Vronskaya S, Montgomery N, Mura D, Balfour DJK (2010) The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on behavioural responses to nicotine. Psychopharmacology 211:33–42
Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, Zhang ZF, Crooks PA, Bardo MT et al (2011) bPiDI: a novel selective alpha 6 beta 2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br J Pharmacol 163:346–357
Zale EL, Dorfman ML, Hooten WM, Warner DO, Zvolensky MJ, Ditre JW (2015) Tobacco smoking, nicotine dependence, and patterns of prescription opioid misuse: results from a nationally representative sample. Nicotine Tob Res 17:1096–1103
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on Conditioned Determinants of Reward Seeking
The author Anthony R. Caggiula is retired
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sved, A.F., Caggiula, A.R. & Donny, E.C. Elucidating the reinforcing effects of nicotine: a tribute to Nadia Chaudhri. Psychopharmacology 240, 417–430 (2023). https://doi.org/10.1007/s00213-022-06266-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06266-7