Abstract
Rationale
Nicotine and bupropion have been demonstrated to enhance the value of other reinforcers, and this may partially account for nicotine reward and dependence. Evidence suggests that the sexes differ in their sensitivity to the primary and secondary reinforcing effects of nicotine and nicotine-associated stimuli. Whether the sexes also differ in sensitivity to the reward-enhancing effects of nicotine (and bupropion) is yet unclear.
Objectives
The present study evaluated potential sex differences in the enhancement effects of nicotine and bupropion using a reinforcer demand approach. Furthermore, we sought to investigate the role that D1- and D2-type dopamine receptors play in the reward-enhancing effects of nicotine and bupropion.
Methods
Demand for sensory reinforcement was assessed in male and female rats responding on a progression of fixed ratio schedules. The effects of nicotine and 10 or 20 mg/kg bupropion on reinforcer demand were assessed within subjects. Subsequently, the effects of SCH-23390 and eticlopride were assessed on the enhancing effects of nicotine and bupropion on progressive ratio responding.
Results
Nicotine and bupropion enhanced demand metrics of reinforcement value in both sexes. Females were more sensitive to the enhancement effects of bupropion assessed by reinforcer demand and progressive ratio performance. D2-like dopamine receptor antagonism by eticlopride attenuated the enhancement effects of bupropion, but not of nicotine.
Conclusions
Nicotine and bupropion both enhance reinforcement value in both sexes, though females may be more sensitive to the reward-enhancing effects of bupropion. D2- and possibly D1-type receptors appear to be involved in the reward-enhancing effects of bupropion, but not necessarily nicotine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco use is associated with over 480,000 deaths annually in the USA alone and is the leading cause of preventable death and disease in the world (United States Department of Health and Human Services [USDHHS], 2014). Although nicotine has been accepted for decades as the constituent of tobacco smoke that motivates smoking behavior, the behavioral and neuropharmacological mechanisms whereby nicotine reinforces smoking are more complex than simply primary reinforcement by the effects of nicotine (USDHHS, 1988; Caggiula et al. 2009). A growing body of research indicates that full characterization of the mechanisms whereby nicotine motivates smoking must include primary reinforcement by nicotine, secondary reinforcement by nicotine-associated environmental stimuli, Pavlovian conditioning of the interoceptive stimulus effects of nicotine with non-nicotine rewards, and nicotine-mediated enhancement of the value of non-nicotine rewards [for a review, see Bevins and Palmatier (2004)].
Notably, research has revealed that smoking behavior and nicotine dependence differ between males and females and that a complete understanding of nicotine reward must also consider the individual’s sex (Lynch et al. 2002; Roth et al. 2004; Perkins et al. 1994, 2002; Perkins, 2009). In particular, further research attention needs to be directed toward understanding how the varied behavioral and neurobiological mechanisms of nicotine reward differ between males and females. That is, how do the primary reinforcing, secondary reinforcing, reward-enhancing, and interoceptive stimulus effects of nicotine differentially drive smoking and relapse between males and females (Bevins and Palmatier, 2004)?
To this end, the present work investigated potential sex differences in the reward-enhancing effects of nicotine, as well as those of the smoking-cessation aid bupropion (Zyban®). Research indicates that nicotine increases the value of non-nicotine reinforcers and this enhancement effect (and the loss of enhancement effects during quit attempts) likely contributes to smoking maintenance [see Caggiula et al. (2009) for a review]. Inasmuch as previous studies indicate females are less sensitive to the primary reinforcing effects of nicotine and more sensitive to the sensory and contextual elements of smoking than males, sex differences may also be apparent in the reward-enhancing effects of nicotine on sensory reinforcement (Chaudhri et al. 2005; Perkins, 2009).
Bupropion (i.e., Zyban®) is a commonly prescribed smoking-cessation aid. Reward enhancement has also been demonstrated with bupropion, and the replacement of the reward-enhancing effects of nicotine with those of bupropion may partially account for its efficacy as a smoking cessation aid (Palmatier et al. 2009). Additionally, bupropion is effective in treating depression and weight gain (Caixàs et al. 2014; Maneeton et al. 2013), which are two reasons for smoking relapse that are more commonly reported by women than men (Luostarinen et al. 2013; USDHHS, 2001). These findings suggest the possibility that reward enhancement by bupropion may also differ between males and females or play a different role in smoking cessation between sexes. Palmatier et al. (2009) found that treatment with the alpha noradrenergic antagonist prazosin attenuated the reward-enhancing effects of bupropion, but not of nicotine, suggesting noradrenergic receptors play a disparate role in the reward-enhancing effects of these drugs. Additionally, dopamine transmission is known to be involved in reward detection generally, but it is yet unclear how dopamine receptors are involved in reward-enhancing effects of drugs (cf. Stauffer et al. 2015). For instance, nicotine and bupropion both increase dopamine transmission in brains associated with reward detection, but do so via different means (Benowitz, 2009; Stahl et al. 2004). Whether the role of dopamine receptors is similar or disparate in the enhancement of reward by these agents is not apparent. The present study extended investigation of the neuropharmacological mechanisms of reward enhancement by nicotine and bupropion to the dopamine D1 and D2 receptor families using SCH-23390 (D1-family antagonist) and eticlopride (D2-family antagonist).
In the present study, we used reinforcer demand modeling to investigate reward enhancement by nicotine and bupropion in male and female rats. The basic framework of the reinforcer demand approach is rooted in behavioral economic theory, where reinforcement value is conceptualized in terms of reinforcer consumption in relation to its price in units of response cost. As the price of each unit of the reinforcer increases, consumption of the reinforcer decreases, and the rate of these decreases in consumption represents what is termed elasticity of demand. Inelastic demand refers to decreases in consumption that are relatively insensitive to increases in reinforcer price; elastic demand is characterized by relatively dramatic decreases in consumption with increases in unit price (Madden, 2000; Hursh and Silberburg, 2008).
Importantly, demand elasticity characterizes reinforcement value as it relates to two primary constraints on consumption: satiation and price. Inelastic demand represents consumption that is primarily constrained by satiation, and elastic demand characterizes consumption that is principally limited by reinforcer price. Inasmuch as reinforcer magnitude affects the rate of satiation and unit cost accounts for reinforcer size, Hursh and Silberburg (2008) proposed a model that characterizes reinforcement value as the rate of change in elasticity of demand after accounting for scalar differences in reinforcer magnitude. This model relates reinforcer consumption to unit response cost via the following equation:
where Q represents units of reinforcer consumption, Q 0 is predicted consumption when the reinforcer costs nothing to obtain (i.e., the ordinate intercept), k is a constant reflecting the range of the demand function in log units of consumption, e is the base of the natural logarithm, C is the response cost per reinforcer delivery, and α represents the rate of change in decline of consumption in standardized price (Q 0 * C). The values of Q 0 and α are adjusted to maximize the fit of the demand model to the data and may be conceptualized to represent basal intensity of demand (Q 0) and sensitivity to price (α; Hursh and Silberburg, 2008; Hursh 2014). That is, Q 0 represents consumption where the only constraint is satiation, and α reflects the limiting effects of both satiation and price on consumption by representing the rate at which consumption shifts from being limited by satiation to constrained by reinforcer price (Hursh and Silberburg, 2008; Bickel et al. 2000; Johnson and Bickel, 2006). Importantly, the essential value of a reinforcer is inversely related to sensitivity to price (α) and can be calculated from the demand model as
where essential value (EV) is conceptualized as the strength of a reinforcer to maintain behavior independent of scalar manipulations of reinforcer magnitude and accounting for individual sensitivity to response cost (Hursh and Silberburg, 2008; Hursh 2014).
Previous work has found that nicotine increased consumption of primary and secondary reinforcers and that reinforcer demand modeling effectively characterized this nicotine-induced change in reinforcement value (Barrett and Bevins, 2012; Cassidy and Dallery, 2012, 2014). Specifically, nicotine has been shown to increase intensity of demand (i.e., Q 0) and enhance the essential value of food pellets (Cassidy and Dallery, 2012), food-associated conditioned reinforcers (Cassidy and Dallery, 2014), and mildly reinforcing visual stimuli (Barrett and Bevins, 2012). The present study further extended previous work by evaluating the reward-enhancing effects of nicotine alongside those of bupropion using the reinforcer demand approach and investigated how the enhancement effects of each drug varied as a function of sex. Furthermore, the present study investigated the involvement of D1-type and D2-type dopamine receptors as mechanisms for the reward-enhancing effects of nicotine and bupropion using SCH-23390 and eticlopride.
Methods
Subjects
Twenty-four experimentally naïve Sprague Dawley rats (n = 12 per sex; Harlan, Indianapolis, IN), 9 weeks upon arrival, were individually housed in clear polycarbonate tubs lined with TEK Fresh® cellulose bedding in a temperature- and humidity-controlled colony. The rats were given 2 days to acclimate to the colony followed by three additional days of handling before initiation of training. Water was continuously available and the rats were given 12 g (female) or 15 g (males) of laboratory chow daily, unless otherwise specified. Sessions were conducted during the light phase of a 12:12 h light/dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
We used 16 conditioning chambers (ENV-008CT; Med-Associates, Inc., St. Albans, VT; measuring 30.5 × 24.1 × 21.0 cm, L × W × H) enclosed in light- and sound-attenuating cubicles fitted with an exhaust fan. Sidewalls were aluminum; the ceiling and front and back walls were clear polycarbonate. One sidewall featured a dipper receptacle, occupying a 5.2 × 5.2 × 3.8 cm (L × W × H) recessed space, into which a dipper arm provided 0.1 mL of sucrose solution when raised. Retractable response levers were featured on either side of the dipper receptacle, approximately 5 cm above the rod floor. White 28-V DC (100-mA) lamps were located 3 cm above each lever, hereafter termed lever lights. Two 28-V DC (100-mA) lamps were also located above the conditioning chamber, but within the sound-attenuating cubicle, hereafter termed house-light. An infrared emitter/detector unit, positioned 4 cm above the floor, bisected the chamber 14.5 cm from the sidewall featuring the dipper receptacle and functioned to monitor chamber activity. Data collection and presentation of experimental events were controlled via personal computer with Med Associates interface and software (MedPC for Windows, IV).
Drugs
(−)-Nicotine hydrogen tartrate [0.4 mg/kg, 5-min injection-to-placement interval (IPI)], bupropion hydrochloride (10 and 20 mg/kg; 15-min IPI), SCH-23390 (10 and 30 μg/kg; 45-min IPI), and eticlopride hydrochloride (10 and 30 μg/kg; 45-min IPI) were obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in 0.9 % saline. All injections were at 1 mL/kg. Per field standards, nicotine dose was reported as base form; all other drug doses were reported as salt form. The pH for nicotine was adjusted to 7.0 ± 0.2 with a NaOH solution. All doses and IPIs were based on published research, including previous work from our laboratory (Wilkinson et al. 2010; Liu et al. 2010; Wooters et al. 2009). Nicotine was injected subcutaneously; all other drugs were injected intraperitoneally.
Acquisition
A timeline of all behavioral training and testing phases is shown in Fig. 1. The rats were trained to lever press over four “auto-shaping” sessions using 26 % (weight/volume) liquid sucrose (cf. Charntikov et al. 2013). Each session began with random insertion of one of the two levers. After a lapse of 15 s or a lever press, the response lever was immediately retracted and the dipper arm was raised for 4 s. Following a variable length timeout (average 60 s, range = 30–90 s), the opposite lever was inserted into the chamber initiating a new trial as just described. The lever inserted on odd-numbered trials was always randomly determined, and the opposite lever always followed on even-numbered trials. Thus, over a 60-trial session, each lever was inserted 30 times but never presented more than 2 times in succession. Each session was conducted in continuous house-light illumination and no other stimuli were presented.
Timeline of behavioral training and testing phases through the entirety of the experiment. Note that the design of the experiment is fully within subjects, apart from the variable of sex. Therefore, the n/condition throughout equals 12 males and 12 females. The duration of the demand assessment phase varied between individuals depending upon when each reached termination (i.e., the FR at which the mean number of VS presentations earned was <1 across all drug conditions). Session duration during the four auto-shaping sessions varied between 60 and 75 min, depending on individual performance. The duration of all other sessions was 60 min
Over the following 10 days, the rats were trained to lever-press maintained by visual stimuli (VS) consisting of 60-s termination of house-light illumination compounded with 5-s illumination of lever lights. Hereafter, daily sessions were 60 min. Active and inactive lever assignments were pseudo-randomly determined and counterbalanced. VS reinforcement was delivered on a fixed ratio (FR) one schedule (one response per reinforcer) for responding on the active lever; responses on the inactive lever were recorded but had no programmed consequences. In order to familiarize the rats to injection procedures and to provide sufficient nicotine pre-exposure to minimize the response-suppressant effects of nicotine, each rat received an injection of saline 5 min preceding placement into the chamber and an injection of nicotine 15 min following termination of each session.
Reinforcer demand assessment
Following the tenth day of FR1 training, the rats continued to lever-press maintained by VS reinforcement in 60-min sessions as described earlier. The response requirement was now systematically increased after completion of each block of 16 sessions. The sequence of response costs followed an exponential base 2 sequence ranging from FR1 to FR512. Over different sessions within each FR block, the rats received injections of 0.9 % saline, nicotine (0.4 mg/kg), or bupropion (10 or 20 mg/kg) before placement in the apparatus. Sessions proceeded with the restriction that each drug condition was experienced once before repeating and no drug condition was experienced 2 days in succession. Each drug was tested four times within each FR block. However, only the last three were included in analyses to capture stable performance on each FR schedule (i.e., the terminal 12 sessions of each 16-session FR block). Demand assessment continued for each rat until the last session of FR512 or until the last session of a FR block in which the mean number of VS presentations earned was <1 across all drug conditions.
Progressive ratio and antagonist testing
This phase began 24 h after the last demand assessment session. Within a single session, lever pressing was reestablished via the same sucrose-maintained auto-shaping procedure previously described. Over the next 15 sessions, responding for VS stabilized on a progressive ratio (PR) schedule of reinforcement. The PR sequence followed an exponential base 2 sequence in one-third logarithmic steps, rounded to the nearest whole number (i.e., 2, 3, 4, 5, 6, 8, 10, 13, 16, etc.). This sequence was chosen because it included the ratios experienced in the demand assessment phase and progressed slowly enough to minimize ratio strain in the beginning of ratio progression. This progression also afforded the possibility of encountering schedules as high as, or higher than, each rat’s termination schedule in the demand assessment phase.
Over 36 sessions, the rats continued to respond on the PR schedule described above. On these sessions, the rats received an injection of either SCH-23390 or eticlopride followed by administration of saline, nicotine (0.4 mg/kg), or bupropion (20 mg/kg). Only the higher dose of bupropion from the demand assessment phase was included in this phase to ensure a high baseline for observing potential decreases in responding wrought by dopamine receptor antagonism. Each antagonist was assessed at three different doses (including a vehicle benchmark), and in combination with saline, nicotine, or bupropion across two determinations, requiring 18 days of testing for each antagonist. Drug and antagonist dose testing order was randomized and counter-balanced across individuals, and each dose-drug combination was tested once before repeating for a second determination. Testing with SCH-23390 was completed before testing began with eticlopride.
Dependent measures and analyses
The number of active and inactive lever presses, infrared beam breaks (activity), and the number of VS presentations earned within each session were recorded throughout the experiment. The number of VS presentations earned over sessions of the demand assessment phase was analyzed using the exponential reinforcer demand model proposed by Hursh and Silberberg (2008), and the values of Q 0 (consumption at price zero) and the essential value (EV) were calculated from the model fits to the consumption data of individual rats using nonlinear least squares regression. To ensure comparability of EV estimates, the range parameter, k, was constrained to be shared across all eight conditions of sex * drug (k = 1.95). Analyses of the effects of nicotine and bupropion on Q 0 or EV were performed via mixed factorial ANOVA with sex as a between-subjects factor and drug as a within-subjects factor. Post hoc pairwise comparisons were performed on significant main effects of drug or sex * drug interactions.
The primary measures of interest during the antagonist testing phase were the number of active lever presses and the number of beam breaks in a session. These measures were subjected to three-factor, mixed-measures ANOVA with sex as a between-subjects factor and with drug and antagonist dose conditions as within-subjects factors. The datasets from each antagonist-testing phase (SCH-23390 and eticlopride) were analyzed separately. A priori comparisons were conducted on the effects of drug and sex in the absence of antagonist (i.e., the saline control condition of dose). Additional post hoc pairwise comparisons were conducted upon detection of additional significant interactions where appropriate. All pairwise comparisons corrected family-wise error rates using Tukey’s HSD method with significance set at adjusted p values <0.05 (Tukey, 1949). Fits of the reinforcer demand model were performed using GraphPad Prism v7.01 (GraphPad Software, Inc., La Jolla, CA). All other analyses were performed using the lme4, lsmeans, and pbkrtest packages for R version 3.3.1 (Bates et al. 2015; Halekoh and Højsgaard, 2014; Lenth, 2016; R Core Team, 2016).
Results
Assessment of demand for VS
Figure 2 presents the demand functions for VS reinforcement between saline, nicotine, and both bupropion dose conditions for males (left panel) and females (right panel). Fits of the reinforcer demand model in Fig. 1 are presented as a representation via fits to data averaged across rats within each condition. Males completed the demand assessment phase by reaching termination criteria earlier than the females; 50 % of the males had reached termination criteria by FR128, whereas the females reached it at FR256.
VS consumption as a function of FR schedule between males (left, n = 12) and females (right, n = 12), and across the four administration conditions of nicotine (filled circles), saline (open circles), 10 mg/kg bupropion (open triangles), and 20 mg/kg bupropion (closed triangles). The presented demand curves are representative fits to averaged data, and not the curves used to generate metrics for statistical analysis. Because individual rats exited the demand assessment phase upon reaching termination criteria at different FR schedules, not all of the data are presented in Fig. 1; only data from those FR schedules where at least a quarter of the rats of each sex remained in the demand assessment phase are presented (FR 128 for males; FR 512 for females)
The model-estimated values of Q 0 and essential value (EV) between conditions of sex and drug are shown in Fig. 3. Analysis of Q 0 (Fig. 3A) revealed a main effect of drug [F(3,66) = 26.8; p < 0.001] and of sex [F(1,22) = 4.32; p = 0.049]. The sex * drug interaction approached conventional levels of significance and was therefore further explored [F(3,66) = 2.72; p = 0.052]. Post hoc analysis on the sex * drug interaction revealed that nicotine, and both doses of bupropion, increased Q 0 relative to saline in both sexes [ps ≤ 0.019]. Q 0 in the 20-mg/kg bupropion condition did not differ from nicotine or 10 mg/kg bupropion in either sex [ps ≥ 0.202]. Nicotine increased Q 0 relative to 10 mg/kg bupropion in the males [p = 0.010], but not the females [p = 0.943]. Consequently, Q 0 differed between males and females in the 10-mg/kg bupropion condition [p = 0.002], but not in any other drug condition [ps ≥ 0.190].
a–d Estimates of Q 0 and essential value obtained by fits of the reinforcer demand model to the data with a shared k = 1.95. The top panels present the raw values obtained by the model; the bottom panels present change scores relative to the saline condition. For all panels, females are represented by filled bars and males by open bars. All data is presented as the mean ± 1 SEM. Asterisks represent significant differences between the sexes within condition of drug. Differences within the sexes denoted by s and n represent significant differences from the saline and nicotine conditions of drug, respectively
Analysis of EV (Fig. 3B) discovered significant main effects of sex [F(1,22) = 5.63; p = 0.027] and of drug [F(3,66) = 60.8; p < 0.001], as well as a significant sex * drug interaction [F(3,66) = 3.93; p = 0.012]. Analysis of the interaction revealed greater EV in females than males at the 10- and 20-mg/kg bupropion conditions [ps ≤ 0.029]. No sex differences in essential value were detected for saline or nicotine [ps ≥ 0.088]. In females, nicotine and both doses of bupropion enhanced EV of VS reinforcement relative to saline [ps < 0.001], but did not differ from each other [ps ≥ 0.854]. In males, nicotine and both bupropion doses also enhanced EV relative to saline [ps < 0.001]. However, EV in the nicotine condition was also greater than the 10-mg/kg bupropion dose condition in males [ps = 0.003]. EV in the 20-mg/kg bupropion condition did not differ from nicotine or 10 mg/kg bupropion in males [ps ≥ 0.246].
Although not significant in the saline condition, females showed a consistent tendency toward higher sensitivity to VS reinforcement on Q 0 and EV. This observation suggests the possibility that females and males may be equally sensitive to the enhancing effects of nicotine and bupropion, and that any observed sex differences may reflect a carryover effect of differential baseline sensitivity that is easier to detect in conditions yielding higher response rates. To address this possibility, we represented the effects of nicotine and bupropion on Q 0 and EV as change scores from the saline condition (Fig. 3C and D) and then examined potential differences between the sexes as discrepancies in those change scores (i.e., differences in the magnitude of change from saline). Mixed factorial (sex * drug) ANOVAs were conducted on both demand model metric change scores. For change in Q 0 (Fig. 3C), no significant effects of sex or drug were detected [Fs ≤ 1.79; ps ≥ 0.180], but a significant sex * drug interaction was detected [F(2,44) = 3.45; p = 0.041]. Post hoc analysis on the interaction found that females showed greater changes from saline in Q 0 than males in the 10-mg/kg bupropion condition [p = 0.028]. Additionally, increases by nicotine in Q 0 were greater than by 10 mg/kg bupropion in males [p = 0.016]. Analysis on change in EV from saline (Fig. 3D) found a significant main effect of sex [F(1,22) = 8.40; p = 0.008] and a significant sex * drug interaction [F(2,44) = 4.55; p = 0.016], but no main effect of drug [F(2,44) = 1.23; p = 0.302]. Post hoc tests revealed that females showed greater changes from saline in EV than males in both bupropion dose conditions [ps ≤ 0.041]. In males, increases in EV by nicotine were greater than by 10 mg/kg bupropion [p = 0.009].
SCH-23390 testing on progressive ratio responding
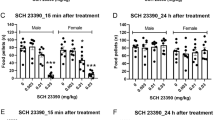
Active lever-pressing and locomotor activity under the PR schedule of VS reinforcement during the SCH-23390 testing phase is shown in Fig. 4. Nicotine and bupropion both increased active lever pressing (top left panel) relative to saline in each sex, and the D1 receptor family antagonist SCH-23390 attenuated this effect. Analysis revealed significant main effects of sex [F(1,22) = 5.99; p = 0.023], drug [F(2,44) = 37.1; p < 0.001], and dose [F(2,44) = 17.5; p < 0.001], as well as significant sex * drug interaction [F(2,44) = 7.73; p = 0.001]. Analysis of the main effect of dose revealed significant decreases by 10 and 30 μg/kg SCH-23390 relative to saline, and found that the magnitude of this decrease was dose dependent [ps ≤ 0.007]. Further analysis of the sex * drug interaction revealed significantly higher active lever pressing in females than males under the 20-mg/kg bupropion condition [p = 0.001], but not in the saline or nicotine conditions; the latter approached significance [ps ≥ 0.052]. In both sexes, bupropion and nicotine increased active lever pressing relative to saline [ps ≤ 0.006], and bupropion increased responding relative to nicotine in females [p = 0.001], but not in males [p = 0.837]. Finally, a priori analyses on the effects of sex and drug in the absence of SCH-23390 (Fig. 4, top right) also revealed higher responding in females than males in the saline, nicotine, and bupropion conditions [ps ≤ 0.030]. Similarly, nicotine and bupropion enhanced active lever pressing relative to saline in both sexes [ps ≤ 0.007], but nicotine and bupropion did not differ from each other for either sex when tested in the absence of SCH-23390 [ps ≥ 0.219].
Active lever pressing (top panels) and locomotor activity (bottom panels) under the conditions of saline, nicotine, or 20 mg/kg bupropion on behavior maintained by a PR schedule of VS reinforcement during the D1 antagonist testing phase. The left panels represent data following administration of either saline (filled), 10 μg/kg (open), or 30 μg/kg SCH-23390 (shaded) injection, 45 min preceding different experimental sessions. The right panels present the same data, but only sessions in the absence of SCH-23390, and arranged so as to highlight the effects of sex (n = 12/sex) under the saline, nicotine, and bupropion conditions. In all panels, asterisks indicate differences between the sexes within the same conditions of drug and antagonist dose. Differences within the sexes denoted by s and n represent significant differences from the saline and nicotine conditions of drug, respectively
Analysis of locomotor activity (Fig. 4, bottom left) uncovered significant effects of sex [F(1,22) = 13.6; p = 0.001], drug [F(2,44) = 21.3; p < 0.001], and dose [F(2,44) = 27.4; p < 0.001]. The sex * drug [F(2,44) = 8.93; p < 0.001] and sex * dose [F(2,44) = 4.35; p = 0.019] interactions were also significant. Analysis on the main effect of dose revealed that 30 μg/kg SCH-23390 decreased locomotor activity relative to saline and 10 μg/kg [ps < 0.001]. Analysis of the sex * drug interaction revealed higher locomotor activity in females compared to males under nicotine and bupropion conditions [ps ≤ 0.001], but not at saline [p = 0.359]. Relative to saline, nicotine increased locomotor activity in females [p = 0.028] but did not significantly impact activity in males [p = 0.086]. Bupropion also increased activity in females [p < 0.001] but did not differ from saline in males [p = 0.242]. Consequently, locomotor activity under bupropion and nicotine conditions was significantly different in both sexes [ps < 0.006]. A priori analyses on the effects of sex and drug in the absence of SCH-23390 (Fig. 4, bottom right) revealed higher activity for females in nicotine and bupropion conditions [ps ≤ 0.002], but not at saline [p = 0.310]. Bupropion increased activity relative to nicotine and to saline in females [ps ≤ 0.003], but produced neither of these effects in males [ps ≥ 0.176]. Nicotine had no effects on activity in either sex in the absence of SCH-23390 [ps ≥ 0.130].
Eticlopride testing on progressive ratio responding
Active lever pressing and locomotor activity under the PR schedule of VS reinforcement during the eticlopride testing phase are shown in Fig. 5. Analysis of active lever pressing (top left panel) revealed significant main effects of sex [F(1,22) = 7.85; p = 0.010], drug [F(2,44) = 29.2; p < 0.001], and dose [F(2,44) = 12.1; p < 0.001]. The sex * drug [F(2,44) = 6.78; p = 0.003] and drug * dose interactions [F(4,88) = 3.40; p = 0.012] were also significant. Post hoc analyses found higher active lever pressing in females at the nicotine and bupropion conditions [ps ≤ 0.030], but not at saline [p = 0.280]. In both sexes, nicotine and bupropion increased active lever pressing relative to saline [ps ≤ 0.032], and bupropion was higher than nicotine in females [p = 0.015], but not in males [p = 0.553]. Treatment with 10 or 30 μg/kg eticlopride did not affect responding under saline or nicotine conditions [ps ≥ 0.188]. In the bupropion condition, 30 μg/kg eticlopride decreased responding relative to saline and 10 μg/kg [ps < 0.001]; 10 μg/kg eticlopride did not differ from saline [p = 0.409]. Finally, a priori analyses of the effects of drug and sex in the absence of eticlopride (Fig. 5, top right) revealed higher responding in females in the bupropion condition [p < 0.001], but not the saline or nicotine condition; the latter approached significance [p ≥ 0.053]. Furthermore, nicotine and bupropion increased responding relative to saline in both sexes [ps ≤ 0.017], and bupropion responding was higher than nicotine in females [p = 0.005], but not in males [p = 0.655].
Active lever pressing (top panels) and locomotor activity (bottom panels) under the conditions of saline, nicotine, or 20 mg/kg bupropion on behavior maintained by a PR schedule of VS reinforcement during the D2 antagonist testing phase. The left panels represent data following administration of either saline (filled), 10 μg/kg (open), or 30 μg/kg eticlopride (shaded) injection, 45 min preceding different experimental sessions. The right panels present the same data, but only sessions in the absence of eticlopride, and arranged so as to highlight the effects of sex (n = 12/sex) under the saline, nicotine, and bupropion conditions. In all panels, asterisks indicate differences between the sexes within the same conditions of drug and antagonist dose. Differences within the sexes denoted by s and n represent significant differences from the saline and nicotine conditions of drug, respectively. Finally, ampersands denote that 30 μg/kg eticlopride differed from both saline and 10 μg/kg eticlopride under bupropion conditions in both sexes
Analysis of locomotor activity (Fig. 5, bottom left) found significant main effects of sex [F(1,22) = 20.1; p < 0.001] and drug [F(2,44) = 31.2; p < 0.001], and a significant sex * drug interaction [F(2,44) = 13.0; p < 0.001]. Post hoc tests on the sex * drug interaction found higher locomotor activity in females under the nicotine and bupropion conditions [ps < 0.001], but not at saline [p = 0.091]. In females, bupropion increased activity relative to both saline and nicotine [ps < 0.001], but nicotine and saline did not differ [p = 0.060]. In males, neither activity under neither nicotine nor bupropion condition differed from saline [ps ≥ 0.345], but activity in the bupropion condition was higher than nicotine [p = 0.025]. A priori analyses of the effects of sex and drug in the absence of eticlopride (Fig. 5, bottom right) revealed higher activity in females at the nicotine and bupropion conditions [ps < 0.001] and increased activity by bupropion in females relative to nicotine and saline [ps < 0.001]. Opposing non-significant effects of bupropion and nicotine on activity were observed in males [ps ≥ 0.247], with significantly increased activity by bupropion relative to nicotine [p = 0.020].
Discussion
Nicotine and bupropion increased consumption of VS in males and females across a wide range of FR schedules. The reinforcer demand analysis revealed that nicotine and bupropion increased intensity of demand (Q 0) and essential value (EV) of VS in both sexes. This finding is notable because the Q 0 and EV of demand curves represent different facets of reinforcement value as a construct. Q 0 represents value as consumption of the reinforcer when the only constraint on consumption is satiation, and for this reason may be considered a hedonic set point. By contrast, EV represents reinforcement value as the rate at which elasticity of demand (i.e., sensitivity to cost) increases. In the presently employed model, EV was normalized with respect to Q 0 so as to remain independent of scalar changes in sheer reinforcer magnitude. That is, EV represents the value of a reinforcer as sensitivity to cost independent of changes in reinforcer quantity but varies with reinforcer quality (cf. Barrett and Bevins, 2012; Hursh and Silberburg, 2008). Thus, the finding that nicotine and bupropion enhanced both Q 0 and EV is interesting because it suggests that these drugs affected reinforcer value by decreasing both the impact of satiation and sensitivity to escalating cost, regardless of sex.
Interestingly, bupropion engendered greater enhancement in females than in males, which is consistent with previous findings that females are more sensitive to the behaviorally activating effects of many psychomotor stimulants (Van Swearingen et al. 2013; Reichel et al. 2012; Eubig et al. 2014). Indeed, females also showed pronounced and consistent locomotor activation by nicotine and bupropion, whereas males showed no such effects (Figs. 4 and 5). Rather, nicotine decreased (though non-significantly) locomotor activity in males and simultaneously increased VS-maintained lever pressing on the PR schedule. These dissociations are consistent with previous work in males, which found that enhanced lever pressing by nicotine and bupropion was not principally caused by locomotor activation (Barrett and Bevins, 2012, 2013; Palmatier et al. 2009). The present findings demonstrate that the behavioral activating effects of nicotine and bupropion differ between males and females, and suggest that the relative role that locomotor activation may play in enhancing the frequency and persistence of responding for sensory reinforcement may also differ between the sexes. That is, the present study cannot wholly parse apart whether differences between the sexes in the effects of nicotine and bupropion on active lever pressing were driven by sex differences in the locomotor-activating effects of these drugs. Future experiments will have to be designed specifically to unravel this conundrum.
Generally, females appeared to show greater sensitivity to VS reinforcement than males. Active lever pressing throughout the experiment and estimates of Q 0 and essential value were consistently higher in females than males, though not always statistically significant. Females were also more sensitive to the reward-enhancing effects of bupropion than males. Change scores in essential value were greater in females at the 10-mg/kg bupropion condition, which may reflect different dose-response functions between the sexes to the reward-enhancing effects of bupropion. Indeed, whereas the sexes did not differ in the enhancing effects of nicotine on either Q 0 or essential value, males showed a greater response to nicotine than they did to bupropion on these measures. Possibly, a higher dose of bupropion than was tested may have wrought nicotine-like levels of change in VS reinforcement value in males. Regardless, the sexes appear to differ in their sensitivity to the effects of bupropion on VS reinforcement value. Notably, sex differences in the enhancing effects of bupropion were far more pronounced on essential value than Q 0, suggesting that females may be more sensitive than males to the reward-enhancing effects of bupropion as pertaining to the consumption constraining effects of response cost than satiation. That is, reinforcer consumption of males and females was affected similarly at low response cost, where the primary constraint on consumption was satiation. However, the effect of bupropion to increase perseverance of responding in the face of escalating response cost was greater in females than in males. Inasmuch as value may be well characterized as the price one is willing to pay to maintain consumption of a reinforcer (Hursh and Silberburg, 2008), these findings suggest that females may be more sensitive than males to the primary reinforcing effects of VS generally, that nicotine and bupropion enhance the value of VS reinforcement in both sexes, and that bupropion enhancement is more pronounced in females.
Gonadal hormones have been implicated as an important factor in the sex differences in the pharmacokinetics of nicotine (Harrod et al., 2007; Benowitz et al. 2006; Benowitz et al. 2009). Some evidence suggests that gonadal hormones may cause differences in nicotine pharmacodynamics as well. That is, ovarian steroid hormones have been shown to have regulatory effects on nAChR density and function (see Pauly, 2008 for a review). Ovariectomized rats show decreases in the density of α7 nAChRs in the hypothalamus, amygdala, raphe nucleus, and cerebellum; estrogen replacement attenuates this effect (Morley et al. 1983; Miller et al. 1982, 1984; Miller and Billiar, 1986; Arimatsu et al. 1985; Koylu et al. 1997; Centeno et al. 2006). However, gonadal hormones do not appear to regulate the density of non-α7 nAChRs. Furthermore, the number, synaptic location, subtype distribution, and nicotine-induced upregulation of nAChRs do not appear to reliably differ between male and female rats (cf. Pauly, 2008). Given the finding that antagonism of α7 receptors does not attenuate the reward-enhancing effects of nicotine (Liu et al. 2007), the relative influence of gonadal hormones on the reward-enhancing effects of nicotine is uncertain. The effects of hormone levels or estrous cycling are beyond the scope of this study; the present studies did not monitor hormone levels or estrous cycling. However, future research should investigate the role of estrous or other sex hormones or their metabolites in the reward-enhancing effects of nicotine and other drugs.
In the present study, the greatest differences between the sexes were observed under bupropion conditions. Given that the mechanisms of bupropion enhancement are particularly mediated by dopamine and norepinephrine receptors (cf. Palmatier et al. 2009), sex differences in the dopaminergic or adrenergic responses to nicotine and bupropion may be informative in the context of reward enhancement. There is some evidence that gonadal hormones, specifically estrogen, regulate dopaminergic response in striatal tissue (Becker, 1999; cf. Roth et al. 2004; Carroll and Anker, 2010). For instance, nicotine-evoked dopamine release has been shown to be greater in estrogen-treated ovariectomized rats (Dluzen and Anderson, 1997). Further, the density of dopamine uptake sites varies with estrogen level through phases of estrous cycling (Morissette and Di Paolo, 1993). Differences in dopaminergic function between the sexes have been implicated as a mechanism for sex differences in the primary reinforcing effects of psychomotor stimulants, including nicotine (cf. Roth et al. 2004; Carroll and Anker, 2010). Future research should investigate the relation between estrogen-regulated differences in dopaminergic tone in the midbrain and sensitivity to the reward-enhancing effects of nicotine, bupropion, or other psychomotor stimulants.
Antagonism of the D1 receptor family by SCH-23390 (Hyttel and Arnt, 1987) decreased lever pressing and locomotor activity in males and females. However, antagonism of the D2 receptor family by eticlopride (Hall et al. 1985) decreased only active lever pressing and only in the bupropion condition for both sexes. These findings suggest that pretreatment with eticlopride partially attenuated the reward-enhancing effects of bupropion without impacting the enhancing effects of nicotine or the basal reinforcement value of the VS. D2-family receptors may play a critical role in the enhancing effects of bupropion that are not shared with nicotine. This finding echoes a similar finding by Palmatier et al. (2009) that α1-noradrenergic receptors play a role in bupropion enhancement effects but not in enhancement by nicotine in male rats. Activation of D1-like receptors may also be involved in the reward-enhancing effects of nicotine and bupropion. However, assessing this possibility will require further investigation using techniques that can parse apart the role of these receptors in locomotor behavior versus processing of reinforcement value.
A limitation of the present work is that we did not investigate the possibility of cooperativity between D1-type and D2-type receptors in the reward-enhancing effects of nicotine or bupropion. Cooperative interaction between D1 and D2 receptors in the nucleus accumbens (NAc) has been shown to be involved in a number of dopamine-mediated behaviors. For instance, animals will self-administer D1 and D2 agonists into the NAc in combination but not separately (Ikemoto et al. 1997). Lever pressing maintained by amphetamine (Phillips et al. 1994), ethanol (Hodge et al. 1997), and a conditioned reinforcer (Chu and Kelley, 1992) has also been demonstrated to involve coactivation of NAc D1-type and D2-type receptors. To what extent coactivation of D1-type and D2-type receptors might be involved in the reward-enhancing effects of nicotine, bupropion, or other drugs is not clear. The present study did not include the tests required to identify the possibility of cooperative interaction between these receptors’ families, something that future studies should take into careful consideration.
The present findings add to a growing body of literature demonstrating differences between males and females in the behavioral effects of nicotine (e.g., Chaudhri et al. 2005; Perkins, 2009; O’Dell and Torres, 2014) and other drugs of abuse (Lynch et al. 2002; Roth et al. 2004; Carroll and Anker, 2010; Mitchell and Potenza, 2015). Furthermore, the present findings suggest that females are more sensitive to the reward-enhancement effects of bupropion and that dopaminergic mechanisms (i.e., D2 family receptors) may play a greater role in this enhancement in females than in males. Indeed, a notable body of literature implicates the heightened dopaminergic response of striatal cells in females as a mechanism for sex differences in the rewarding effects of a variety of drugs of abuse (Becker, 1990; Castner et al. 1993; Walker et al. 2012; Cummings et al. 2014). Future research may investigate the role that differential dopaminergic response in the striatum plays in sex differences to the reward-enhancing effects of nicotine, bupropion, and other drugs. In addition, future research should investigate the role that replacement of nicotine reward enhancement with reward enhancement by bupropion and other smoking cessation aids, such as varenicline, may play in the efficacy of these agents in reducing smoking. If replacing the reward-enhancing effects of nicotine with reward enhancement by an alternative is effective in reducing smoking, the present findings suggest that bupropion may be an effective treatment in females for different reasons than in males. Future studies should investigate whether pharmacological aids for smoking cessation that share reward-enhancing effects with nicotine show differential efficacy between males and females in both pre-clinical and clinical settings.
References
Arimatsu Y, Kondo S, Kojima M, Arimatsu Y, Kondo S, Kojima M (1985) Enhancement by estrogen treatment of α-bungarotoxin binding in fetal mouse amygdala cells reaggregated in vitro. Neuroscience Research 2(4):211–220
Barrett ST, Bevins RA (2012) A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behav Pharmacol 23:781–789
Barrett ST, Bevins RA (2013) Nicotine enhanced operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacol Biochem Behav 114-115:9–15
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Becker JB (1990) Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse 5:157–164
Becker JB (1999) Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64:803–812
Benowitz NL (2009) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 49:57–71
Benowitz NL, Lessov-Schlagger CN, Swan GE, Jacob P III (2006) Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther 79:480–488
Benowitz NL, Hukkanen J, Jacob P III (2009) Nicotine chemistry, metabolism, kinetics, and biomarkers. Handb Exp Pharmacol 192:29–60
Bevins RA, Palmatier MI (2004) Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev 3:143–158
Bickel WK, Marsch LA, Carroll ME (2000) Deconstructing relative reinforcing efficacy and situating the measure of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology 153:44–56
Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009) The role of nicotine in smoking: a dual reinforcement model. In: Bevins RA, Caggiula AR (eds) The motivational impact of nicotine and its role in tobacco use. Springer Science and Business Media, New York, pp. 91–109
Caixàs A, Albert L, Capel I, Rigla M (2014) Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Design, Development and Therapy 8:1419–1427
Carroll ME, Anker JJ (2010) Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav 58:44–56
Cassidy RN, Dallery J (2012) Effects of economy type and nicotine on the essential value of food in rats. J Exp Anal Behav 97:183–202
Cassidy RN, Dallery J (2014) Quantifying nicotine’s value-enhancement effect using a behavioral economic approach. J Exp Anal Behav 102:353–362
Castner SA, Xiao L, Becker JB (1993) Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 610:127–134
Centeno ML, Henderson JA, Pau KY, Bethea CL (2006) Estradiol increases alpha7 nicotinic receptor in serotonergic dorsal raphe and noradrenergic locus coeruleus neurons of macaques. J Comp Neurol 497:489–501
Charntikov S, Swalve N, Pittenger ST, Fink K, Schepers S, Hadlock GC, Fleckenstein AE, Hu G, Li M, Bevins RA (2013) Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology 75:138–144
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA (2005) Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180:258–266
Chu B, Kelley AE (1992) Potentiation of reward-related responding by psychostimulant infusion into the nucleus accumbens: role of dopamine receptor subtypes. Psychobiology 20:153–162
Cummings JA, Jagannathan L, Jackson LR, Becker JB (2014) Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Pharmacol Biochem Behav 135:22–28
Dluzen DE, Anderson LI (1997) Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett 230:140–142
Eubig PA, Noe TE, Fioresco SB, Sable JJ, Schantz SL (2014) Sex differences in response to amphetamine in adult Long-Evans rats performing a delay-discounting task. Pharmacol Biochem Behav 118:1–9
Halekoh U, Højsgaard S (2014) A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models—the R package pbkrtest. J Stat Softw 59:1–30
Hall H, Köhler C, Gawell L (1985) Some in vitro receptor binding properties of [3H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain. Eur J Pharmacol 111:191–199
Harrod SB, Booze RM, Mactutus CF (2007) Sex differences in nicotine levels following repeated injection in rats are attenuated by gonadectomy. Pharmacol Biochem Behav 86:32–36
Hodge CW, Samson HH, Chappelle AM (1997) Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res 21:1083–1091
Hursh SR (2014) Behavioral economics and the analysis of consumption and choice. In: McSweeney FS, Murphy ES (Eds) The wiley blackwell handbook of operant and classical conditioning. John Wiley & Sons, Ltd, Oxford, UK, pp. 275–305
Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115:186–198
Hyttel J, Arnt J (1987) Characterization of binding of 3H-SCH-23390 to dopamine D1 receptors. Correlation to other D1 and D2 measures and effect of selective lesions. J Neural Transm 68:171–189
Ikemoto S, Glazier BS, Murphy JM, McBride WJ (1997) Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci 17:8580–8587
Johnson MW, Bickel WK (2006) Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav 85:73–93
Koylu E, Demirgoren S, London ED, Pogun S (1997) Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci 61:185–190
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33
Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF (2007) Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology 194:463–473
Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF (2010) Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol 21:153–160
Luostarinen M, Tuovinen EL, Saarni SE, Kinnunen T, Hukkinen M, Haukkala A et al (2013) Weight concerns among Finnish ever-smokers: a population based study. Nicotine and Tobacco Research 15:1696–1704
Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164:121–137
Madden GJ (2000) A behavioral economics primer. In: Bickel WK, Vuchinich RE (eds) Reframing health behavior change with behavioral economics. Lawrence Erlbaum Associates, New Jersey, pp. 3–26
Maneeton N, Maneeton B, Eurviriyanukul K, Srisurapanont M (2013) Efficacy, tolerability, and acceptability of bupropion for major depressive disorder: a meta-analysis of randomized-controlled trials comparison with venlafaxine. Drug Design, Development and Therapy 7:1053–1062
Miller MM, Billiar RB (1986) A quantitative and morphometric evaluation of 125I-alpha-bungarotoxin binding in the rat hypothalamus. Brain Res Bull 16:681–688
Miller MM, Silver J, Billiar RB (1982) Effects of ovariectomy on the binding of [125I]-alpha bungarotoxin (2.2 and 3.3) to the suprachiasmatic nucleus of the hypothalamus: an in vivo autoradiographic analysis. Brain Res 247:355–364
Miller MM, Silver J, Billiar RB (1984) Effects of gonadal steroids on the invivo binding of [125I]-alpha bungarotoxin to the suprachiasmatic nucleus. Brain Res 290:67–75
Mitchell MR, Potenza MN (2015) Importance of sex differences in impulse control and addictions. Frontiers in Psychiatry 6:24
Morissette M, Di Paolo T (1993) Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology 58:16–22
Morley BJ, Rodriguez-Sierra JF, Clough RW (1983) Increase in hypothalamic nicotinic acetylcholine receptors in prepuberal female rats administered estrogen. Brain Res 278:262–265
O’Dell LE, Torres OV (2014) A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology 76:566–580
Palmatier MI, Levin ME, Mays KL, Donny EC, Caggiula AR, Sved AF (2009) Bupropion and nicotine enhance responding for nondrug reinforcers via dissociable pharmacological mechanisms in rats. Psychopharmacology 207:381–390
Pauly JR (2008) Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci 13:505–516
Perkins KA (2009) Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. In: Bevins RA, Caggiula AR (eds) The motivational impact of nicotine and its role in tobacco use. Springer Science and Business Media, New York, pp. 143–169
Perkins KA, Epstein LH, Grobe J, Fonte C (1994) Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav 47:107–112
Perkins KA, Jacobs L, Sanders M, Caggiula AR (2002) Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology 163:194–201
Phillips GD, Robbins TW, Everitt BJ (1994) Bilateral intra-accumbens self-administration of D-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpride. Psychopharmacology 114:477–485
R Core Team (2016). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/
Reichel CM, Chan CH, Ghee SM, See RE (2012) Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology 223:371–380
Roth ME, Cosgrove KP, Carroll ME (2004) Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 28:533–546
Stahl SM, Pradko JF, Haight BR, Model JG, Rockett CB, Learned-Coughlin S (2004) A review of the neuropharmacology of bupropion, a duel norepinephrine and dopamine reuptake inhibitor. The Primary Care Companion to the Journal of Clinical Psychiatry 6:159–166
Stauffer WR, Lak A, Kobayashi S, Schultz W (2015) Components and characteristics of the dopamine reward utility signal. J Comp Neurol 524:1699–1711
Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 5:99–114
U.S. Department of Health and Human Services (2001) Women and smoking: a report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2001, Atlanta
U.S. Department of Health and Human Services (2014) The health consequences of smoking—50 years of progress: a report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014, Atlanta
U.S. Department of Health and Humans Services (1988) The health consequences of smoking: nicotine addiction: a report of the surgeon general. U.S. Department of Health and Humans Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1988, Atlanta
Van Swearingen AED, Walker QD, Kuhn CM (2013) Sex differences in novelty- and psychostimulant-induced behaviors in C57BL/6 mice. Psychopharmacology 225:707–718
Walker QD, Johnson ML, Van Swearingen AED, Arrant AE, Caster JM, Kuhn CM (2012) Individual differences in psychostimulant responses of female rats are associated with ovarian hormones and dopamine neuroanatomy. Neuropharmacology 62:2264–2277
Wilkinson JL, Carroll FI, Bevins RA (2010) An investigation of bupropion substitution for the interoceptive stimulus effects of nicotine. J Psychopharmacol 24:817–828
Wooters TE, Bevins RA, Bardo MT (2009) Neuropharmacology of the interoceptive stimulus properties of nicotine. Current Drug Abuse Reviews 2:243–255
Acknowledgments
We also thank Dr. Jeffrey Stevens, Dr. Ming Li, and Dr. Samuel Allgood for providing insightful comments on an earlier version of this manuscript. Finally, we also thank Cindy Pudiak, Olivia Loh, Tiffany Schultz, Hannah Sellyer, and Aly Lange for help with conducting daily experimental sessions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors and this research were in part supported by a grant from the National Institute on Drug Abuse (DA034389).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Barrett, S.T., Geary, T.N., Steiner, A.N. et al. Sex differences and the role of dopamine receptors in the reward-enhancing effects of nicotine and bupropion. Psychopharmacology 234, 187–198 (2017). https://doi.org/10.1007/s00213-016-4448-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4448-x