Abstract
Rationale and objective
Recent work has shown that the novel compound N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB) may selectively block nicotinic acetylcholine receptors involved in regulating dopamine release. The current experiments examined the acute effect of bPiDDB on nicotine self-administration, sucrose-maintained responding, and nicotine-induced changes in acute and sensitized locomotor activity.
Methods
Rats were first trained to respond for either nicotine (i.v.) or sucrose pellets using a standard two-lever operant conditioning procedure using a fixed ratio 5 schedule of reinforcement and were then pretreated with bPiDDB (0, 0.3, 1, or 3 mg kg−1) 15 min prior to the session. In separate experiments, rats were assessed for nicotine-induced changes in locomotor activity following pretreatment with bPiDDB (1 or 3 mg kg−1) or mecamylamine (1 mg kg−1); pretreatments were assessed with both acute and repeated nicotine (0.4 mg kg−1) treatment.
Results
Results showed that bPiDDB dose-dependently decreased nicotine self-administration, but not sucrose-maintained responding. In the locomotor experiments, bPiDDB attenuated the hyperactivity produced by acute and repeated nicotine; however, this effect was not robust compared to mecamylamine. In contrast to mecamylamine, bPiDDB did not block the initial hypoactivity produced by acute nicotine.
Conclusion
Since bPiDDB decreased nicotine self-administration specifically, this novel nicotinic receptor antagonist may constitute a lead for the development of a clinically useful treatment for tobacco dependence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While the reinforcing and dependence liability of nicotine involves a host of neurotransmitters, the mesolimbic dopamine (DA) system is thought to be critically involved in these processes (Benwell and Balfour 1992; Corrigall et al. 1992). Given the role of mesolimbic DA in nicotine addiction, the specific nicotinic acetylcholine receptors (nAChRs) modulating DA release may offer a viable molecular target for the development of novel medications to treat tobacco dependence. Mecamylamine is a nonselective, noncompetitive antagonist at all nicotinic receptors, whereas dihydro-β-erythroidine (DHβE) is a preferential antagonist for high affinity nicotinic receptors, including the α4β2* nAChR, and methyllycaconitine (MLA) is relatively selective for the low affinity α7* nAChR. Importantly, mecamylamine and DHβE decrease nicotine self-administration (Grottick et al. 2000), indicating that high affinity nAChRs are critically involved in the reinforcing effect of nicotine. This conclusion is corroborated by evidence showing that mice lacking the β2 subunit do not self-administer nicotine (Epping-Jordan et al. 1999; Picciotto et al. 1998). In addition, since MLA also diminishes nicotine self-administration and the nicotine-induced decrease in brain stimulation reward threshold (Markou and Paterson 2001; Panagis et al. 2000), low affinity α7* nAChRs may also be involved in nicotine reward.
Although there is no subtype-selective nAChR antagonist that targets the specific nAChR subtype(s) mediating nicotine-evoked DA release currently commercially available, a selective nAChR antagonist might be a potential candidate medication for reducing tobacco dependence and minimizing relapse to tobacco use. Indeed, clinical evidence suggests that nAChR antagonists may serve as effective smoking pharmacotherapies. For example, the noncompetitive nAChR antagonist mecamylamine reverses both positive and negative subjective effects induced by intravenous nicotine in human smokers (Lundahl et al. 2000). In a randomized, double-blind placebo-controlled study, mecamylamine combined with a nicotine transdermal patch was also shown to improve smoking cessation outcome for up to 1 year compared to nicotine alone (Rose et al. 1994). Unfortunately, due to the lack of subtype selectivity of this noncompetitive antagonist at both central and peripheral high-affinity nAChRs, the clinical utility of mecamylamine is limited. Blocking peripheral nAChRs may be beneficial in reducing smoking satisfaction associated with the oropharyngeal sensations resulting from the smoke (Rose et al. 1999). However, the therapeutic effect of mecamylamine is offset by the untoward anticholinergic side effects, most notably constipation and dry mouth, which may severely limit long-term patient compliance. A nAChR subtype selective antagonist targeted at central nAChRs that specifically mediate nicotine-evoked DA release is expected to retain the beneficial therapeutic effects of a nonselective nAChR antagonist, while circumventing peripherally mediated side effects.
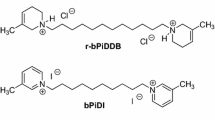
Our laboratory has recently synthesized a library of bis-azaaromatic quaternary ammonium analogs as nAChR antagonists (Ayers et al. 2002). This library of compounds includes the bis-picolinium series of which the 12-carbon analog, N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; see Fig. 1), is the most potent in inhibiting nicotine-evoked DA release from superfused rat striatal slices in vitro (Dwoskin et al. 2004). Since bPiDDB alone did not alter DA release, it appears that bPiDDB acts as an antagonist, rather than as a partial agonist, in inhibiting nicotine-evoked DA release. However, bPiDDB has a lower IC50 value than mecamylamine (5 nM for bPiDDB and 1 μM for mecamylamine; Dwoskin et al. 2004; Teng et al. 1997) for inhibiting nicotine-evoked DA release from rat striatal slices. In contrast to the full inhibition produced by mecamylamine in this assay, bPiDDB inhibited the response to nicotine only by a maximum of 60%. bPiDDB has been suggested to act specifically at a subset of nAChRs that directly mediate nicotine-evoked DA release (e.g., α6β2* or α3β2*), whereas mecamylamine acts as an antagonist at multiple nAChRs that mediate nicotine-evoked DA release. Alternatively, in contrast to bPiDDB, mecamylamine may also interact with nonnicotinic receptors (e.g., N-methyl-d-aspartate receptors), thus modulating DA release through secondary mechanisms. Although bPiDDB may not block nicotine-evoked DA release completely, its high selectivity may be sufficient to decrease the reinforcing effect of nicotine without inducing the nonspecific side effects that are noted with mecamylamine.
The purpose of the present study was to examine the ability of acute bPiDDB to alter intravenous nicotine self-administration in rats. To assess the specificity of the bPiDDB-induced change in nicotine self-administration, we also examined the effect of acute bPiDDB on sucrose-maintained responding and on nicotine-induced locomotor activity. For comparison, the effect of mecamylamine on nicotine-induced locomotor activity was also determined.
Methods
Subjects
Male Sprague–Dawley rats (200 to 225 g) from Harlan Industries (Indianapolis, IN) were used. Rats had unlimited access to food and water in the home cage, except as noted. Rats were maintained on a 14:10 h light/dark cycle in which the lights came on at 0600 hours and went off at 2000 hours. All experiments were conducted during the light phase of the cycle. Rats were acclimated to the animal colony for at least 5 days and were handled briefly on three to five consecutive days prior to the start of the experiment. The Institutional Animal Care and Use Committee of the University of Kentucky approved the experiments described herein. The experiments conformed to the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996 Edition).
Apparatus
Nicotine self-administration and sucrose-maintained responding experiments (experiments 1 and 2) were conducted in operant conditioning chambers (ENV-001; Med Associates, St Albans, VT), housed in sound-attenuated outer chambers using a Med Associates Interface model SG-503 with MED-IV software. The end walls of the operant conditioning chamber were aluminum, front and back walls were made of clear Plexiglas, and the floor consisted of 18 stainless steel rods (4.8 mm in diameter and placed 1.6 cm apart). Located in the bottom center of one of the end walls was an opening (5×4.2 cm) for a recessed food tray, into which a food hopper could dispense sucrose pellets individually. Located on either side of the food tray was a response lever. A 28-V white cue light was located 6 cm above each response lever. An infusion pump (Med Associates) delivered drug reinforcement via a silastic tube attached to a swivel mounted on the outside of the back wall.
Locomotor activity was recorded automatically using an animal activity monitoring system with Versamax System software (AccuScan Instruments Inc., Columbus, OH). Rats were placed into a monitoring chamber (42×42-cm square and 30 cm high) made of clear acrylic walls and floor. The chamber incorporated a horizontal 16×16 grid of photo beam sensors, with each beam 2.5 cm apart and 7.0 cm above the chamber floor. Horizontal activity was recorded for a 60-min period, comprised of six 10-min intervals. Activity was measured by photo beam interruptions and was expressed as total distance traveled (cm).
Drugs
S(−)-nicotine ditartrate (Sigma, St. Louis, MO) was prepared in a 0.9% saline solution, to which NaOH was added to obtain a pH of 7.4. N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; formula weight=514.38) was synthesized according to previously established methods (Ayers et al. 2002). Mecamylamine HCl was purchased from Sigma; both of these drugs were prepared in 0.9% saline and administered via subcutaneous injection. All injections were administered in a volume of 1 ml kg−1 body weight. Doses of bPiDDB and mecamylamine represent the salt weights, and the nicotine doses are represented as the free-base weight.
Experiment 1: nicotine self-administration
Animals were initially given brief lever-press training for sucrose reward (45 mg Noyes pellet). Rats were food deprived to 85% of their ad libitum weights by restricting their intake of rat chow to 8–10 g per day for 5 days. They were then briefly trained to press an active lever in a two-lever operant chamber using a fixed ratio 1 (FR 1) schedule, which was incrementally increased to FR 5 across seven sessions, during 15-min operant sessions. Animals were given 20 g day−1 of food following each lever-press training session. After training for sucrose reinforcement, rats were allowed ad libitum access to food in the home cage for 7 days and were then implanted with an indwelling jugular catheter. Rats were anesthetized by injections of ketamine (80 mg kg−1 i.p.) and diazepam (5 mg kg−1 i.p.) and a silastic catheter was inserted into the jugular vein. The free end of the catheter exited through the skin and was secured to an acrylic head mount attached to the skull. An infusion pump was attached to the head mount via a silastic leash during the self-administration sessions.
The nicotine self-administration procedure was similar to that described previously (Corrigall and Coen 1989). Following recovery from catheter surgery (7 days), rats were reintroduced to the operant conditioning chambers for 60-min daily sessions; food restriction was maintained for the duration of the experiment (17–20 g day−1, given in the home cage after the session). Responses made on one lever (active) were recorded and were followed by an infusion of nicotine (0.03 mg kg−1 infusion−1, 100 μl delivered over 5.9 s), whereas responses made on the other lever (inactive) were recorded, but had no scheduled consequence. The unit dose of nicotine (0.03 mg kg−1 infusion−1) was chosen based on previously published work (Corrigall and Coen 1989). This dose produces optimal responding on an FR schedule with limited access. Completion of the FR requirement resulted in simultaneous activation of the infusion pump and cue lights, which signaled a 20-s time-out period during which responding on either lever had no consequence. The FR 1 schedule was gradually increased across sessions to a terminal FR 5 schedule. Rats were trained on the FR 5 schedule until stable responding was achieved, defined by the following criteria: (1) minimum of 10 infusions per session; (2) less than 20% variability in responding for three consecutive sessions; and (3) minimum of 2:1 (active/inactive) response ratio. After responding for nicotine stabilized, the effect of acute pretreatment with bPiDDB on nicotine self-administration was determined. Separate groups of rats were used to assess one of the doses of bPiDDB (0.3, 1, or 3 mg kg−1 s.c.) or saline, given 15 min prior to the session.
Experiment 2: sucrose-maintained responding
Immediately following initial sucrose training as described in experiment 1, rats (n=30; n=7–8 per group) were trained further to lever press for sucrose reinforcement using a FR 5 schedule of reinforcement during 60-min sessions. To reduce the sucrose reinforcement rate to a level comparable to the rate observed in the nicotine self-administration experiment, a long signaled time-out (100 s, cue lights on) was used as described previously (Paterson et al. 2003). Criteria for pretreatment were the same as those used in the nicotine self-administration experiments. Subsequently, separate groups of rats were used to assess one of the doses of bPiDDB (0.3, 1, or 3 mg kg−1 s.c.) or saline, given 15 min prior to the session.
Experimental 3: acute locomotor activity
Rats (n=66, 8–9 per group) were initially habituated to the locomotor apparatus for a 60-min session. On the following day, rats were pretreated with bPiDDB (1 or 3 mg kg−1 s.c.), mecamylamine (1 mg kg−1 s.c.), or saline 15 min prior to nicotine (0.4 mg kg−1 s.c.) or saline treatment. Immediately following the second injection, each rat was placed into the activity chamber, and locomotor activity was recorded for 60 min in 10-min intervals.
Experiment 4: nicotine-sensitized locomotor activity
In another experiment, the acute effects of bPiDDB or mecamylamine were assessed in rats previously sensitized to the locomotor stimulant effect of nicotine. To be consistent with the nicotine self-administration procedure, all rats in this experiment were food restricted (20 g day−1) for the duration of the experiment. Rats (n=36, n=9 per group) were habituated to the locomotor apparatus for 60 min. Beginning the next day, all rats were treated with nicotine (0.4 mg kg−1 s.c.) immediately prior to being placed into the activity chambers for 24 consecutive days (days 1–24). All locomotor sessions were 60 min. On day 25, all rats were pretreated with saline 15 min prior to nicotine treatment to ensure that the pretreatment injection procedure did not change locomotor activity. On the next day (day 26), rats were pretreated with bPiDDB (1 or 3 mg kg−1 s.c.), mecamylamine (1 mg kg−1 s.c.), or saline 15 min prior to a nicotine injection (0.4 mg kg−1 s.c.).
Data analysis
Separate one-way analyses of variance (ANOVAs) were conducted to determine the effect of acute bPiDDB pretreatment on nicotine self-administration and sucrose-maintained responding (SPSS, Version 10.0; Chicago, IL, USA). In addition, separate two-way repeated measures ANOVAs, with pretreatment as a between-subjects factor and time as a within-subjects factor, were performed to analyze the time course of the effect of bPiDDB on nicotine self-administration and sucrose-maintained responding over the 60-min session. Post hoc comparisons of each pretreatment dose against the saline control were conducted using Dunnett's t (two-tailed) with P<0.05 indicating significance. Planned t tests incorporating a more stringent significance level (P<0.01) were used to compare pairs of means at specific time blocks to compensate for family-wise error across multiple statistical comparisons.
Acute nicotine-induced locomotor activity was analyzed using a three-way mixed-factor ANOVA with pretreatment [saline, mecamylamine (1 mg kg−1), bPiDDB (1 mg kg−1) or bPiDDB (3 mg kg−1)] and treatment (saline or nicotine) as between-subject factors and time block as a within-subject factor. Subsequent two-way mixed-factor ANOVAs were conducted to explore differences within each pretreatment group between nicotine and saline treatments. Planned t tests incorporating a more stringent significance level (P<0.01) was used to compare pairs of means at specific time blocks to compensate for family-wise error across multiple statistical comparisons. To assess the effect of repeated nicotine administration, a one-way within-subjects ANOVA was conducted across days 1 to 25. A two-way mixed-factor ANOVA was used to analyze differences between pretreatment groups over 10-min blocks for the 60-min session on day 26. Subsequent two-way mixed-factor ANOVAs were used to assess the effects of each specific dose compared to saline control over 10-min blocks for the 60-min session. Planned t tests incorporating a more stringent significance level (P<0.01) was used to compare pairs of means at specific time blocks to compensate for family-wise error across multiple statistical comparisons.
Results
Experiment 1: nicotine self-administration
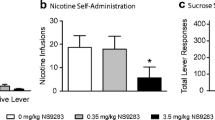
To determine whether baseline responding differed between groups prior to bPiDDB pretreatment, a one-way ANOVA was performed on the average number of responses during the three maintenance sessions when responding for nicotine stabilized. No significant differences in baseline (no pretreatment) responding between groups were found for active or inactive lever presses. There was a significant difference in the number of nicotine infusions earned across bPiDDB pretreatment doses (F 3,23=3.90, P<0.05; see Fig. 2) as indicated by a one-way ANOVA. Post hoc analysis indicated that bPiDDB (1 and 3 mg kg−1) pretreatment significantly decreased the number of nicotine infusions earned during the operant session compared to saline (P<0.05). To determine the time course effect of bPiDDB on nicotine self-administration, analyses were conducted on the number of nicotine infusions earned across 10-min time blocks during the 60-min session (Fig. 3a–c). ANOVA revealed significant main effects of time (F 5,115=9.20, P<0.001) and dose (F 3,23=3.90, P<0.05). Planned comparisons indicated a significant difference between saline and bPiDDB (1 mg kg−1) at the 30-min time block. In addition, there were significant differences found between saline and bPiDDB (3 mg kg−1) at the 40- and 50-min time blocks. Responding on the inactive lever was not significantly different across bPiDDB doses (data not shown).
Effect of bPiDDB on nicotine self-administration. Results are expressed as the total number of infusions earned (mean±SEM) during the operant conditioning session following bPiDDB or saline pretreatment. Asterisk (*) denotes a significant difference compared to the saline group, P<0.05. N=6–8 rats per group
Time course effect of bPiDDB (0.3, 1, or 3 mg kg−1, a–c respectively) on nicotine self-administration. Results are expressed as the number of infusions earned (mean±SEM) during 10-min blocks across the operant conditioning session. Asterisk (*) denotes a significant difference compared to the saline group at the same time block, P<0.05. N=6–8 rats per group
Experiment 2: sucrose-maintained responding
Similar to the nicotine self-administration experiment, no significant differences in baseline responding between groups were found for active or inactive lever presses (data not shown). One-way ANOVA revealed that there was no significant effect of any bPiDDB pretreatment dose on the number of sucrose pellets earned across the 60-min session relative to saline pretreatment (Fig. 4). The sucrose-maintained responding time course across the 60-min session is shown in Fig. 5a–c.
Experiment 3: acute locomotor activity
To determine the effect of bPiDDB on acute nicotine-induced locomotor behavior, rats were pretreated with either bPiDDB (1 or 3 mg kg−1), mecamylamine (1 mg kg−1), or saline, followed 15 min later by treatment with either nicotine (0.4 mg kg−1) or saline. ANOVA revealed a significant Pretreatment × Treatment × Time interaction, F 15,290=3.87, P<0.001. To assess the effect nicotine and saline treatment within each pretreatment group, ANOVAs were conducted within each pretreatment group across the 60-min session (Fig. 6a–d). In the saline pretreatment group, a significant Time × Treatment interaction was revealed, F 5,70=17.48, P<0.001. Planned comparisons within the saline pretreated group revealed that rats treated with nicotine and saline were significantly different at the 10- and 30- to 60-min time blocks. Thus, acute nicotine initially produced a transient period of hypoactivity, followed by a period of hyperactivity. In the bPiDDB (1 mg kg−1) pretreatment group, there was a significant Time × Treatment interaction, F 5,70=51.18, P<0.001. Planned comparisons indicated that in the bPiDDB (1 mg kg−1) pretreated groups, rats treated with nicotine and saline were significantly different at the 10-min time block. In the bPiDDB (3 mg kg−1) pretreatment group, there was a significant Time × Treatment interaction, F 5,80=55.46, P<0.001. Planned comparisons indicated that in the bPiDDB (3 mg kg−1) pretreated group, rats treated with nicotine and saline were significantly different at the 10- and 30- to 60-min time blocks. Thus, bPiDDB did not reliably block the acute locomotor effect of nicotine. In groups pretreated with mecamylamine, only a significant main effect of time was observed, F 5,70=90.21, P<0.001, indicating a complete blockade of the locomotor effects of acute nicotine by mecamylamine.
Time course effect of pretreatment with saline (a), bPiDDB (1 or 3 mg kg−1, b and c, respectively) or mecamylamine (d) in rats given acute nicotine or saline. Results are expressed as the total distance traveled (mean±SEM) during 10-min blocks across the session. Asterisk (*) denotes a significant difference compared to the saline treatment group at the same time block, P<0.01. N=8–9 rats per group. SAL Saline, NIC nicotine, MEC mecamylamine
Experiment 4: nicotine-sensitized locomotor activity
A separate group of rats was used to assess the effect of acute bPiDDB on locomotor activity after 25 repeated injections of nicotine (days 1–25). Prior to mecamylamine or bPiDDB (1 or 3 mg kg−1) pretreatment, rats showed a significant increase in locomotor activity from days 1 to 24 of repeated nicotine treatment (F 24,768=104.05, P<0.0001; data not shown), an effect indicative of sensitization. There were no significant differences in locomotor activity between groups across this sensitization phase (data not shown). On day 25, all animals received a saline injection 15 min prior to nicotine. Post-hoc paired sample t tests indicated that this saline pretreatment did not alter locomotor activity, as there were no significant differences between days 24 and 25. On day 26, animals received either saline, mecamylamine (1 mg kg−1), or bPiDDB (1 or 3 mg kg−1) 15 min prior to their usual nicotine injection. ANOVA indicated a significant interaction of Pretreatment × Time block, F 15,160=1.98, P<0.05. Subsequent ANOVAs between each dose over time blocks analyzed the effects of the individual pretreatments compared to saline on locomotor activity (Fig. 7a–c). In rats pretreated with bPiDDB (1 mg kg−1) on day 26, a significant main effect of time (F 5,25=31.80, P<0.001; Fig. 7b) was observed. In rats pretreated with bPiDDB (3 mg kg−1), a significant Pretreatment × Time interaction was revealed (F 5,80=2.89, P<0.005); post hoc t tests indicated that bPiDDB (3 mg kg−1) significantly decreased activity in nicotine-sensitized rats only at the 30-min time block (P<0.01). When animals were pretreated with mecamylamine on day 26, there was a significant Pretreatment × Time interaction (F 5,80=2.96, P<0.05); post hoc t tests showed that mecamylamine decreased activity in nicotine-sensitized rats on the 30- to 60-min time blocks (P<0.05; Fig. 7d).
Time course effect of pretreatment with mecamylamine (a) or bPiDDB (1 or 3 mg kg−1, b and c, respectively) compared to saline control in rats given repeated nicotine. Results are expressed as the total distance traveled (mean±SEM) during 10-min blocks across the session. Asterisk (*) denotes a significant difference compared to the saline treatment group at the same time block, P<0.01. N=9 rats per group. SAL Saline, MEC mecamylamine
Discussion
The primary purpose of the current study was to characterize the acute effect of bPiDDB on nicotine self-administration, sucrose-maintained responding, and nicotine-induced changes in acute and sensitized locomotor activity. In rats trained to self-administer nicotine intravenously on a limited access schedule, acute bPiDDB pretreatment decreased nicotine self-administration dose dependently. The bPiDDB-induced decrease was specific, as responding on the active (nicotine) lever was decreased, whereas responding on the inactive lever was not altered. In addition, within the dose range shown to decrease nicotine self-administration, acute bPiDDB did not alter sucrose-maintained responding. Following acute nicotine, animals initially displayed decreased activity followed by an increase in activity compared to saline controls; this effect of nicotine was mecamylamine dependent. The initial hypoactive effect of nicotine was not blocked by bPiDDB in the dose range tested (1 or 3 mg kg−1). Moreover, when rats were sensitized to nicotine following repeated treatment and were food restricted under conditions similar to those used in the nicotine self-administration experiment, acute bPiDDB (1 mg kg−1) failed to alter nicotine-induced sensitized locomotor activity; however, bPiDDB (3 mg kg−1) did transiently attenuate nicotine-induced sensitized locomotor activity. Taken together, these results suggest that the ability of bPiDDB (1 mg kg−1) to decrease nicotine self-administration does not simply reflect a nonspecific suppression of behavior.
In assessing the specificity of bPiDDB to decrease nicotine self-administration in the two-lever operant conditioning experiment, it should be noted that the number of responses on the active lever for nicotine was higher than the number of responses on the inactive lever. While a significant bPiDDB-induced decrease in responding on the active lever to earn infusions was evident, no effect on the inactive lever was seen. Therefore, active lever pressing for nicotine infusions was more sensitive to disruption by bPiDDB than inactive lever pressing. However, this may reflect a rate dependency effect, as psychoactive drugs generally decrease high rates of responding to a greater degree than low rates of responding (Dews 1955; Phillips et al. 1991). To further assess nonspecific effects of bPiDDB on lever pressing behavior, the effects of this novel compound were further examined on sucrose-maintained behavior. In a separate group of rats, bPiDDB did not alter the number of sucrose pellets earned, although the rate of sucrose reinforcement was slightly higher than the rate observed with nicotine reinforcement. These results indicate that rate dependency alone cannot provide an explanation for the current results.
Under conditions similar to those used in the current study to assess the effect of bPiDDB, previous research has shown that mecamylamine produces a monophasic dose-dependent decrease in nicotine self-administration on a limited access FR schedule of reinforcement (Corrigall and Coen 1989; Rauhut et al. 2002; Shoaib et al. 1997; Watkins et al. 1999). In those previous studies, the highest dose of mecamylamine tested produced approximately a 50% decrease in number of nicotine infusions earned. The mecamylamine-induced decrease in nicotine self-administration is thought to reflect a decrease of the reinforcing effect of nicotine, as the decrease in responding resembles extinction following saline substitution (Donny et al. 1999). Similar to mecamylamine, the current study found that bPiDDB produced a monophasic dose-dependent decrease in nicotine self-administration, with a maximal decrease in responding of 30–40% within the dose range tested. Since bPiDDB is thought to be a selective antagonist for nAChR subtypes mediating nicotine-evoked DA release (e.g., α3β2* or α6β2*), whereas mecamylamine produces a nonselective antagonism of nAChRs, the similar dose-effect profiles for bPiDDB and mecamylamine suggest that the nicotinic receptor subtype(s) mediating nicotine-evoked DA release are involved in the reinforcing effect of nicotine. Additional work using a progressive ratio schedule of reinforcement is needed to determine the extent to which bPiDDB specifically blocks the reinforcing effect of nicotine.
In the locomotor activity experiments, acute nicotine administration produced an initial hypoactivity that was followed by a rebound period of hyperactivity, defined by a comparison to saline controls. With repeated nicotine treatment, tolerance developed to the hypoactivity and sensitization developed to the hyperactivity, which is consistent with previous literature (Ksir 1994; Miller et al. 2001; Morrison 1967). Importantly, mecamylamine antagonized both the acute and repeated nicotine-induced locomotor effects, findings that are also consistent with previous literature (Clarke and Kumar 1983; Miller et al. 2001). In contrast, bPiDDB did not alter the initial hypoactivity following acute nicotine treatment nor did it reliably alter the acute hyperactive effect. Although the lower dose of bPiDDB (1 mg kg−1) did significantly reduce nicotine-induced hyperactivity, no effect was obtained with the higher dose (3 mg kg−1). Moreover, close examination of these results revealed that the saline control group for comparison to the 1 mg kg−1 bPiDDB group (Fig. 6b) was somewhat higher than the other saline control groups (Fig 6a,c). Thus, the apparent effect of bPiDDB (1 mg kg−1) on acute nicotine-induced hyperactivity may simply reflect this baseline difference, rather than a pharmacological antagonism. In rats sensitized to nicotine, bPiDDB (3 mg kg−1) pretreatment also transiently decreased sensitized locomotor activity, although not to the extent observed with mecamylamine. Previous work indicates that nicotine-induced hyperactivity and locomotor sensitization is dependent on DA systems (Balfour et al. 1998; Damaj and Martin 1993). Thus, these results suggest that bPiDDB may have a selective action on nAChRs that mediate the dopaminergic component following repeated nicotine administration.
The current work demonstrates the ability of bPiDDB to decrease nicotine self-administration, which suggests that this selective nicotinic receptor antagonist constitutes a lead for the development of a new class of therapeutic agents for the treatment of dependence on tobacco. Currently available pharmacotherapies for treating tobacco dependence (i.e., nicotine-replacement therapies and bupropion) are associated with high relapse rates (Cummings and Hyland 2004), indicating the need for new agents with greater efficacy. Since mecamylamine dose-dependently decreases nicotine self-administration in animal models (Rauhut et al. 2002; Watkins et al. 1999) and shows some efficacy in clinical trials (Rose et al. 1994), nicotinic receptor antagonists have potential as tobacco use cessation agents. Further support for this contention is the evidence that bupropion, in addition to inhibiting neurotransmitter transporters, acts as a nicotinic receptor antagonist (Miller et al. 2002; Slemmer et al. 2000), a pharmacological property which may contribute to its efficacy as a tobacco use cessation agent. The current work utilizing animal models demonstrates that selective nicotinic receptor antagonists may afford a viable approach to treat nicotine addiction. In addition, selective antagonists may provide more clinically effective agents with fewer side effects, compared with nonselective antagonists such as mecamylamine, the use of which is associated with limiting side effects. Furthermore, selective nicotinic receptor antagonists that block the specific receptor sites mediating nicotine-evoked DA release may be particularly effective in reducing the pleasurable effects of tobacco use, thus offering another advantage over currently available tobacco-use-cessation therapies. Further research is needed to ensure that this novel compound is acting primarily on the DA system. It is possible that the compound has effects on other neurotransmitter systems that have not yet been revealed.
References
Ayers JT, Dwoskin LP, Deaciuc AG, Grinevich VP, Zhu J, Crooks PA (2002) bis-Azaaromatic quaternary ammonium analogues: ligands for alpha4beta2* and alpha7* subtypes of neuronal nicotinic receptors. Bioorg Med Chem Lett 12:3067–3071
Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M (1998) Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav 59:1021–1030
Benwell ME, Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856
Clarke PB, Kumar R (1983) The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol 78:329–337
Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99:473–478
Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992) The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107:285–289
Cummings KM, Hyland A (2004) Impact of nicotine replacement therapy on smoking behavior. Annu Rev Public Health
Damaj MI, Martin BR (1993) Is the dopaminergic system involved in the central effects of nicotine in mice? Psychopharmacology (Berl) 111:106–108
Dews PB (1955) Studies on behavior. I. Differential sensitivity to pentobarbital of pecking performance in pigeons depending on the schedule of reward. J Pharmacol Exp Ther 113:393–401
Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE (1999) Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 147:135–142
Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA (2004) Subtype-selective nicotinic receptor antagonists: potential as tobacco use cessation agents. Bioorg Med Chem Lett 14:1863–1867
Epping-Jordan MP, Picciotto MR, Changeux JP, Pich EM (1999) Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 147:25–26
Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA (2000) Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther 294:1112–1119
Ksir C (1994) Acute and chronic nicotine effects on measures of activity in rats: a multivariate analysis. Psychopharmacology (Berl) 115:105–109
Lundahl LH, Henningfield JE, Lukas SE (2000) Mecamylamine blockade of both positive and negative effects of IV nicotine in human volunteers. Pharmacol Biochem Behav 66:637–643
Markou A, Paterson NE (2001) The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res 3:361–373
Miller DK, Wilkins LH, Bardo MT, Crooks PA, Dwoskin LP (2001) Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacology (Berl) 156:469–476
Miller DK, Sumithran SP, Dwoskin LP (2002) Bupropion inhibits nicotine-evoked [(3)H]overflow from rat striatal slices preloaded with [(3)H]dopamine and from rat hippocampal slices preloaded with [(3)H]norepinephrine. J Pharmacol Exp Ther 302:1113–1122
Morrison CF (1967) Effects of nicotine on operant behaviour of rats. Int J Neuropharmacol 6:229–240
Panagis G, Kastellakis A, Spyraki C, Nomikos G (2000) Effects of methyllycaconitine (MLA), an alpha 7 nicotinic receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology (Berl) 149:388–396
Paterson NE, Semenova S, Gasparini F, Markou A (2003) The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 167:257–264
Phillips G, Willner P, Sampson D, Nunn J, Muscat R (1991) Time-, schedule-, and reinforcer-dependent effects of pimozide and amphetamine. Psychopharmacology (Berl) 104:125–134
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Rauhut AS, Mullins SN, Dwoskin LP, Bardo MT (2002) Reboxetine: attenuation of intravenous nicotine self-administration in rats. J Pharmacol Exp Ther 303:664–672
Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV (1994) Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther 56:86–99
Rose JE, Westman EC, Behm FM, Johnson MP, Goldberg JS (1999) Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan. Pharmacol Biochem Behav 62:165–172
Shoaib M, Schindler CW, Goldberg SR (1997) Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 129:35–43
Slemmer JE, Martin BR, Damaj MI (2000) Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 295:321–327
Teng L, Crooks PA, Buxton ST, Dwoskin LP (1997) Nicotinic-receptor mediation of S(−)nornicotine-evoked [3H] -overflow from rat striatal slices preloaded with [3H] -dopamine. J Pharmacol Exp Ther 283:778–787
Watkins SS, Epping-Jordan MP, Koob GF, Markou A (1999) Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav 62:743–751
Acknowledgements
This work was supported by USPHS grant U19 DA17548 and by NIDA training grant T32 DA07304. We acknowledge the excellent technical assistance of Laura Fenton and Andrew Meyer and the expert advice of Dr. William Corrigall. For purposes of full disclosure, the University of Kentucky holds patents on bPiDDB, and a potential royalty stream to LPD and PAC may occur consistent with the University of Kentucky policy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neugebauer, N.M., Zhang, Z., Crooks, P.A. et al. Effect of a novel nicotinic receptor antagonist, N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology 184, 426–434 (2006). https://doi.org/10.1007/s00213-005-0163-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0163-8