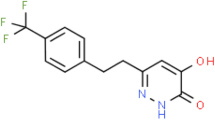
Abstract
Rationale
Glibenclamide (GD) is a widely used medical drug; therefore, identifying the mechanisms underlying its pleiotropic effects in the central nervous system is urgent.
Objectives
The aim of this work was to determine the ability of GD to modulate serotonin (5-hydroxytryptamine, 5-HT) and dopamine (DA) transmission and to assess the dose-dependent effect of GD on cognitive function in rats during natural ageing.
Methods
In Experiment 1, rats received 10, 25, or 50 μg/kg GD intraperitoneally for 10 days. In Experiment 2, rats received 50 μg/kg GD intraperitoneally for 30 days. Spatial and working memory was assessed in the MWM and Y-maze tests, respectively. In both experiments, the levels of DA and 5-HT, their metabolites, and turnover rate were analysed by HPLC-ED in the rat hippocampus and striatum.
Results
Changes in DA and 5-HT levels occurred only with a dose of 50 μg/kg GD. Therefore, in the second experiment, we administered a dose of 50 μg/kg GD. At this dose, GD prevented the development of impairments in spatial and working memory. The hippocampal concentrations of DA and DOPAC decreased, and the striatal concentrations of DA, DOPAC, 5-HT, and 5-HIAA increased.
Conclusion
One of the possible mechanisms of the precognitive effect of GD is its ability to modulate monoamine transmission. Thus, in translating our results to humans, GD can be recommended as a prophylactic agent for natural ageing to reduce the risk of developing cognitive impairments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive function is realized through integrated brain activity (Hugo and Ganguli 2014), and cognitive impairments develop in a variety of focal and diffuse brain lesions (Kalaria et al. 2016; Solé et al. 2017; Lao et al. 2021; Paitel et al. 2021; Sood and Raji 2021). According to the World Health Organization, more than 20 million people worldwide suffer from dementia and cognitive impairment, and this number is constantly increasing, both in older people and also in the working-age population (WHO 1948). Therefore, the preservation and restoration of impaired cognitive function are global problems for modern medicine.
Practically, all neurotransmitter systems are involved in the biochemical processes of memory (Stern and Alberini 2013; Atherton et al. 2015; Alhowail 2021). For example, cognitive impairment is associated with 5-HT system dysfunction, which leads to impaired glutamate and GABA transmission in the hippocampus and frontal cortex (Nazar et al. 1999). The modulatory effects of various 5-HT receptors on cognitive function are also mediated by the influence of 5-HT on other neurotransmitter systems. For example, the activation of 5-HT1A, 5-HT2A, 5-HT4, and 5-HT6 receptors enhances cognition and memory (Roth et al. 2004), and the activation of 5-HT1B and 5-HT3 receptors in the hippocampus causes memory impairment (Buhot et al. 2003). Recent studies have shown that activation of 5-HT receptors in the CA1 region of the hippocampus potentiates excitatory signalling at CA3-to-CA1 synapses and enhances spatial memory, while suppression of 5-HT synapse activity in CA1 leads to memory impairment (Teixeira et al. 2018). These data show a significant modulatory effect of 5-HT on memory processes. Dopamine (DA) is implicated in the regulation of cognitive function, including cognitive control processes and working memory (Cools and D'Esposito 2011). Traditionally, the cognitive effects of DA have been attributed to the mesocortical DA pathway. However, the involvement of striatal DA in working memory and cognitive control has recently received increasing attention (McNab and Klingberg 2008). However, no consistent conclusion has been reached. The involvement of DA in modulating cognitive function is associated with the innervation of the hippocampus by dopaminergic mesencephalic neurons from the ventral tegmental area and the substantia nigra (Atherton et al. 2015). In addition, DAT-KO rats, which cannot reuptake dopamine and thus have elevated extracellular striatal dopamine levels, demonstrate a deficit in working memory (Kurzina et al. 2020).
Serotonergic/dopaminergic drugs are assumed to positively influence cognition, but their effects appear to be dependent on baseline serotonin/dopamine levels (Gibbs and D’Esposito 2005); thus, they cannot be prescribed for preventing cognitive dysfunction. Drugs that modulate 5-HT and DA transmission through indirect effects may be useful for preventing or correcting cognitive impairment. In particular, glibenclamide (GD) is an attractive option. This drug was introduced into clinical practice in 1969 (for treating type 2 diabetes mellitus) and has an established neuroprotective effect. A few studies have reported that GD has a procognitive effect on a model of type 2 diabetes and Alzheimer’s disease in rats (Esmaeili et al. 2020) and attributes its effect to a decrease in neuroinflammation in the hippocampus. However, studies have yet to investigate the effects of GD during natural ageing. Moreover, the mechanism underlying GD’s procognitive effect may be much more complex. Given that GD modulates DA (Patel et al. 2010; Zubov et al. 2020) and 5-HT transmission (Soliman et al. 2020) and the evidence that the striatum is involved in cognition (Cools 2011), the aim of this work was to demonstrate the ability of glibenclamide to modulate 5-HT and DA transmission and to assess the dose-dependent effect of glibenclamide on the cognitive function of rats during natural ageing.
Materials and methods
Animals
Adult male Wistar rats (180 ± 20 g) were used in all experiments. The rats were purchased from the Rappolovo nursery (Leningrad Region, Russia). The animals were housed in cages (4–5 animals/cage) in a room with controlled conditions (temperature of 24 ± 1 °C, 45–65% humidity, and a 12 h light/12 h dark cycle). In the experimental period, pelleted rat chow and water were available ad libitum. All procedures with rats were carried out according to institutional guidelines and in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals as well as national laws (Ministry of Health of Russian Federation N267, June 19, 2003; Guide for the Use of Laboratory Animals, Moscow, 2005).
Experimental design
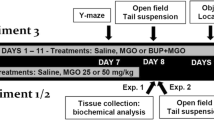
The experiments were carried out in two stages. A schematic diagram of the experiments is shown in Fig. 1.
Stage 1
Forty male rats were divided into 4 groups of 10 animals each by the block randomization method; the groups were as follows: GD-10, GD-25, GD-50, and the control group. The rats in the GD-10 group received an intraperitoneal injection of 10 µg/kg GD daily for 10 days. The GD-25 and GD-50 groups received intraperitoneal injections of 25 µg/kg and 50 µg/kg GD, respectively, daily for 10 days. The control group received an intraperitoneal injection of 1 ml of saline. At the end of the experiment, the rats were deeply anaesthetized by intraperitoneal administration of Zoletil (15 mg/kg) and decapitated with a guillotine (OpenScience AE1601, RPC OpenScience Ltd, Russia). The rat brains were quickly excised and dissected from the skull according to previously described methods (Paul et al. 2008). Using a brain slicer matrix, the whole hippocampus and striatum were collected. Brain areas were identified with a brain atlas (Paxinos and Watson 2013). All collected tissue was immediately frozen and stored at − 80 °C until analysis. The monoamine levels in the samples were analysed by HPLC-ED.
Stage 2
At the age of 4 months, twenty Wistar males were tested in the Morris water maze test and in the Y-maze test (as described below). At the age of 7 months, the rats were divided into two groups of 10 animals each by the block randomization method, forming the GD-50 and control groups. The rats in the GD-50 group received an intraperitoneal injection of 50 µg/kg GD daily for 30 days. The control group received an intraperitoneal injection of saline on the same schedule. At the age of 8 months, the cognitive function of the rats was re-assessed with the MWM and Y-maze tests. At the end of the experiment, the animals were decapitated, and striatum and hippocampus samples were collected as described above.
Evaluation of behaviour in the Morris water maze (MWM) test
Spatial learning and memory were investigated using the MWM test. In the MWM, rats are placed in a large circular pool of water from which they can escape onto a hidden platform located in the middle of one of the four quadrants of the pool. The dimensions of the pool were 150 cm in diameter by 60 cm high, with the water level set at a height of 43 cm above the base. The pool was filled with water at a temperature of 22 ± 1 °C. The dimensions of the platform were 10 × 10 cm. Four points around the circumference of the pool were arbitrarily designated north, south, east, or west, and on this basis, the pool area was divided into 4 quadrants (NW, SW, etc.). Geometric shapes on the walls of the pool in the positions NW, NE, SE, and SW serve as cues for the animal.
The platform was located in the centre of the northwestern (NW) sector. The platform was rendered invisible by ensuring that its surface was just beneath the water surface (at a depth of 1.0–1.5 cm) and by making the water opaque (by adding a small quantity of milk). Thus, there were no local cues to guide escape behaviour. The northeastern (NE), eastern (E), southern (S), and southwestern (SW) regions of the pool were chosen as the starting positions. Normal rats very quickly learned to swim directly towards the platform from any starting position at the circumference of the pool.
Over 4 days, each rat was given 4 trials to find the platform, each trial from a different position. The attempt ended when the rat found the platform or if the rat did not find the platform within 90 s. In the latter case, the rat was carefully directed to the platform, where it was allowed to sit for 30 s. The new trial started 90–120 s after removing the rat from the platform in the previous trial. The final test without a platform (the probe trial) was carried out on the 5th day for 90 s. Several measures were used to estimate the efficiency of the rat’s performance: directionality, latency to find the platform on each trial, length of the swim path, and the distribution of swimming time and path in the four quadrants of the pool during the probe trial, in which the platform was removed from the pool.
Animals were recorded, and path processing was carried out in the “Pavlovian Tracking” program, developed by employees of the I.P. Pavlov Department of Physiology, FSBSI “Institute of Experimental Medicine”. The time the animal spent in the platform area was recorded.
Y-maze preference test
Spontaneous alternation behaviour was tested in the Y-maze to evaluate the working memory performance of animals as described elsewhere (Yoon et al. 2013). A rat was placed into the Y-maze (arms angled at 120°, 10 cm width × 45 cm length × 30 cm wall height) for 8 min and video recorded. The sequence of arm entries was registered, and alternation was defined as consecutive entry into three different arms without repeats. The percentage of alternation behaviour was calculated relative to the maximal number of alternations (number of arm entries minus 2).
Measurements of brain monoamines and their metabolites
Measurement of monoamine levels was carried out as previously described (Tseilikman et al. 2020). Briefly, brain tissue was homogenized in 0.1 M perchloric acid. After homogenization, the samples were centrifuged (7000 × g for 15 min at 4 °C), and the supernatants were filtered through a Whatman 0.2-micron pore size syringe filter (Millipore Sigma, Burlington, USA). High-performance liquid chromatography (HPLC) was performed on a Hypersil BDS C18 reversed-phase column (250 × 4.6 mm, 5 μm) under isocratic conditions with electrochemical detection. The mobile phase consisted of 75 mM phosphate buffer containing 2 mM citrate acid, 0.1 mM octane sulfonic acid, and 15% (v/v) acetonitrile (pH 4.6). Electrochemical detection was achieved by setting a glassy carbon working electrode at + 780 mV. Twenty microlitres of supernatant was injected directly into the HPLC system. Each sample was injected 3 times, and the results were averaged. Monoamine levels were corrected for the total protein concentration of the final sample, as assessed by the Pierce bicinchoninic acid (BCA) assay according to the manufacturer’s instructions (Thermo Fisher Scientific, Inc), and were expressed in ng/mg of total protein content using an external calibration curve. The turnover of 5-HT was calculated as follows:
The turnover of dopamine was calculated as follows:
The minimum detectable monoamine concentration was 5 ng/20 μl. The coefficient of monoamine variation in a single sample did not exceed 5%. Quality control of the measurement results was carried out by an internal quality control service.
Statistical analysis
Statistical analysis was conducted using Statistica 8.0 (StatSoft). Sample size calculation was performed with G*Power 3.1.9.2 software with an effect size of 0.55, a 5% significance level, and a power of 0.8. The normality of the distribution was verified by the Shapiro–Wilk test. Data are expressed as the mean ± standard error of the mean (SEM) for monoamines and the median (q1/4; q3/4) for behavioural assessment data. A one-way ANOVA (factor, GD dose (0, 10, 25, 50)) was used to detect the dose-dependent effect of GD. The effect of GD on spatial memory and working memory was determined by two-way ANOVA including time (4 or 8 months) as the dependent variable and group (control versus GD) as the independent variable. Statistical significance of differences in behavioural assessment data was determined with the Wilcoxon signed-rank test. To find a linear relationship between the changes in monoamine levels and cognitive performance, the Spearman correlation coefficient was calculated. p < 0.05 was considered a statistically significant value.
Results
Dose-dependent effect of GD on brain monoamines, their metabolites, and turnover rates
Figure 2 shows the dose-dependent effect of GD on the concentration of DA and its metabolites (DOPAC and HVA) in the hippocampus (A) and striatum (B). Exposure to GD led to a change in the DOPAC concentration in the hippocampus (F (3,36) = 3.00, p = 0.044). Hippocampal DOPAC concentrations were significantly decreased in GD-50 rats compared to those in controls (p = 0.034, Tukey post hoc test) but not those in GD-10 and GD-25 rats. Hippocampal DA and HVA concentrations were not altered by GD. In the striatum, exposure to GD led to a change in DA concentration (F (3,36) = 4.71, p = 0.007) and DOPAC concentration (F (3,36) = 6.03, p = 0.002). Striatal DA and DOPAC concentrations were significantly increased in GD-50 rats compared to those in controls (p = 0.018 and p = 0.002, respectively, Tukey post hoc test) but not those in GD-10 and GD-25 rats.
Figure 3 shows the dose-dependent effect of GD on the concentration of 5-HT and its metabolite 5-HIAA in the hippocampus (A) and striatum (B). Hippocampal concentrations of 5-HT and 5-HIAA were not altered by GD. Striatal 5-HT concentrations were significantly different (F (3,36) = 3.57, p = 0.023). The concentration of 5-HT was significantly increased in GD-50 rats compared to that in controls (p = 0.024, Tukey post hoc test) but not that in GD-10 and GD-25 rats.
Figure 4 shows the lack of an effect of GD on monoamine turnover rates in the hippocampus (A) and striatum (B). The one-way ANOVA did not detect significant differences among the groups.
Effect of chronic intraperitoneal injection of GD on spatial memory
In the MWM test, the time spent in the target quadrant was 41.0 (35.3; 45.3) s in 4-month-old animals. Three months after the random division of these animals into two groups, this indicator was retrospectively recalculated for each subgroup and amounted to 40.4 (35.9; 45.4) s in the control group versus 41.0 (32.8; 45.2) s in the GD-50 group (U = 50.0, p = 0.999). Thus, the groups did not differ in this indicator before the onset of exposure.
The effect of chronic GD injection on spatial memory in rats is summarized in Fig. 5A and B. A two-way ANOVA (on time and GD administration) showed that GD administration during ageing changed the time spent in the target quadrant during the MWM test (F (1,18) = 11.3, p = 0.003). The contribution of each factor is presented in Fig. 5B.
With normal ageing, the time spent in the target quadrant decreased from 40.4 (35.9; 45.4) s at 4 months to 36.6 (27.8; 36.6) s at 8 months, p = 0.019. Administration of GD negates this effect, as the time spent in the target quadrant was 41.0 (32.8; 45.2) s at 4 months vs. 44.7 (39.3; 47.7) s at 8 months, p = 0.222. The control group of 8-month-old rats spent less time in the target quadrant than the GD-50 group, p = 0.008.
Effect of chronic intraperitoneal injection of GD on working memory
In the Y-maze test, the percentage of spontaneous alternation was 66.1 (65.3; 72.7)% in 4-month-old animals. Three months after the random division of these animals into two groups, this indicator was retrospectively recalculated for each subgroup and amounted to 67.7 (64.3; 70.6)% in the control group vs. 66.1 (65.0; 72.7)% in the GD-50 group (U = 47.0, p = 0.859). Thus, the groups did not differ in this indicator before the onset of exposure. The effect of chronic GD injection on the working memory of rats is summarized in Fig. 5C. A two-way ANOVA (including time and GD administration) showed that GD administration during natural ageing changed the rate of spontaneous alternation (F (1,18) = 7.6, p = 0.013). The contribution of each factor is presented in Fig. 5D. With normal ageing, the percentage of spontaneous alternation decreased from 67.7 (64.3; 70.6)% at 4 months to 53.3 (41.8; 61.7)% at 8 months, p = 0.044. The administration of GD negates this effect, as the percentage of spontaneous alternation was 66.1 (65.0; 72.7)% at 4 months vs. 73.5 (61.1; 84.4)% at 8 months, p = 0.429. The percentage of spontaneous alternation of 8-month-old animals in the control was lower than that in the GD-50 group, p = 0.007.
Effect of chronic intraperitoneal injection of GD on brain monoamines, their metabolites, and turnover rates
Figure 6 shows the effect of chronic intraperitoneal injection of 50 µg/kg GD on DA concentration and its metabolites (DOPAC and HVA) in the hippocampus (A) and striatum (B). Exposure to GD resulted in a twofold decrease in DA concentration in the hippocampus (t = 3.6, p = 0.002) and a 2.1-fold decrease in DOPAC concentration (t = 3.7, p = 0.002 compared to control). In the striatum, the opposite changes were observed: the concentrations of DA and DOPAC increased by 1.8 (t = 7.7, p = 0.000 compared to the control) and 2.4 times (t = 6.7, p = 0.000 compared to the control), respectively.
Figure 7 shows the effect of chronic intraperitoneal injection of GD on the concentration of 5-HT and its metabolite 5-HIAA in the hippocampus (A) and striatum (B). Hippocampal concentrations of 5-HT and 5-HIAA were not altered by GD. Striatal 5-HT concentrations were 1.6 times higher in the GD-50 group (t = 4.1, p = 0.001 compared to control). The concentration of 5-HIAA in the GD-50 group was 1.4 times higher than that in the control group (t = 3.2, p = 0.005 compared to the control).
Chronic intraperitoneal injection of GD had no effect on monoamine turnover rates in the hippocampus (Fig. 8A) and striatum (Fig. 8B).
Correlation between changes in monoamine levels and cognitive performance
The most notable correlations between changes in monoamine levels and time spent in the target quadrant during the MWM (A, B, C) or the percentage of spontaneous alternation in the Y-maze (D, E, F) are presented in Fig. 9. There was a moderate negative correlation between hippocampal DA concentration and the time spent in the target quadrant during the MWM (p = 0.014, R = − 0.536). Moderate positive correlations were found between striatal DA concentration and the time spent in the target quadrant during the MWM (p = 0.002, R = 0.653) and between 5-HT concentrations and the MWM indicator (p = 0.015, R = 0.536). A similar pattern was observed when comparing the changes in monoamine levels with changes in the percentage of spontaneous alternation during the Y-maze test. The R values of all other indicators can be found in Supplemental Table 1.
Discussion
This research examined the dose-dependent effect of chronic intraperitoneal administration of GD on the concentrations of dopamine and serotonin, their metabolites, and their turnover rate in the hippocampus and striatum of Wistar rats. It also examined the cognitive performance of animals under chronic administration of GD. Of all the analysed doses of GD, only the dose of 50 μg/kg led to significant changes in DA and 5-HT concentrations. After intraperitoneal administration, the DOPAC level decreased in the hippocampus, and the DA, DOPAC, and 5-HT levels increased in the striatum, though the turnover rate of these monoamines remained unchanged. Therefore, for the subsequent experiment, we administered a dose of 50 μg/kg GD. The administration of GD over 30 days prevented cognitive decline in rats at 8 months of life. The procognitive effect of GD was exhibited in the MWM and Y-maze tests. Therefore, GD prevented the development of impairments in spatial and working memory. In animals that received 50 μg/kg GD for 30 days, there was a decrease in DA and DOPAC levels in the hippocampus and an increase in DA, DOPAC, 5-HT, and 5-HIAA levels in the striatum. The turnover rates of DA and 5-HT in both structures remained unchanged. We also found a moderate negative correlation between hippocampal dopamine concentration and the time spent in the target quadrant during the MWM and moderate positive correlations between striatal dopamine and the time spent in the target quadrant during the MWM as well as between 5-HT concentrations and the MWM indicator.
All higher cognitive functions are regulated by complex circuits that span multiple areas of the brain. Ascribe a certain functional ability to any particular region, or even several regions, would be an oversimplification. However, the basic brain structures for each function can be identified. The hippocampus is one of the regions critical for spatial memory (Vorhees and Williams 2014; Zameer et al. 2019). Therefore, in this research, we analysed the dose-dependent effect of GD on DA and 5-HT, their metabolites, and their turnover rate in the hippocampus. The hippocampus is innervated by dopaminergic neurons from the ventral tegmental area, the region that also innervates the striatum. In addition, dopamine is released from the locus coeruleus (LC) to the dorsal hippocampus (Kempadoo et al. 2016). The striatum has been shown to mediate associations between stimuli and responses (Goodman and Packard 2018; Packard and Knowlton 2002). According to recent studies, the striatum, along with the hippocampus, promotes extinction through various learning mechanisms (Goodman and Packard 2018). Thus, the influence of GD on DA, 5-HT and their metabolite concentrations was analysed not only in the hippocampus but also in the striatum.
GD has long been used in clinical practice as a hypoglycaemic drug, but its pleiotropic effects on the brain have recently been demonstrated (Zubov et al. 2020; Pestereva et al. 2021; Rosado et al. 2021; Qiu et al. 2021). According to classic theories, the target of GD is KATP channels (Babenko et al. 1998). GD causes KATP channels to close, but its effect is dose-dependent (Groop et al. 1991). In the clinic, drugs based on GD are administered in a narrow dose range, so the additional effects may not be apparent. Therefore, we tested three doses of GD: 10, 25, and 50 μg/kg (Fig. 1A). Only 50 μg/kg GD had a modulating effect on monoamines (Figs. 2B, 3B, 4B). The effect we found can be explained as follows: KATP channels are found in the brain, particularly in the basal ganglia (Dunn-Meynell et al. 1998), and their opening leads to inhibition of dopamine release from nerve endings in the striatum (Avshalumov and Rice 2003) but not an overall decrease in dopamine in this area of the CNS (Bao et al. 2005). Therefore, by promoting the closure of KATP channels, GD can activate dopamine release. However, in the striatum, we found an increase in DA and DOPAC concentrations without changes in their turnover rate (Figs. 2B, 4B), which most likely indicates an increase in dopamine synthesis. An increase in DA synthesis is possibly postsynaptically mediated via D1/D2 dopamine receptors on medium spiny GABA neurons or through activation of 5-HT2A receptors (by a GD-induced increase in 5-HT (Fig. 3B)), which are located on glutamatergic neurons projecting from the striatum or nucleus accumbens to nigral or VTA regions (Di Matteo et al. 2008). Indeed, in our study, it was shown that with the administration of 50 μg/kg GD in the striatum, an increase in 5-HT concentration was observed without changes in its turnover rate (Figs. 3B, 4B). This increase may be a direct effect of GD since inhibitors of various K + channels have previously been shown to increase the concentration of 5-HT in the striatum (Dawson and Routledge 1995). 5-HT in the striatum originates from the raphe nuclei and can have both inhibitory and excitatory effects on DA neurons, depending on the type of receptor it interacts with (Moukhles et al. 1997). For example, an increase in DA levels in the dorsal striatum may be observed in response to stimulation of the 5-HT1B receptor by 5-HT, which modulates L-DOPA metabolism to produce DA (Knobelman et al. 2000). When comparing the concentrations of DA and 5-HT and the results on the behavioural tests, we discovered moderate positive correlations between striatal dopamine concentration and the time spent in the target quadrant during the MWM as well as between striatal dopamine concentration and the percentage of spontaneous alternation during the Y-maze test. Additionally, we compared 5-HT concentrations and the MWM/Y-maze indicators (Fig. 9). These findings are similar to those previously described. For example, lower dopamine release in the striatum was found to be correlated with worse working memory and performance during probabilistic category learning (van de Giessen et al. 2017), and higher striatal dopamine release predicted better working memory performance (Landau et al. 2009) and probabilistic category learning (Wilkinson et al. 2014). Methamphetamine was shown to reduce striatal 5-HT and impair learning and memory in adult male rats (Gutierrez et al. 2018). Therefore, our results are consistent with these reports, suggesting a positive relationship between striatal dopamine/5-HT concentrations and the preservation of cognitive function.
In the hippocampus, 50 μg/kg GD caused a decrease in the DOPAC concentration but did not result in changes in the turnover rate of hippocampal DA (Fig. 3A), which indicates a possible decrease in the rate of DA synthesis in dopaminergic terminals. The opposite effects of GD on DA content in the striatum and hippocampus can be explained by the following two reasons, both of which are based on the fact that DA enters the hippocampus from the ventral tegmental area, substantia nigra pars compacta, and from LC (Kempadoo et al. 2016; McNamara and Dupret 2017); thus, the sources of DA in the striatum and hippocampus partially overlap. First, the administration of GD may cause a redistribution of the DA flow, with predominant outflow into the nigrostriatal pathway. Second, GD could directly or indirectly increase the activity of dopamine-beta-hydroxylase (DBH), the enzyme that converts dopamine to norepinephrine (NE). An increase in DBH activity will lead to an increase in NE and a corresponding decrease in DA, which is consistent with our findings. Increased NE in the hippocampus has a positive effect on spatial and working memory (Mei et al. 2015; Guerrero et al. 2020; Wang et al. 2020). While this assumption requires further verification, it is supported by studies showing an increase in DBH activity in the hippocampus during cognitive load (Murata 2013; Xiao et al. 2018). When we compared the levels of DA and 5-HT and the results of the behavioural tests, moderate negative correlations were observed between hippocampal dopamine concentration and the time spent in the target quadrant during the MWM as well as between hippocampal dopamine concentration and the percentage of spontaneous alternation during the Y-maze test (Fig. 9). This pattern persisted for both control rats and animals receiving GD (Fig. 9). Other authors have shown that, in contrast, an increase in dopamine (and norepinephrine) release from the LC to the hippocampus promotes spatial learning and memory (Kempadoo et al. 2016). After comparing our data with those from Kempadoo et al. (2016), Guerrero et al. (2020) and Wang et al. (2020), we believe that the procognitive effect of GD is explained by its effect on hippocampal DBH activity, as discussed above.
Thus, despite the opposite effects of GD on the levels of DA in the hippocampus and striatum, its cumulative effect is procognitive. Moreover, since we found that chronic administration of GD leads to an increase in DA in the striatum, GD can likely be used to treat Parkinson’s disease. Experimental confirmation of this assumption has previously been obtained (Ren et al. 2016; Sarookhani et al. 2018).
The raphe nuclei are the source of hippocampal 5-HT; therefore, as in the striatum, we expected an increase in the concentration of 5-HT in the hippocampus. However, this did not occur (Figs. 3A, 4A), probably because 5-HT was redistributed to the striatum.
In this study, we showed that chronic (30-day) intraperitoneal administration of GD prevented memory impairment during natural ageing in the MWM and Y-maze tests (Fig. 5), which was associated with changes in the concentration of monoamines in the hippocampus and striatum. The procognitive effect of GD has previously been experimentally demonstrated (in a model of type 2 diabetes, a model of Alzheimer’s disease, and a model of traumatic brain injury) (Stokum et al. 2017; Esmaeili et al. 2020) and demonstrated in the clinic (Slingerland et al. 2008). However, the mechanism underlying the effect of GD on cognitive function remains unclear. According to reports, GD treatment reduces glial activation in the dentate gyrus of the hippocampus (Stokum et al. 2017) by increasing insulin levels in the brain (Esmaeili et al. 2020). At the same time, it is undeniable that monoamine neurotransmitters are essential for the formation and retention of memory (Kurian et al. 2011). Dopamine is involved in many processes, such as executive function, learning, reward, and motivation. 5-HT is involved in the modulation of many CNS-mediated functions, including arousal, motor activity, and stress; therefore, it is not surprising that the GD-induced modulation of monoamine transmission that we identified (Figs. 6, 7, and 8) affected cognition. This effect was confirmed by a correlation analysis between working and spatial memory and monoamine content in the hippocampus and striatum (Fig. 9). We expected to see a preferential effect of GD on hippocampal monoamine concentrations but found only a decrease in DA and DOPAC concentrations (Fig. 6A). Moreover, some authors believe that the cause of cognitive impairment may be a decrease in DA and 5-HT levels in the hippocampus (Zhang et al. 2020). The concentration of monoamines in the striatum under such conditions has not yet been studied. We believe that the demonstrated changes in the monoamine levels in the striatum can reduce extinction in learning and reduce memory impairment.
Conclusions
GD is widely administered; therefore, identifying the mechanisms underlying its pleiotropic effects in the CNS is urgent. We showed that administration of 50 μg/kg GD to rats for 30 days during natural ageing leads to reduced memory impairment, which was associated with an increase in DA and 5-HT concentrations in the striatum and a decrease in DA concentration in the hippocampus. Therefore, one of the possible mechanisms of the procognitive effect of GD is its ability to modulate monoamine transmission. Thus, translating our results to humans, GD is recommended as a prophylactic agent in natural ageing to reduce the risk of developing cognitive impairments.
Data availability
The data that support the findings of this study are available from the corresponding author, D. S. Traktirov, upon reasonable request.
References
Alhowail A (2021) Molecular insights into the benefits of nicotine on memory and cognition (review). Mol Med Rep 23(6):398. https://doi.org/10.3892/mmr.2021.12037
Atherton LA et al (2015) Memory trace replay: the shaping of memory consolidation by neuromodulation. Trends Neurosci 38(9):560–570. https://doi.org/10.1016/j.tins.2015.07.004
Avshalumov MV, Rice ME (2003) Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc Natl Acad Sci U S A 100(20):11729–11734. https://doi.org/10.1073/pnas.1834314100
Babenko AP et al (1998) A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60:667–687. https://doi.org/10.1146/annurev.physiol.60.1.667
Bao L et al (2005) Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J Neurosci 25(43):10029–10040. https://doi.org/10.1523/JNEUROSCI.2652-05.2005
Buhot MC et al (2003) Spatial learning in the 5-HT1B receptor knockout mouse: selective facilitation/impairment depending on the cognitive demand. Learn Mem 10(6):466–477. https://doi.org/10.1101/lm.60203
Cools R (2011) Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol 21(3):402–407. https://doi.org/10.1016/j.conb.2011.04.002
Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69(12):e113–e125. https://doi.org/10.1016/j.biopsych.2011.03.028
Dawson LA, Routledge C (1995) Differential effects of potassium channel blockers on extracellular concentrations of dopamine and 5-HT in the striatum of conscious rats. Br J Pharmacol 116(8):3260–3264. https://doi.org/10.1111/j.1476-5381.1995.tb15133.x
Di Matteo V et al (2008) Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog Brain Res 172:7–44. https://doi.org/10.1016/S0079-6123(08)00902-3
Dunn-Meynell AA et al (1998) Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res 814(1–2):41–54. https://doi.org/10.1016/s0006-8993(98)00956-1
Esmaeili MH et al (2020) Glibenclamide mitigates cognitive impairment and hippocampal neuroinflammation in rats with type 2 diabetes and sporadic Alzheimer-like disease. Behav Brain Res 379:112359. https://doi.org/10.1016/j.bbr.2019.112359
Gibbs SEB, D’Esposito M (2005) Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cogn Affect Behav Neurosci 5(2):212–221. https://doi.org/10.3758/cabn.5.2.212
Goodman J, Packard MG (2018) The role of the dorsal striatum in extinction: a memory systems perspective. Neurobiol Learn Mem 150:48–55. https://doi.org/10.1016/j.nlm.2018.02.028
Groop LC et al (1991) Dose-dependent effects of glyburide on insulin secretion and glucose uptake in humans. Diabetes Care 14(8):724–727. https://doi.org/10.2337/diacare.14.8.724
Guerrero et al (2020) Serotonin and noradrenaline content and release in the dorsal hippocampus during learning and spatial memory in prenatally stressed rats. Acta Neurobiol Exp (wars) 80(4):400–410
Gutierrez A et al (2018) A single high dose of methamphetamine reduces monoamines and impairs egocentric and allocentric learning and memory in adult male rats. Neurotox Res 33(3):671–680. https://doi.org/10.1007/s12640-018-9871-9
Hugo J, Ganguli M (2014) Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 30(3):421–442. https://doi.org/10.1016/j.cger.2014.04.001
Kalaria RN et al (2016) Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta 1862(5):915–925. https://doi.org/10.1016/j.bbadis.2016.01.015
Kempadoo KA et al (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113(51):14835–14840. https://doi.org/10.1073/pnas.1616515114
Knobelman DA et al (2000) Regulation of extracellular concentrations of 5-hydroxytryptamine (5-HT) in mouse striatum by 5-HT(1A) and 5-HT(1B) receptors. J Pharmacol Exp Ther 292(3):1111–1117
Kurian MA et al (2011) The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol 10(8):721–733. https://doi.org/10.1016/S1474-4422(11)70141-7
Kurzina NP et al (2020) Deficit in working memory and abnormal behavioral tactics in dopamine transporter knockout rats during training in the 8-arm maze. Behav Brain Res 390:112642. https://doi.org/10.1016/j.bbr.2020.112642
Landau SM et al (2009) Striatal dopamine and working memory. Cereb Cortex 19(2):445–454. https://doi.org/10.1093/cercor/bhn095
Lao Y et al (2021) Association between alcohol intake, mild cognitive impairment and progression to dementia: a dose-response meta-analysis. Aging Clin Exp Res 33(5):1175–1185. https://doi.org/10.1007/s40520-020-01605-0
McNab F, Klingberg T (2008) Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 11(1):103–107. https://doi.org/10.1038/nn2024
McNamara CG, Dupret D (2017) Two sources of dopamine for the hippocampus. Trends Neurosci 40(7):383–384. https://doi.org/10.1016/j.tins.2017.05.005
Mei Y et al (2015) Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline. Aging Cell 14(4):659–668. https://doi.org/10.1111/acel.12345
Moukhles H et al (1997) Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 76(4):1159–1171. https://doi.org/10.1016/s0306-4522(96)00452-6
Murata M (2013) Surface modification of liposomes using polymer-wheat germ agglutinin conjugates to improve the absorption of peptide drugs by pulmonary administration. J Pharm Sci 102(4):1281–1289. https://doi.org/10.1002/jps.23463
Nazar M et al (1999) The role of the hippocampus and 5-HT/GABA interaction in the central effects of benzodiazepine receptor ligands. J Neural Transm (vienna) 106(5–6):369–381. https://doi.org/10.1007/s007020050165
Packard MG, Knowlton BJ (2002) Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25:563–593. https://doi.org/10.1146/annurev.neuro.25.112701.142937
Paitel ER et al (2021) A systematic review of cognitive event-related potentials in mild cognitive impairment and Alzheimer’s disease. Behav Brain Res 396:112904. https://doi.org/10.1016/j.bbr.2020.112904
Patel AD et al (2010) Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol 69(12):1177–1190. https://doi.org/10.1097/NEN.0b013e3181fbf6d6
Paul CA et al (2008) Dissection of rat brains. CSH Protoc: pdb.prot4803. https://doi.org/10.1101/pdb.prot4803
Paxinos G, Watson C (2013) The rat brain in stereotaxic coordinates, 7th edn. Academic Press, Amsterdam
Pestereva N et al (2021) m-Calpain is released from striatal synaptosomes. Int J Neurosci. https://doi.org/10.1080/00207454.2021.1901697
Qiu X et al (2021) Inhibition of NLRP3 inflammasome by glibenclamide attenuated dopaminergic neurodegeneration and motor deficits in paraquat and maneb-induced mouse Parkinson’s disease model. Toxicol Lett 349:1–11. https://doi.org/10.1016/j.toxlet.2021.05.008
Ren A et al (2016) CD200 Inhibits inflammatory response by promoting KATP channel opening in microglia cells in Parkinson’s disease. Med Sci Monit 22:1733–41. https://doi.org/10.12659/msm.898400
Rosado AF et al (2021) Glibenclamide treatment prevents depressive-like behavior and memory impairment induced by chronic unpredictable stress in female mice. Behav Pharmacol 32(2&3):170–181. https://doi.org/10.1097/FBP.0000000000000599
Roth BL et al (2004) Serotonin receptors represent highly favorable molecular targets for cognitive enhancement in schizophrenia and other disorders. Psychopharmacology 174(1):17–24. https://doi.org/10.1007/s00213-003-1683-8
Sarookhani MR et al (2018) Involvement of adenosine triphosphate-sensitive potassium channels in the neuroprotective activity of hydrogen sulfide in the 6-hydroxydopamine-induced animal model of Parkinson’s disease. Behav Pharmacol 29(4):336–343. https://doi.org/10.1097/FBP.0000000000000358
Slingerland AS et al (2008) Sulphonylurea therapy improves cognition in a patient with the V59M KCNJ11 mutation. Diabet Med 25(3):277–281. https://doi.org/10.1111/j.1464-5491.2007.02373.x
Solé B et al (2017) Cognitive impairment in bipolar disorder: treatment and prevention strategies. Int J Neuropsychopharmacol 20(8):670–680. https://doi.org/10.1093/ijnp/pyx032
Soliman E et al (2020) Impact of some oral hypoglycemic agents on type 2 diabetes-associated depression and reserpine-induced depression in rats: the role of brain oxidative stress and inflammation. Naunyn Schmiedebergs Arch Pharmacol 393(8):1391–1404. https://doi.org/10.1007/s00210-020-01838-w
Sood A, Raji MA (2021) Cognitive impairment in elderly patients with rheumatic disease and the effect of disease-modifying anti-rheumatic drugs. Clin Rheumatol 40(4):1221–1231. https://doi.org/10.1007/s10067-020-05372-1
Stern SA, Alberini CM (2013) Mechanisms of memory enhancement. Wiley Interdiscip Rev Syst Biol Med 5(1):37–53. https://doi.org/10.1002/wsbm.1196
Stokum JA et al (2017) Glibenclamide pretreatment protects against chronic memory dysfunction and glial activation in rat cranial blast traumatic brain injury. Behav Brain Res 333:43–53. https://doi.org/10.1016/j.bbr.2017.06.038
Teixeira CM et al (2018) Hippocampal 5-HT input regulates memory formation and Schaffer collateral excitation. Neuron 98(5):992-1004.e4. https://doi.org/10.1016/j.neuron.2018.04.030
Tseilikman V et al (2020) High and low anxiety phenotypes in a rat model of complex post-traumatic stress disorder are associated with different alterations in regional brain monoamine neurotransmission. Psychoneuroendocrinology 117:104691. https://doi.org/10.1016/j.psyneuen.2020.104691
van de Giessen E et al (2017) Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry 22(1):68–75. https://doi.org/10.1038/mp.2016.21
Vorhees CV, Williams MT (2014) Assessing spatial learning and memory in rodents. ILAR J 55(2):310–332. https://doi.org/10.1093/ilar/ilu013
Wang FX et al (2020) Norepinephrine in the dentate gyrus is involved in spatial learning and memory alteration induced by chronic restraint stress in aged rats. NeuroReport 31(18):1308–1314. https://doi.org/10.1097/WNR.0000000000001547
Wilkinson L et al (2014) Probabilistic classification learning with corrective feedback is associated with in vivo striatal dopamine release in the ventral striatum, while learning without feedback is not. Hum Brain Mapp 35(10):5106–5115. https://doi.org/10.1002/hbm.22536
World Health Organization (1948) Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 13 March 2020
Xiao LY et al (2018) Acupuncture rescues cognitive impairment and upregulates dopamine-β-hydroxylase expression in chronic cerebral hypoperfusion rats. Biomed Res Int 2018:5423961. https://doi.org/10.1155/2018/5423961
Yoon SY et al (2013) Oroxylin A improves attention deficit hyperactivity disorder-like behaviors in the spontaneously hypertensive rat and inhibits reuptake of dopamine in vitro. Arch Pharmacal Res 36(1):134–140. https://doi.org/10.1007/s12272-013-0009-6
Zameer S et al (2019) Behavioral experimental paradigms for the evaluation of drug’s influence on cognitive functions: interpretation of associative, spatial/nonspatial and working memory. CNS Neurol Disord Drug Targets 18(3):185–204. https://doi.org/10.2174/1871527318666190112143834
Zhang L et al (2020) Neuroprotective effect of different physical exercises on cognition and behavior function by dopamine and 5-HT level in rats of vascular dementia. Behav Brain Res 388:112648. https://doi.org/10.1016/j.bbr.2020.112648
Zubov A et al (2020) Glibenclamide as a neuroprotective antidementia drug. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2020.1789170
Funding
This work was supported by the Russian Foundation for Basic Research under Grant 20–015-00168.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zubov, A., Ivleva, I., Pestereva, N. et al. Glibenclamide alters serotonin and dopamine levels in the rat striatum and hippocampus, reducing cognitive impairment. Psychopharmacology 239, 2787–2798 (2022). https://doi.org/10.1007/s00213-022-06159-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06159-9