Abstract
In previous studies we have demonstrated that the γ-aminobutryic acid-A (GABA-A) receptor antagonist oroxylin A has an awakening effect and it also represses ADHD-like behaviors (hyperactivity, impulsivity and inattention) in the spontaneously hypertensive rat (SHR) model of attention-deficit hyperactivity disorder (ADHD). We hypothesized that the effects of oroxylin A were exerted via the GABA-A receptor given the important role of the GABAergic system in ADHD. However, it is possible that aside from the GABAergic system, oroxylin A may influence other systems especially those implicated in ADHD (e.g. DAergic, etc.). To test this hypothesis, we evaluated the effects of GABA agonist, or dopamine (DA) antagonist in oroxylin A-induced alleviation of ADHD-like behaviors in SHR. SHR showed inattention and impulsivity as measured by the Y-maze and the electro-foot shock aversive water drinking tests, respectively. Oroxylin A significantly improved these behaviors, furthermore, its effect on SHR impulsivity was attenuated by haloperidol, a DA antagonist, but not by baicalein, an agonist of the GABA-A receptor. In vitro studies showed that oroxylin A inhibited DA uptake similar to methylphenidate, a dopamine transporter blocker, but did not influence norepinephrine uptake unlike atomoxetine, a selective NE reuptake inhibitor. Collectively, the present findings suggest that oroxylin A improves ADHD-like behaviors in SHR via enhancement of DA neurotransmission and not modulation of GABA pathway as previously reported. Importantly, the present study indicates the potential therapeutic value of oroxylin A in the treatment of ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a heterogeneous developmental disorder characterized by three core symptoms: hyperactivity, impulsiveness, and sustained inattention (Dellu-Hagedorn et al. 2004; Mannuzza et al. 1993). Although it is considered as the most commonly diagnosed neurobehavioural disorder in childhood, in many cases, it also persists into adolescence and adulthood (Kessler et al. 2006; Mannuzza et al. 1993). Epidemiologic data indicated that the prevalence of ADHD is approximately 5–8 % worldwide (Kessler et al. 2006; Wilens et al. 2002).
A number of theories have been put forward to explain the origin of ADHD symptoms. The dynamic developmental theory explains that ADHD is caused by a dysfunction in dopamine (DA) neurotransmission resulting in alterations in reinforcement and extinction processes, producing the behavioral aberrations seen in this disorder (Sagvolden et al. 2005a, b, 2009). Furthermore, the role of DA in the etiology of ADHD has been suggested based on findings of molecular genetic studies, neuroimaging studies of patients, neuropharmacology of psychostimulants prescribed to treat ADHD, and behavior and biochemistry of animal models (for review see van der Kooij and Glennon 2007).
Stimulants such as methylphenidate and amphetamines are the most widely used medications approved by the US Food and Drug Administration for the treatment of ADHD in children (Findling 2008). Methylphenidate, the most prescribed stimulant ADHD drug, causes behavioral normalization by enhancing DA levels in the striatum and nucleus accumbens (NAc) by targeting and blocking the actions of the DA transporter (DAT). Although methylphenidate is efficacious in 70–80 % of ADHD patients (Spencer et al. 2004; Elia et al. 1999), the major concern with the use of this drug is the potential for diversion and abuse (Heal et al. 2009). More recently, the norepinephrine (NE) reuptake inhibitor, atomoxetine has been introduced for ADHD treatment. Several trials, however, indicated the inferiority of atomoxetine to psychostimulants on efficacy endpoints (Heal et al. 2009). The weak selective DA reuptake inhibitor, bupropion has also been used for treating ADHD, but it only showed very moderate efficacy (Conners et al. 1996; Kuperman et al. 2001). Therefore, there is currently substantial interest in developing alternative ADHD therapies which effectively alleviate ADHD outcomes without causing adverse behavioral effects.
The spontaneously hypertensive rat (SHR), bred from normotensive Wistar Kyoto rat (WKY) strain initially proposed as an animal model of hypertension, is the most validated animal model of ADHD (Sagvolden et al. 2005a, b). The SHR shows good face, construct and predictive validity, displaying the behavioral characteristics of ADHD (hyperactivity, impulsivity and inattention) (Russell et al. 2005). Furthermore, DA functioning was found to be downregulated, i.e., electrical and chemical stimulation in mesolimbic, mesocortical and nigrostriatal DAergic pathways were attenuated in SHR (Linthorst et al. 1990; Russell et al. 1998, 2000). Also, drugs used in the pharmacological treatment of ADHD, usually catecholamine agonists, have been shown to reduce ADHD-like behaviors in this animal model (Sagvolden and Xu 2008; Sagvolden 2011).
Oroxylin A is a flavonoid isolated from the root of Scutellaria baicalensis Georgi. It has antioxidant (Jiwajinda et al. 2002), anti-inflammatory (Chen et al. 2000), and anti-allergy effects (Taniguchi et al. 2000) and ameliorates memory impairment and neuronal damage induced by transient cerebral hypo-perfusion (Kim et al. 2006), scopolamine (Kim et al. 2007) or Aβ (25–35) (Kim et al. 2008). Heun et al. (2003) demonstrated through receptor binding studies that oroxylin A acts as a γ-aminobutyric acid-A (GABA-A) receptor antagonist. Our previous studies showed the awakening effect of oroxylin A as it significantly improved motor coordination in rota-rod test and decreased thiopental sodium-induced sleeping time (Park et al. 2006). Moreover, we have also demonstrated that oroxylin A attenuated hyperactivity and motor inattention in SHR (Yoon et al. 2008). Thus, given the important role of the GABAergic system in ADHD (Brummelte et al. 2008), we hypothesized that the ameliorating effects of oroxylin A on ADHD-like behaviors in SHR were exerted via antagonism of the GABA-A receptor. However, it is also possible that aside from GABAergic system, oroxylin A may influence other systems especially those implicated in ADHD (e.g. DAergic, etc.). To test this hypothesis, we evaluated the effects of GABA agonist, or DA antagonist in oroxylin A-induced alleviation of ADHD-like behaviors in SHR.
Materials and methods
Animals
We used male SHR and WKY (4 weeks of age) obtained from Hanlim Laboratory Animals Co. (Hwasung, Korea). All animals were maintained on a standard light–dark cycle, at ambient temperature (22 ± 2 °C) and humidity (55 ± 5 %) with free access to chow pellets and water. All animals were acclimated to their home cages for at least 7 days before testing. The experimental groups, consisting of 8–10 animals per drug and dose, were chosen by means of a randomized schedule. All tests took place between 9:00 and 18:00 h. Animal treatment and maintenance were carried out in accordance with the Principle of Laboratory Animal Care (NIH publication No. 85-23 revised 1985) and the Animal Care and Use Guidelines of Sahmyook University, Korea.
Materials
Oroxylin A and baicalein were obtained from the National Center for Standardization of Herbal Medicine. Haloperidol was purchased from Sigma-Aldrich Co. Methylphenidate was generously supplied by Hwanin Pharm. Co. Ltd. Oroxylin A and baicalein were dissolved in DMSO, and suspended in a 10 % solution of cremophor. Methylphenidate and haloperidol were dissolved in physiologic saline (0.9 % w/v of NaCl).
Y-maze test: attention
Spontaneous alternation behavior requires attention (Katz and Schmaltz 1980) and working memory (Sarter et al. 1988). Y-maze test was used to identify the influence of oroxylin A on sustained inattention behavior of the SHR. The methods used were similar to those described previously (Sarter et al. 1988) with some modifications. Each arm of the Y-maze was 45 cm long, 10 cm wide, and 20 cm high, and both arms were positioned at equal angles. Thirty minutes after saline or drug [oroxylin A or methylphenidate, intraperitoneal (i.p.)] administration, rats were placed individually at the end of an arm and allowed to enter the maze freely for an 8-min test session. An arm entry was defined as the entry of all four paws and the tail into one arm. The sequence of arm entries was recorded using automated systems (Ethovision system Noldus IT b.v., Netherlands). The alternation behavior (actual alternations) was defined as the consecutive entry into three arms, i.e., the combination of three different arms, with stepwise combinations in the sequence. The maximum number of alternations was considered as the total number of arms entered minus 2, and the percentage of alternation behavior was calculated as (actual alternations/maximum alternations) × 100.
Electro-foot shock aversive water drinking test: impulsivity
Impulsivity tests were conducted in a test box described previously (Kim et al. 2012). The methods of the impulsivity tests were patterned after those described by Kim et al. (2012) with some modifications. Tests consisted of a training phase for 1 day and a test phase. In order to increase motivation to drink water, rats were administered orally with 1 ml of 8 % NaCl solution. During the test phase, saline-or drug- (oroxylin A or methylphenidate, i.p.) administered rats were tested for impulsive behaviors, i.e., the persistence to drink water despite presentation of an electroshock (50 pulses/s, 0.5 ms of pulse width, 4 mA). In some tests, the effects of DAergic antagonism or GABA-A receptor modulation on oroxylin A-induced alleviation of SHR impulsivity were investigated. Haloperidol (0.005 mg/kg, i.p.), a DA antagonist, or baicalein (5 mg/kg, i.p.), a GABA agonist, was injected 20 min before administering oroxylin A. Experiments were conducted as described above.
Intracellular DA or NE uptake assay
HEK-293 cells were transiently transfected with human DA transporter in pcDNA 3.1. After 48 h of transfection, the medium was removed and cells were treated with test compounds for 30 min. The uptake was measured following incubation of cells for 5 min with 250 ml of uptake buffer (5 mM Tris base, 7.5 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM ascorbic acid, and 5 mM glucose, pH 7.1) containing 20 nM [3H]DA. After rinsing with 1 ml of uptake buffer, cells were solubilized in 0.5 ml of 1 % SDS, and the radioactivity incorporated into the cells was measured by liquid scintillation counting. For NE uptake assay, HEK-293 cells were transfected with the cDNA of human NE transporter. Uptake procedure was virtually the same as DA uptake assay except that 20 nM [3H]NE was used instead of [3H]DA.
Statistical analysis
All data were expressed as the mean ± SEM. One-way ANOVA was used for statistical analysis. When significant differences were found, Newman–Keuls test was used as a post hoc test. Differences were considered statistically significant when p < 0.05.
Results
Oroxylin A improves inattention in SHR
Figure 1a shows marked inattention in SHR as evidenced by lower spontaneous alternation behavior (%) compared with WKY. SHR also showed significant increase in total arm entries compared with WKY (Fig. 1b). Oroxylin A (5 and 10 mg/kg) significantly increased spontaneous alternation in SHR and also in WKY (Fig. 1a). Treatment with oroxylin A did not affect total arm entries of either strain (Fig. 1b). On the other hand, methylphenidate (2 mg/kg) significantly improved spontaneous alternation behavior in SHR (Fig. 1a).
Effects of oroxylin A on a spontaneous alternation behavior and b total arm entries in SHR and WKY. Oroxylin A (1, 5 and 10 mg/kg, i.p.) or methylphenidate (MPH, 2 mg/kg, i.p.) was administered 30 min prior to tests. Data are expressed as the mean ± S.E.M. # p < 0.05, versus WKY, *p < 0.05 versus control, n = 9 animals per group
Oroxylin A reduces impulsiveness in SHR: effects of baicalein and haloperidol
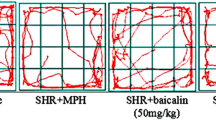
As shown in Fig. 2, SHR showed marked levels of impulsivity as indicated by more number of drinking attempts (frequency in the water area, Fig. 2a) and also drinking frequency (Fig. 2b) compared with WKY. Oroxylin A (1 and 5 mg/kg) significantly decreased drinking attempts and drinking frequency in SHR but not in WKY. In a similar fashion, methylphenidate (2 mg/kg) reduced impulsivity in SHR but not in WKY. Figure 3 shows that the GABA-A agonist baicalein (5 mg/kg, i.p.) did not block the effects of oroxylin A but enhanced further its efficacy in alleviating impulsiveness in SHR. On the other hand, the DA receptor antagonist haloperidol (0.005 mg/kg) inhibited the effects of oroxylin A. These results suggest that behavioral effects of oroxylin A were mediated through the DAergic system.
Effects of oroxylin A on a water area frequency and b drinking frequency in SHR and WKY. Oroxylin A (1 and 5 mg/kg, i.p.) or methylphenidate (MPH, 2 mg/kg, i.p.) was administered 30 min prior to tests. Data are expressed as the mean ± S.E.M. ## p < 0.01, versus WKY, *p < 0.05, **p < 0.01 versus control, n = 9 animals per group
Effects of baicalein or haloperidol on impulsive behavior in SHR. Impulsivity in the SHR is defined by more number of drinking attempts (frequency in the water area) compared with WKY. Baicalein (5 mg/kg, i.p.) or haloperidol (5 mg/kg, i.p.) was given 20 min before oroxylin A injection. # p < 0.05 versus oroxylin A; **p < 0.01, versus control, n = 9 animals per group
Oroxylin A selectively inhibits DA reuptake
Figure 4a shows that oroxylin A concentration-dependently inhibited intracellular DA uptake similar to methylphenidate, a DAT blocker. Atomoxetine, a selective NE inhibitor, also inhibited DA uptake coinciding with the results of a previous study (Bymaster et al. 2002). However, unlike atomoxetine, oroxylin A did not influence NE uptake (Fig. 4b).
Discussion
Hyperactivity, sustained inattention, and impulsivity are the main clinical symptoms of ADHD (American Psychiatric Association 1994). In the present and previous study (Yoon et al. 2008), we have demonstrated that the SHR, considered as the most validated animal model of ADHD (Sagvolden et al. 2009), showed overactivity, inattentiveness and impulsivity relative to its normotensive progenitor strain, the WKY. More importantly, we have also shown that oroxylin A alleviated ADHD-like behaviors SHR. Furthermore, the effects of oroxylin A on SHR impulsivity were attenuated by haloperidol, a DA antagonist, but not by baicalein, an agonist of GABA receptor. In vitro studies showed that oroxylin A selectively blocked DA reuptake, a mechanism exerted by typical ADHD drugs (e.g. psychostimulants such as methylphenidate and amphetamines).
The prior studies conducted by Huen et al. (2003) found that oroxylin A is a GABA-A antagonist. Because GABA is the main inhibitory neurotransmitter in the central nervous system (Roberts 1986), GABA antagonism produces stimulatory effects on behavior as exemplified by the effects of the GABA antagonists such as bicuculline and flumazenil (for review see Armijo et al. 2005). Furthermore, GABA antagonists have been explored as potential ADHD pharmacotherapies (Miyazaki et al. 2006; Weisler 2007), given the roles of GABAergic dysfunctions in the pathogenesis of ADHD and the interconnectivity between GABAergic and DAergic systems (Brummelte et al. 2008). Indeed, we have shown the potential therapeutic value of oroxylin A as an ADHD drug as it ameliorated ADHD-like behaviors of SHR. We noticed, however, that haloperidol but not baicalein blocked the effects of oroxylin A in the impulsivity test. This indicates that enhancement of DA neurotransmission but not GABA-A antagonism could be suggested as the mechanism of oroxylin A-induced behavioral improvement in SHR.
DA exerts powerful control over both motor and cognitive behavior (Carlsson 1987). The contribution of DAergic dysfunction in the production of ADHD outcomes has been reviewed elsewhere (Sagvolden et al. 2005a, b, 2009). In the NAc and frontal cortex, DA is released by vesicular fusion mechanisms and is cleared by cocaine-sensitive DAT. Methylphenidate and amphetamine, the most prescribed stimulant drugs for ADHD, target the DAT via different mechanisms (Volkow et al. 2001). Considering that the effects of oroxylin A were mediated by enhancement of DA neurotransmission, we conducted in vitro studies to determine the potential underlying mechanism. The results showed that oroxylin A inhibited DA uptake similar to methylphenidate, but did not influence NE uptake unlike atomoxetine. This suggests that oroxylin A may be a DAT blocker although further studies are required to confirm this. Furthermore, it would also be beneficial to demonstrate in vivo the DAT function or properties which are influenced by oroxylin A. In case of methylphenidate, chronic exposure to this drug has been shown to reduce DAT density in SHR (Roessner et al. 2010; Simchon et al. 2010). Elevated DAT density has been observed in untreated children and adults with ADHD (Spencer et al. 2005; Krause 2008; Weiss et al. 2003) and also in adolescent SHR (Roessner et al. 2010). The ability of methylphenidate to reduce high DAT density in SHR (Roessner et al. 2010; Simchon et al. 2010) as well as in individuals with ADHD (Vles et al. 2003) has been considered as a potential mechanism underlying the beneficial effects of this drug.
One concern with the use pharmaceutical stimulants in ADHD is the abuse and dependence potential of these drugs given their pharmacological actions on neurotransmitters, especially DAergic systems (Kaye and Darke 2012). Although the safety profile of short-term stimulant (e.g. methylphenidate) therapy in clinical trials has been well established, the long-term effects of these drugs are not yet very much understood (Marco et al. 2011). In preclinical studies, we have demonstrated, however, that chronic methylphenidate treatment in SHR did not enhance self-administration of the drug indicating the lack of psychological dependence following chronic methylphenidate use (dela Peña et al. 2011). While this finding may suggest the safety of methylphenidate as an ADHD intervention, of course, other factors need to be considered as well as the effects of chronic methylphenidate treatment on responsivity to other drugs of abuse. Since oroxylin A shares mechanism of action similar to methylphenidate, the next subject of research is to identify the potential side effects of the compound if used as an ADHD intervention.
In summary, we have shown that oroxylin A improved ADHD-like behaviors in SHR through modulation of the DAergic pathway probably via blockade of the DAT. These findings demonstrate the therapeutic value of oroxylin A as an alternative drug for the treatment of ADHD.
References
Armijo, J.A., M. Shushtarian, E.M. Valdizan, A. Cuadrado, I. de las Cuevas, and J. Adín. 2005. Ion channels and epilepsy. Curr Pharm Des 11(15): 1975–2003.
American Psychiatric Association. 1994. Diagnostic and statistical manual of mental disorders: DSM-IV. 4, 78–85. Washington, D.C.: American Psychiatric Association.
Brummelte, S., T. Grund, G.H. Moll, G. Teuchert-Noodt, and R.R. Dawirs. 2008. Environmental enrichment has no effect on the development of dopaminergic and GABAergic fibers during methylphenidate treatment of early traumatized gerbils. Journal of Negative Results in BioMedicine 7: 2.
Bymaster, F.P., J.S. Katner, D.L. Nelson, S.K. Hemrick-Luecke, P.G. Threlkeld, J.H. Heiligenstein, S.M. Morin, D.R. Gehlert, and K.W. Perry. 2002. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27(5): 699–711.
Carlsson, A. 1987. Perspectives on the discovery of central monoaminergic neurotransmission. Annual Review of Neuroscience 10: 19–40.
Chen, Y.C., L.L. Yang, and T.J. Lee. 2000. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-kappaB activation. Biochemical Pharmacology 59(11): 1445–1457.
Conners, C.K., C.D. Casat, C.T. Gualtieri, E. Weller, M. Reader, A, Reiss, R.A. Weller, M, Khayrallah, and J, Ascher. 1996. Bupropion hydrochloride in attention deficit disorder with hyperactivity. Journal of the American Academy of Child & Adolescent Psychiatry 35: 1314–1321.
dela Peña, I.C., S.Y. Yoon, J.C. Lee, J.B. dela Peña, A.R. Sohn, J.H. Ryu, C.Y. Shin, and J.H. Cheong. 2011. Methylphenidate treatment in spontaneously hypertensive rat: Influence on methylphenidate self-administration and reinstatement in comparison with Wistar rats. Psychopharmacology 221(2): 217–226.
Dellu-Hagedorn, F., S. Trunet, and H. Simon. 2004. Impulsivity in youth predicts early age-related cognitive deficits in rats. Neurobiology of Aging 25: 525–537.
Elia, J., P.J. Ambrosini, and J.L. Rapoport. 1999. Treatment of attention-deficit hyperactivity disorder. New England Journal of Medicine 11(340): 780–788.
Findling, R. 2008. Evolution of the treatment of attention-deficit/hyperactivity disorder in children: A review. Clinical Therapeutics 30(5): 942–957.
Heal, D.J., S.C. Cheetham, and S.L. Smith. 2009. The neuropharmacology of ADHD drugs in vivo: Insights on efficacy and safety. Neuropharmacology 57: 608–618.
Jiwajinda, S., V. Santisopasri, A. Murakami, O.K. Kim, H.W. Kim, and H. Ohigashi. 2002. Suppressive effects of edible Thai plants on superoxide and nitric oxide generation. Asian Pacific Journal of Cancer Prevention 3(3): 215–223.
Kim, D.H., S.J. Jeon, K.H. Son, J.W. Jung, S. Lee, B.H. Yoon, J.J. Lee, Y.W. Cho, J.H. Cheong, K.H. Ko, and J.H. Ryu. 2007. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobio Learning Memory 87(4): 536–546.
Kim, D.H., S. Jeon, K. Son, J. Jung, S. Lee, B. Yoon, J. Choi, J. Cheong, K.H. Ko, and J.H. Ryu. 2006. Effect of the flavonoid, oroxylin A, on transient cerebral hypoperfusion-induced memory impairment in mice. Pharmacology, Biochemistry and Behavior 85: 658–668.
Kim, D.H., S. Kim, S. Jeon, K.H. Son, S. Le, B. Yoon, J.H. Cheong, K.H. Ko, and J.H. Ryu. 2008. The effects of acute and repeated oroxylin A treatments on Abeta (25–35)-induced memory impairment in mice. Neuropharmacology 55: 639–647.
Katz, R.J., and K. Schmaltz. 1980. Dopaminergic involvement in attention: A novel animal model. Progress in Neuropsychopharmacology 4: 585–590.
Kessler, R.C., L. Adler, R. Barkley, J. Biederman, C.K. Conners, O. Demler, et al. 2006. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. American Journal of Psychiatry 163(4): 716–723.
Krause, J. 2008. SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder. Expert Review of Neurotherapeutics 8: 611–625.
Kuperman, S., P.J. Perry, G.R. Gaffney, B.C. Lund, K.A. Bever-Stille, S. Amdt, T.L. Holman, D.J. Moser, and J.S. Paulsen. 2001. Bupropion SR vs. methylphenidate vs. placebo for attention deficit hyperactivity disorder in adults. Annals of Clinical Psychiatry 13(3): 129–134.
Kim, P., I. Choi, I.C. dela Pena, H.J. Kim, K.J. Kwon, J.H. Park, S. Han, J.H. Ryu, J.H. Cheong, and C.Y. Shin. 2012. A simple behavioral paradigm to measure impulsive behavior in an animal model of Attention deficit hyperactivity disorder (ADHD) of the spontaneously hypertensive rats. Biomolecules & Therapeutics 20(1): 125–131.
Kaye, S., and S. Darke. 2012. The diversion and misuse of pharmaceutical stimulants: What do we know and why should we care? Addiction 107: 467–477.
Huen, M.S., J.W. Leung, W. Ng, W.S. Lui, M.N. Chan, J.T. Wong, et al. 2003. 5,7-Dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from Scutellaria baicalensis Geogi with selective antagonistic properties. Biochemical Pharmacology 66(1): 125–132.
Linthorst, A.C., M. van den Buuse, W. De Jong, and D.H. Versteeg. 1990. Electrically-stimulated [3H]dopamine and [14C]acetylcholine release from nucleus caudatus slices: Differences between spontaneously hypertensive rats and Wistar–Kyoto rats. Brain Research 509: 266–272.
Mannuzza, S., R.G. Klein, A. Bessler, P. Malloy, and M. LaPadula. 1993. Adult outcome of hyperactive boys: Educational achievement, occupational rank and psychiatric status. Archives of General Psychiatry 50: 565–576.
Marco, E.M., W. Adriani, L.A. Ruocco, R. Canese, A.G. Sadile, and G. Laviola. 2011. Neurobehavioral adaptations to methylphenidate: The issue of early adolescent exposure. Neuroscience and Biobehavioral Reviews 35: 1722–1739.
Miyazaki, M., H. Ito, T. Saijo, K. Mori, S. Kagami, and Y. Kuroda. 2006. Favorable response of ADHD with giant SEP to extended release valproate. Brain Development 28: 470–472.
Park, H.G., J.Y. Choi, G.S. Lee, J.H. Choi, K.H. Son, H.S. Ko, K.H. Ko, J.H. Ryu, and J.H. Cheong. 2006. Different effects of flavonoids in Scutellaria baicalensis on anxious and sedative behaviors. Toxicology and Applied Pharmacology 14: 83–89.
Roberts E. 1986. GABA: the road to neurotransmitter status. In Benzodiazepine/GABA receptors and chloride channels: Structural and functional properties, Olsen, R.W. & Venter, J.C. eds, 1–39. New York: Liss.
Roessner, V., T. Sagvolden, T. Dasbanerjee, F.A. Middleton, S.V. Faraone, S.I. Walaas, A. Becker, A. Rothenberger, and N. Bock. 2010. Methylphenidate normalizes dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience 167(4): 1183–1191.
Russell, V., A. de Villiers, T. Sagvolden, M. Lamm, and J. Taljaard. 1998. Differences between electrically-, ritalin- and d-amphetamine-stimulated release of [3H]dopamine from brain slices suggest impaired vesicular storage of dopamine in an animal model of attention-deficit hyperactivity disorder. Behavioural Brain Research 94(1): 163–171.
Russell, V.A., A.S. de Villiers, T. Sagvolden, M.C. Lamm, and J.J. Taljaard. 2000. Methylphenidate affects striatal dopamine differently in an animal model for attention deficit/hyperactivity disorder—The spontaneously hypertensive rat. Brain Research Bulletin 53: 187–192.
Russell, V.A., T. Sagvolden, and E.B. Johansen. 2005. Animal models of attention-deficit hyperactivity disorder. Behavioral and Brain Functions 1: 9.
Sagvolden, T. 2011. Impulsiveness, overactivity and poor sustained attention improve by chronic treatment with low doses of l-amphetamine in an animal model of attention-deficit/hyperactivity disorder (ADHD). Behavioral and Brain Functions 7: 6.
Sagvolden, T., E.B. Johansen, H. Aase, and V.A. Russell. 2005a. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences 28: 397–419.
Sagvolden, T., V.A. Russell, H. Aase, E.B. Johansen, and M. Farshbaf. 2005b. Rodent models of attention-deficit/hyperactivity disorder. Biological Psychiatry 57: 1239–1247.
Sagvolden, T., E.B. Johansen, G. Wøien, S.I. Walaas, J. Storm-Mathisen, L.H. Bergersen, O. Hvalby, V. Jensen, H. Aase, V.A. Russell, P.R. Killeen, T. Dasbanerjee, F.A. Middleton, and S.V. Faraone. 2009. The spontaneously hypertensive rat model of ADHD—The importance of selecting the appropriate reference strain. Neuropharmacology 57(7–8): 619–626.
Sagvolden, T., and T. Xu. 2008. l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of attention-deficit/hyperactivity disorder (ADHD). Behavioral and Brain Functions 23(4): 3.
Sarter, M., G. Bodewitz, and D.N. Stephens. 1988. Attenuation of scopolamine-induced impairment of spontaneous alternation behavior by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology 94: 491–495.
Simchon, Y., A. Weizman, and M. Rehavi. 2010. The effect of chronic methylphenidate administration on presynaptic dopaminergic parameters in a rat model for ADHD. European Neuropsychopharmacology 20(10): 714–720.
Spencer, T., J. Biederman, B.K. Madras, S.V. Faraone, D.D. Dougherty, A.A. Bonab, and A.J. Fischman. 2005. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: A focus on the dopamine transporter. Biological Psychiatry 57: 1293–1300.
Spencer, T., J. Biederman, and T. Wilens. 2004. Stimulant treatment of adult attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America 27(2): 361–372.
Taniguchi, C., M. Homma, O. Takano, T. Hirano, K. Oka, Y. Aoyagi, T. Niitsuma, and T. Hayashi. 2000. Pharmacological effects of urinary products obtained after treatment with saiboku-to, a herbal medicine for bronchial asthma, on type IV allergic reaction. Planta Medica 66(7): 607–611.
van der Kooij, M.A., and J.C. Glennon. 2007. Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews 31: 597–618.
Vles, S., F.J. Feron, J.G. Hendriksen, J. Jolles, M.J. van Kroonenburgh, and W.E. Weber. 2003. Methylphenidate down-regulates the dopamine receptor and transporter system in children with attention deficit hyperkinetic disorder (ADHD). Neuropediatrics 34: 77–80.
Volkow, N.D., G. Wang, J.S. Fowler, J. Logan, M. Gerasimov, L. Maynard, Y. Ding, S.J. Gatley, A. Gifford, and D. Franceschi. 2001. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. The Journal of Neuroscience 21: RC121.
Weisler, R.H. 2007. Emerging drugs for attention-deficit/hyperactivity disorder. Expert Opinion on Emerging Drugs 12: 423–434.
Weiss, M., D. Worling, and M. Wasdell. 2003. A chart review study of the inattentive and combined types of ADHD. Journal of Attention Disorders 7: 1–9.
Wilens, T.E., J. Biederman, and T.J. Spencer. 2002. Attention deficit/hyperactivity disorder across the lifespan. Annual Review of Medicine 53: 113–131.
Yoon, S.Y., M.S. Chun, Y.S. Lee, H.I. Park, C.Y. Shin, J.H. Ryu, and J.H. Cheong. 2008. The Scutellaria flavones, oroxylin A, improves attention-deficit/hyperactivity disorder related behaviors in spontaneously hypertensive rats. Biomolecules & Therapeutics 16: 343–350.
Acknowledgments
This research was supported by grants funded by the Korean Health Technology R&D Project, Ministry of Health and Welfare (A120013) and Sahmyook University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Seo Young Yoon and Ike dela Peña contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yoon, S.Y., dela Peña, I., Kim, S.M. et al. Oroxylin A improves attention deficit hyperactivity disorder-like behaviors in the spontaneously hypertensive rat and inhibits reuptake of dopamine in vitro. Arch. Pharm. Res. 36, 134–140 (2013). https://doi.org/10.1007/s12272-013-0009-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0009-6