Abstract
Methamphetamine (MA) alters dopamine markers and cognitive function in heavy users. In rodents, there are MA dosing regimens that induce concordant effects using repeated administration at spaced intervals. These regimens are effective but complicate experiments designed to disentangle the effects of the drug on different brain regions in relation to their cognitive effects because of treatment spacing. In an effort to simplify the model, we tested whether a single dose of MA could induce the same monoamine and cognitive effects as multiple, spaced dosing without affecting survival. Adult male Sprague-Dawley rats were treated with 40 mg/kg MA subcutaneously once and tested starting 2 weeks later. MA-treated rats showed deficits in egocentric navigation in Cincinnati water maze, in spatial navigation in the Morris water maze, and in choosing a consistent problem-solving strategy in the Star water maze when given the option to show a preference. MA-treated rats had persistent dopamine and serotonin reductions in the neostriatum and nucleus accumbens, and serotonin reductions in the hippocampus of the same magnitude as in repetitive treatment models. The data demonstrate that a single dose recapitulates the neurocognitive and monoamine effects of multiple-dose regimens, thereby simplifying the model of MA-induced neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic methamphetamine (MA) abuse results in cognitive deficits in attention, learning, and memory (Ghahremani et al. 2011; Simon et al., 2000; Moon et al. 2007; Salo et al. 2002; Volkow et al. 2001; Zhong et al. 2016). These deficits are accompanied by reductions in labeled ligand binding to the dopamine (DA) and serotonin (5-HT) transporters (DAT and SERT, respectively) in the caudate-putamen by positron emission tomography and postmortem reductions in striatal DA levels (Chang et al. 2007; Kish et al. 2009; McCann et al. 1998; Sekine et al. 2006; Volkow et al. 2001; Wilson et al. 1996). In rodents, as in human MA users, MA treatment reduces DAT and SERT (Friend and Keefe 2013; Gross et al. 2011) and depletes monoamines (Cappon et al. 2000; Fukumura et al. 1998; Herring et al. 2008b, 2010; Sonsalla et al. 1986). In rodents, MA also increases glial fibrillary acidic protein, causes argyrophilia as shown by silver staining, and increases Fluoro-jade labeling of dying cells (Herring et al. 2008b; Marshall et al. 2007; O’Callaghan and Miller 1994).
The most common neurotoxic dosing regimen is administration of three or four doses of MA spaced 2–3 h apart on a single day. These MA regimens induce cognitive impairments (Bortolato et al. 2009; Cheng et al. 2007; Daberkow et al. 2005; Gutierrez et al. 2017; Herring et al. 2008a; Heysieattalab et al. 2016; Marshall et al. 2007; Reichel et al. 2012; Schroder et al. 2003; Son et al. 2011; Vorhees et al. 2011). They also induce neurochemical changes associated with human MA neurotoxicity and cognitive deficits (see Gutierrez et al. (2017), Herring et al. (2008a), and Vorhees et al. (2011)). Understanding how MA-induced cognitive deficits relate to the neurochemical changes remains unclear because the effects occur concomitantly. Multi-dose regimens do not lend themselves to experiments aimed at dissecting which neurochemical changes are associated with which cognitive deficit. For this, a simpler model would be beneficial. Here, we present a single-dose model that induces the same effects as the multiple-dose models, both behaviorally and neurochemically.
Brain regions most affected by MA are the striatum and hippocampus. In order to assess these regions functionally, we focused on allocentric learning and memory that depends on the hippocampus and medial entorhinal cortex (Buzsaki and Moser 2013; Hafting et al. 2005; McNamara and Skelton 1993; Morris et al. 1982, 1986; O’Keefe and Nadel 1979) and egocentric learning and memory that depends on the striatum and dopamine (Braun et al. 2012, 2015, 2016; Cook and Kesner 1988; Fouquet et al. 2013; Howland et al. 2008; Kelley and Domesick 1982; Kesner et al. 1989; McGeorge and Faull 1989; Packard 2009; Thierry et al. 2000). For allocentric learning and memory, we used the Morris water maze (MWM), and for egocentric learning and memory, we used the Cincinnati water maze (CWM). The MWM and CWM are specific for each type of learning and memory, but are not suitable for determining the relative preference an animal has for one strategy versus the other. To determine if MA shifts this preference, we used a Star water maze (SWM; Fouquet et al. 2013; Rondi-Reig, 2006) that permits rats to choose which strategy to use.
Methods
Subjects
Adult Sprague-Dawley CD IGS male rats (251–275 g) were ordered from Charles River (strain 001, Charles River, Raleigh, NC). Rats were housed in pairs for a week following arrival to acclimate to the vivarium. Rats were then treated with MA or saline (SAL) and separated into individual cages to prevent MA-induced aggression or exaggerated MA-induced hyperthermia. All rats were tested in all three water mazes sequentially. Brain tissue was collected on all rats 24–48 h after completion of behavioral testing.
Drug Treatment and Temperature Monitoring
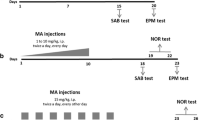
Three days prior to MA treatment, temperature transponders (IPTT-300; BMDS, Seaford, DE) were subcutaneously implanted in rats under isoflurane anesthesia. On the day of treatment, rats received a single injection of SAL (0.9% NaCl) or 40 mg/kg (+)-MA HCl (expressed as freebase and > 98% pure: Sigma, St. Louis, MO) in saline. The dosing volume for both groups was 3 mL/kg. Body temperatures were taken prior to drug treatment and every 30 min thereafter for 8 h. Treatment was conducted with rats housed in cages placed inside infant incubators with ambient temperature maintained at 24.0 ± 0.5 °C for the 8-h monitoring period. If a rat reached a temperature of 41.0 °C or above, it was placed in a cage with shallow water to dissipate excess heat. This intervention is effective in preventing hyperthermia-induced death without interfering with monoamine changes (Herring et al. 2008a). Rats were allowed 14 days of recovery after treatment before testing began. The experimental design is shown in Fig. 1.
Experimental design: Following a week of acclimation to the vivarium, rats were treated with a single injection of (+)-methamphetamine, 40 mg/kg. Two weeks later, they began behavioral testing. All rats received all tests sequentially and were then euthanized and brains taken for regional monoamine determination
Straight Channel
Because of the complexity of the CWM, rats require training to acclimate them to swimming and to find a submerged platform. Therefore, rats were tested in a straight water channel (244 × 15 × 50 cm) for four trials. This procedure exposes rats to swimming for the first time, teaches them that the hidden platform at the opposite end is the escape, and provides an independent measure of swim speed and motivation to escape by timing their transit time prior to being placed in the maze.
Egocentric Learning and Memory
Egocentric learning and memory was tested in the CWM for 18 days (Herring et al. 2008a; Vorhees et al. 2008; Vorhees and Williams 2016). The CWM is a multiple T-maze made of gray PVC with ten T-shaped cul-de-sacs branching from a central corridor. The maze is 50 cm deep and filled with water to a depth of 25 cm. The escape platform was submerged 1.5 cm below the surface. To ensure that rats could not use distal cues, they were tested under infrared light. Rats were given two trials per day for up to 5 min per trial. If a rat found the platform in <5 min, it was given its second trial of the day within 30 s. If a rat reached the 5 min time limit on trial 1, it was given 5–10 min before trial 2. After their trials, rats were placed in a cage with thick towels to wick away excess water and allowed to dry before being moved to their housing room. The dependent measures are errors and latency to escape. An error is head and front leg entry beyond the boundary that marks the beginning of a stem or arm, or return to the start arm.
Learning Strategy
Testing in the eight-arm SWM (Fig. 2) began the day following CWM. Rats were tested for 20 days, two trials/day. The maze was adapted from Rondi-Reig et al. (2006). The purpose of this test was to assess strategy preference. The SWM is designed such that a rat can choose whether to reach the goal on probe trials by whichever strategy it prefers, unlike the other mazes that assess only one strategy at a time. Hence, the SWM was intended to determine if MA treatment altered the rat’s preferred strategy. The apparatus is 210 cm in diameter, 51 cm deep, and filled with water to a depth of 20 cm. The maze has eight connected arms with an octagonal center hub. The room had numerous cues on the walls surrounding the maze. Testing was in two phases: Over the 20 days, there were four training days followed by one probe day; this sequence was repeated four times.
Star water maze: Rats started in Arm-0 during training. On probe trials, rats started from Arm-5. Proximal cues were moved on the center octagon on probe trials so they faced the opposite way as they had during training relative to the rat’s start position. On probe trials, an egocentric strategy would cause the rat to end in Arm-0; a cued strategy would cause the rat to end in Arm-2; a spatial strategy would cause the rat to end in Arm-3. Any other pattern would result in “undetermined” meaning no discernable strategy was used
Training
Rats were initially placed in the start (Arm-0) and allowed 90 s to find the hidden platform located in Arm-3. The intertrial interval (ITI) was 10 s on the platform. If a rat failed to find the platform within 90 s, it was placed there for 10 s. Latency to the hidden platform and errors (entry into arms without the platform) were recorded.
Probe
On days 5, 10, 15, and 20, rats were given one training trial prior to the probe trial. For the probe, rats were started from a novel location (Arm-5) to determine what strategy they used (see below). Platforms were placed in all the other arms. Right and left proximal cues were mounted on the hub walls facing Arm-0 and Arm-5 (but left-right reversed). Rats were then given an additional training trial, starting from Arm-0, with proximal cues returned to their original training positions and with all platforms removed except for the Arm-3 platform. As with the original protocol, this was done in order to reinforce the location of the Arm-3 platform when starting from Arm-0.
Strategies
Learning criteria were set so that only strategies that met the criteria were considered. Those strategies that did not meet the criteria were categorized under undetermined. For the initial learning trials, the criterion was set to at least four successful training trials out of eight. A successful trial was where the rat found the platform without committing more than one error. After the first probe, the criterion was increased to seven out of the eight trials before each of the probe trials on days 10, 15, and 20. Sequential egocentric navigation was identified if a rat used the same sequence of turns that it used to get to Arm-5 and therefore ended in Arm-0. If the rat navigated by the reversed proximal cues on the hub, it would turn right (rather than left, as was the cued path during training), would swim past Arm-1, and would end in Arm-2; this was defined as the guidance method. If the rat swam to Arm-3, it used a spatial strategy, navigating using distal room cues. If a rat went to any other arm, this was defined as undetermined.
Allocentric Learning and Memory
Allocentric navigation testing was conducted in a 244-cm-diameter MWM. The apparatus was made of black polypropylene with a drain in the center. The tank was 51 cm deep and was filled halfway with water. The walls surrounding the maze had spatial cues mounted on them consisting of geometric shapes and posters. Testing consisted of four phases: acquisition, reversal, shift, and cued. The acquisition phase assesses basic spatial learning. The reversal phase tests cognitive flexibility when the location of the platform is moved to the opposite quadrant from where it was during acquisition (i.e., from the southwest to the northeast quadrant). For the shift phase, the platform is moved to an adjacent quadrant (i.e., from northeast to northwest). This phase also requires cognitive flexibility but also tests interference because the rat has two prior reinforced locations while learning a third one. In effect, the rat must extinguish memory for the prior locations and stop searching in those quadrants and begin a new search and remember it (for review, see Vorhees and Williams (2006)). The acquisition, reversal, and shift phases each consisted of 6 days of learning trials followed by a memory probe on day 7 of each phase. Days 1–6 of each phase consisted of four trials/day with a 120 s trial time limit. Probe trials had no platform and lasted 45 s. The diameter of the platform was 10 cm for acquisition, 7 cm for reversal, and 5 cm for shift. This progressively increased task difficulty as rats moved from one phase to the next. The cued phase consisted of 2 days, four trials each day, where black curtains were pulled around the maze to block the distal cues and the platform was marked with a yellow ball raised 12 cm above the water on a steel rod attached to the 10 cm diameter platform. To prevent use of distal cues, the start and platform positions were changed on every trial. Behavior was tracked using ANY-maze software (Stoelting, Wood Dale, IL).
Monoamine Analysis
Brain tissue was collected 1–2 days after the last day of behavior. Rats were decapitated and brains were removed over ice and placed in a cooled brain block. The brain was sliced coronally at the decussation of the optic chiasm followed by a second cut 2 mm anterior to the first. From the remaining section, the neostriatum and nucleus accumbens were dissected bilaterally. The hippocampus was then dissected from the cerebral hemispheres. Tissue was stored at −80 °C until assayed. Monoamines were quantified by high-pressure liquid chromatography with electrochemical detection. Tissue was weighed and sonicated in ice-cold 0.1 N perchloric acid and centrifuged at 20,800 rcf at 4.0 °C for 13 min. The supernatant was loaded onto a Dionex UltiMate® 3000 Analytical Autosampler (Thermo Scientific). The pump was an ESA 5840 set at a flow rate of 0.5 mL/min and temperature of 28.0 °C. The Coulochem III electrochemical detector (Thermo Scientific) was set to − 150 mV for E1 and + 250 mV for E2. The guard cell was set to + 350 mV. The column was a Supelco Supelcosil™ LC-18 column (15 cm × 4.6 mm, 3 μm; Sigma-Aldrich Co.). The mobile phase was commercially available MD-TM Mobile Phase (Thermo Fisher Scientific) that consisted of 89% water, 10% acetonitrile, and 1% sodium phosphate monobasic (monohydrate). Standards for DA, 5-HT, and norepinephrine (NE) were pre-calculated and serially diluted in order to create a standard curve.
Statistical Procedures
All data, except SWM probe trails, were analyzed by mixed linear ANOVA models (SAS Proc Mixed, SAS Institute, Cary, NC, v9.3 TS Level 1M2) with Kenward-Rogers first-order degrees of freedom. Straight channel, SWM training trials, MWM probe trials, and brain monoamines were analyzed by one-way ANOVA. For designs with repeated measures factors, mixed linear ANOVA models were used with group as the fixed factor and day or trial as the repeated factor. These models used the compound symmetry (CS) covariance structure. Significant interactions were further analyzed using slice-effect ANOVAs at each level of the repeated measure factor. Significance was set at p ≤ 0.05. A priori predictions were made where we had prior data, i.e., that MA-treated rats would perform worse in the MWM and CWM compared with controls. For these, the statistical test was one-tailed. For all other outcomes, all F tests were two-tailed. SWM probe trials were analyzed by Fisher’s exact tests with Bonferroni adjustments for multiple comparisons. Data analyzed by mixed linear ANOVA are presented as least square (LS) mean ± SEM to be consistent with assumptions of such models.
Results
Temperature
There was a main effect of drug (F(1,33.7) = 428.53, p < 0.0001; Fig. 3a). Rats treated with MA had higher temperatures than rats treated with SAL. There was also a drug × time interaction (F(16, 525) = 15.93, p < 0.0001). MA increased temperatures at all times except at time-0.
Straight Channel
There was no drug effect on straight channel swimming latency (Fig. 3b), indicating no motoric or motivational differences between the groups.
Cincinnati Water Maze
There was a significant drug main effect on latency (F(1,25) = 25.51, p < 0.0001; Fig. 4a) and errors (F(1,25) = 13.70, p < 0.01; Fig. 4b). Drug × day interactions for both latency and errors showed that rats treated with MA performed similarly to rats treated with SAL for the first 6 days; thereafter, SAL rats improved daily reaching almost no errors by day 18 and finding the escape in < 15 s, whereas MA-treated rats showed little improvement even by day 18.
Star Water Maze
There was a main effect of drug on latency (F(1,25) = 12.13, p < 0.01; Fig. 5a) and errors (F(1,25) = 12.33, p < 0.01; Fig. 5b) on training trials. MA-treated rats had longer latencies and made more errors compared with SAL rats. Probe trials were given on days 5, 10, 15, and 20. MA-treated rats had significantly fewer successful trials in the block prior to the day 5 probe trial (F(1,26) = 3.99, p < 0.05; Fig. 5c) and day 10 probe trial (F(1,26) = 20.31, p < 0.001; Fig. 5c) but not on blocks prior to the probe trials on days 15 and 20. As can be seen in Fig. 5d, there was no drug effect on the percent of rats that used an allocentric strategy. However, most SAL rats used an egocentric strategy, whereas MA-treated rats used this strategy less than SAL rats, and this was most evident on the first probe trial on day 5 (p < 0.01, Fisher’s exact; Fig. 5d). Rats without a consistent strategy were grouped as “Other.” Post hoc comparisons showed that MA-treated rats predominantly fell into this category. There were also similar differences on the second probe trial on day 10 (p < 0.05). Probe trials on days 15 and 20 were not significantly affected; however, the latter showed a trend (p < 0.09).
Star water maze (SWM): Rats were tested for 16 days, two training trials/day with a probe trial every 5th day. Data are shown in blocks, 4 days/block. a Latency on training trials. b Errors on training trials. c Number of successful trials where success was defined as reaching the goal within the time limit making not more than one error. d Probe trials categorized by strategy on successful trials. Group sizes: SAL = 13, MA = 14. Data in a–c are mean ± SEM. *p < 0.05 vs. SAL; **p < 0.01 vs. SAL; ***p < 0.001 vs. SAL
Morris Water Maze
There was a main effect on acquisition latency (F(1,27.1) = 7.55, p < 0.05; Fig. 6a) and path efficiency (F(1,27.3) = 3.80, p < 0.05; Fig. 6b). Rats treated with MA took longer and were less efficient to locate the platform than SAL rats. For the probe trial after acquisition, there were no effects (Fig. 6c). There were no differences between the groups during reversal learning (Fig. 6d, e). However, there was a drug effect on the reversal probe trial (F(1,26) = 5.08, p < 0.05; Fig. 6f) with MA rats further from the former platform site than SAL rats. There was a drug main effect during shift trials on latency (F(1,26) = 7.84, p < 0.01; Fig. 6g) and path efficiency (F(1,26) = 4.00, p < 0.05; Fig. 6h), but no effect on the shift probe trial (Fig. 6i). The MA-treated rats had longer latencies and less efficiency than SAL-treated rats. No significant differences in latency were detected between the groups in the cued phase: mean ± SEM: day 1: SAL 24.0 ± 3.4 s, MA 31.8 ± 3.7 s and day 2: SAL 28.9 ± 3.4 s, MA 23.5 ± 3.7 s.
Morris water maze (MWM): Acquisition (a–c). a Latency. b Path efficiency. c Average distance to platform site on the acquisition probe trial. Reversal (d–f). d Latency. e Path efficiency. f Average distance to the platform site on the reversal probe trial. Shift (g–i). g Latency. h Path efficiency. i Average distance to the platform site on the shift probe. Data presented are mean ± SEM. Group sizes: SAL = 15, MA = 14. *p < 0.05 vs. SAL; **p < 0.01 vs. SAL
Monoamines
In the neostriatum, MA significantly reduced DA (F(1,26) = 85.69, p < 0.0001; Fig. 7a) and 5-HT (F(1,22) = 52.73, p < 0.0001; Fig. 7b), but not NE (Fig. 7c) compared with SAL. Striatal DA levels were negatively correlated with dosing temperature (r = − 0.82, p < 0.0001), CWM latency (r = − 0.79, p < 0.0001) and errors (r = − 0.62, p < 0.001), as well as MWM acquisition latency (r = − 0.42, p < 0.05), reversal latency (r = − 0.38, p < 0.05), shift latency (r = − 0.38, p < 0.04), and reversal probe performance (r = − 0.40, p < 0.05). Striatal 5-HT levels were also negatively correlated with dosing temperature (r = − 0.81, p < 0.0001) and MWM acquisition latency (r = − 0.46, p < 0.05). In the n. accumbens, MA significantly reduced DA (F(1,24) = 7.95, p < 0.01; Fig. 7d) and 5-HT (F(1,19) = 12.54, p < 0.01; Fig. 7e) but not NE (Fig. 7f) relative to SAL. DA in the n. accumbens was negatively correlated with temperature (r = − 0.43, p < 0.05) as was 5-HT (r = − 0.60, p < 0.01). DA was also negatively correlated with CWM latency (r = − 0.79, p < 0.0001) and errors (r = − 0.62, p < 0.001). N. accumbens 5-HT was similarly negatively correlated with CWM latency (r = − 0.72, p < 0.001) and errors (r = − 0.60, p < 0.01) and with MWM shift latency (r = − 0.52, p < 0.05). In the hippocampus, MA significantly reduced 5-HT (F(1,26) = 244.52, p < 0.0001; Fig. 7g) but not NE (Fig. 7h) compared with SAL. Hippocampal 5-HT was negatively correlated with temperature (r = − 0.90, p < 0.0001) and with MWM acquisition latency (r = − 0.46, p < 0.05), reversal probe (r = − 0.48, p < 0.01), and shift latency (r = − 0.51, p < 0.01).
Monoamines: a neostriatal DA, b neostriatal 5-HT, c neostriatal NE. Group sizes: DA: SAL = 15, MA = 13; 5-HT: SAL = 12, MA = 12; NE: SAL = 14, MA = 9. d Nucleus accumbens DA, e nucleus accumbens 5-HT, f nucleus accumbens NE. Group sizes: DA: SAL = 14, MA = 12; 5-HT: SAL = 11, MA = 10; NE: SAL = 11, MA = 10. g Hippocampal 5-HT, h hippocampal NE. Group sizes: 5-HT: SAL = 15, MA = 13; NE: SAL = 15, MA = 13. **p < 0.01; ****p < 0.0001 vs. SAL
Discussion
The present study tested the effects of a single dose of MA on egocentric and allocentric learning and memory and on strategy preference. This dose was previously shown to reduce striatal DA; however, those experiments did not test behavior (Cappon et al. 2000; Fukumura et al. 1998). Here, we examined the effects of the single dose on learning, memory, and monoamines up to 76 days post-treatment.
Deficits in egocentric navigation after 10 mg/kg MA given every 2 h were reported previously (Herring et al. 2008a, 2010; Vorhees et al. 2011). The present data show that a single MA dose produces the same deficits in the CWM and the same DA reductions in the neostriatum and n. accumbens as the 10 mg/kg × 4 regimen. This is consistent with data that show striatal 6-hydroxydopamine injections also impair CWM learning (Braun et al. 2012, 2015, 2016).
The SWM was developed by Rondi-Reig and colleagues (2006; 2013), as a five-arm maze that we modified to have eight arms. MA-treated rats exhibited deficits learning this maze and failed to use a consistent strategy on probe trials. Probe trials showed that SAL-treated rats relied predominantly on an egocentric strategy. This was apparent on the first and second probe trials. It was less evident on the third and fourth probes apparently because after receiving many repetitive learning trials, the rats began to explore other possible exits. This is a known effect of over-training (Gasbarri et al. 2014; Yin et al. 2004). This apparently did not occur in the experiments by Rondi-Reig et al. because they used mice. Interestingly, MA-treated rats did not show the same egocentric preference as controls, perhaps because of the severe DA reductions in the neostriatum, a region that plays an important role in egocentric navigation.
In terms of spatial learning and memory, we have shown that 4 × 10 mg/kg MA at 2-h intervals impairs spatial navigation but only in a large MWM (244 cm in diameter) (Gutierrez et al. 2017), with no effects in smaller tanks (Friedman et al. 1998; Herring et al. 2008a; Schroder et al. 2003). For this study, deficits were found during acquisition and shift platform trials, but not on reversal, but were seen on the reversal probe trial. Patterns such as these are not unusual. We previously found with other treatments that reversal is sometimes less sensitive than acquisition or shift, perhaps because of positive transfer of training that can occur as rats become more familiar with the task requirements (Vorhees et al. 2009).
In some experiments with the 4 × 10 MA dosing regimen, impaired MWM acquisition, reversal, and shift platform trials were found (Gutierrez et al. 2017). Previous studies using multiple-injection regimens (Friedman et al. 1998; Gutierrez et al. 2017; Herring et al. 2010) found 5-HT reductions in the hippocampus of MA-treated rats as was found here after a single dose. 5-HT reductions in the hippocampus may contribute to the MWM deficits since allocentric learning is hippocampally mediated. This single-dose approach used here could be used to test the specificity of 5-HT hippocampal effects in future studies.
The pattern of effects found here with one dose versus experiments using the 10 × 4 regimen is minor. These differences are most likely the result of slight variations in the rats, housing conditions, room temperatures, or other procedures that, while every effort was made to maintain the same conditions, cannot be totally controlled over the years that intervened between experiments. Changes in the vivarium alone could account for some minor differences, but for all intents and purposes, the outcomes from this single-MA-dose method and the four-dose method show remarkable convergence and will permit future experiments to be done with pharmacological interventions without having to factor in the timing across four doses of MA.
References
Bortolato M, Frau R, Piras AP, Luesu W, Bini V, Diaz G, Gessa G, Ennas MG, Castelli MP (2009) Methamphetamine induces long-term alterations in reactivity to environmental stimuli: correlation with dopaminergic and serotonergic toxicity. Neurotox Res 15(3):232–245. https://doi.org/10.1007/s12640-009-9024-2
Braun AA, Amos-Kroohs RM, Gutierrez A, Lundgren KH, Seroogy KB, Skelton MR, Vorhees CV, Williams MT (2015) Dopamine depletion in either the dorsomedial or dorsolateral striatum impairs egocentric Cincinnati water maze performance while sparing allocentric Morris water maze learning. Neurobiol Learn Mem 118:55–63. https://doi.org/10.1016/j.nlm.2014.10.009
Braun AA, Amos-Kroohs RM, Gutierrez A, Lundgren KH, Seroogy KB, Vorhees CV, Williams MT (2016) 6-Hydroxydopamine-induced dopamine reductions in the nucleus accumbens, but not the medial prefrontal cortex, impair Cincinnati water maze egocentric and Morris water maze allocentric navigation in male Sprague–Dawley rats. Neurotox Res 30(2):199–212. https://doi.org/10.1007/s12640-016-9616-6
Braun AA, Graham DL, Schaefer TL, Vorhees CV, Williams MT (2012) Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol Learn Mem 97(4):402–408. https://doi.org/10.1016/j.nlm.2012.03.004
Buzsaki G, Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci 16(2):130–138. https://doi.org/10.1038/nn.3304
Cappon GD, Pu C, Vorhees CV (2000) Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res 863(1-2):106–111. https://doi.org/10.1016/S0006-8993(00)02107-7
Chang L, Alicata D, Ernst T, Volkow N (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102:16–32. https://doi.org/10.1111/j.1360-0443.2006.01782.x
Cheng RK, Etchegaray M, Meck WH (2007) Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res 1186:255–266. https://doi.org/10.1016/j.brainres.2007.10.002
Cook D, Kesner RP (1988) Caudate nucleus and memory for egocentric localization. Behav Neural Biol 49(3):332–343. https://doi.org/10.1016/S0163-1047(88)90338-X
Daberkow DP, Kesner RP, Keefe KA (2005) Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav 81(1):198–204. https://doi.org/10.1016/j.pbb.2005.03.010
Fouquet C, Babayan BM, Watilliaux A, Bontempi B, Tobin C, Rondi-Reig L (2013) Complementary roles of the hippocampus and the dorsomedial striatum during spatial and sequence-based navigation behavior. PLoS One 8(6):e67232. https://doi.org/10.1371/journal.pone.0067232
Friedman SD, Castañeda E, Hodge GK (1998) Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav 61(1):35–44. https://doi.org/10.1016/S0091-3057(98)00066-5
Friend DM, Keefe K a (2013) A role for D1 dopamine receptors in striatal methamphetamine-induced neurotoxicity. Neurosci Lett 555:243–247. https://doi.org/10.1016/j.neulet.2013.08.039
Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV (1998) A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res 806(1):1–7. https://doi.org/10.1016/S0006-8993(98)00656-8
Gasbarri A, Pompili A, Packard MG, Tomaz C (2014) Habit learning and memory in mammals: behavioral and neural characteristics. Neurobiol Learn Mem 114:198–208. https://doi.org/10.1016/j.nlm.2014.06.010
Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED (2011) Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology 36(5):950–959. https://doi.org/10.1038/npp.2010.233
Gross NB, Duncker PC, Marshall JF (2011) Striatal dopamine D1 and D2 receptors: widespread influences on methamphetamine-induced dopamine and serotonin neurotoxicity. Synapse 65(11):1144–1155. https://doi.org/10.1002/syn.20952
Gutierrez, A., Jablonski, S.A., Amos-kroohs, R.M., Barnes, A.C., Williams, M.T., Vorhees, C. V, 2017. Effects of housing on methamphetamine-induced neurotoxicity and spatial learning and memory. doi:https://doi.org/10.1021/acschemneuro.6b00419
Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436(7052):801–806. https://doi.org/10.1038/nature03721
Herring, N.R., Gudelsky, G. a., Vorhees, C. V., Williams, M.T., 2010. (+)-Methamphetamine-induced monoamine reductions and impaired egocentric learning in adrenalectomized rats is independent of hyperthermia. Synapse 64, 773–785. doi:https://doi.org/10.1002/syn.20784
Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT (2008a) Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology 199(4):637–650. https://doi.org/10.1007/s00213-008-1183-y
Herring NR, Schaefer TL, Tang PH, Skelton MR, Lucot JP, Gudelsky GA, Vorhees CV, Williams MT (2008b) Comparison of time-dependent effects of (+)-methamphetamine or forced swim on monoamines, corticosterone, glucose, creatine, and creatinine in rats. BMC Neurosci 9(1):49. https://doi.org/10.1186/1471-2202-9-49
Heysieattalab S, Naghdi N, Zarrindast MR, Haghparast A, Mehr SE, Khoshbouei H (2016) The effects of GABAA and NMDA receptors in the shell-accumbens on spatial memory of METH-treated rats. Pharmacol Biochem Behav 142:23–35. https://doi.org/10.1016/j.pbb.2015.12.008
Howland JG, Harrison RA, Hannesson DK, Phillips AG (2008) Ventral hippocampal involvement in temporal order, but not recognition, memory for spatial information. Hippocampus 18(3):251–257. https://doi.org/10.1002/hipo.20396
Kelley AE, Domesick VB (1982) The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde and retrograde-horseradish peroxidase study. Neuroscience 7(10):2321–2335. https://doi.org/10.1016/0306-4522(82)90198-1
Kesner RP, Farnsworth G, DiMattia BV (1989) Double dissociation of egocentric and allocentric space following medial prefrontal and parietal cortex lesions in the rat. Behav Neurosci 103(5):956–961. https://doi.org/10.1037/0735-7044.103.5.956
Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang LC, Furukawa Y, Chang LJ, Wickham DJ, Sherwin A, Tong J (2009) Brain serotonin transporter in human methamphetamine users. Psychopharmacology 202(4):649–661. https://doi.org/10.1007/s00213-008-1346-x
Simon L, Domier C, Jennife S, Domier C, Carnell J, Brethen P, Rawson R, Ling W (2000) Cognitive impairment in individuals currently using methamphetamine. Am J Addict 9(3):222–231. https://doi.org/10.1080/10550490050148053
Marshall, J.F., Belcher, A.M., Feinstein, E.M., O’Dell, S.J., 2007. Methamphetamine-induced neural and cognitive changes in rodents. Addiction 102 Suppl, 61–9. doi:https://doi.org/10.1111/j.1360-0443.2006.01780.x, 69
McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA (1998) Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 18(20):8417–8422
McGeorge AJ, Faull RLM (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29(3):503–537. https://doi.org/10.1016/0306-4522(89)90128-0
McNamara RK, Skelton RW (1993) The neuropharmacological and neurochemical basis of place leaning in the Morris water maze. Brain Res Brain Res Rev Jan-Apr 18(1):33–49. https://doi.org/10.1016/0165-0173(93)90006-L
Moon M, Do KS, Park J, Kim D (2007) Memory impairment in methamphetamine dependent patients. Int J Neurosci 117(1):1–9. https://doi.org/10.1080/00207450500535503
Morris RGM, Garrud P, Rawlins JNP, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297(5868):681–683. https://doi.org/10.1038/297681a0
Morris RGM, Hagan JJ, Rawlins JNP (1986) Allocentric spatial learning by Hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J Exp Psychol Sect B 38:365–395. https://doi.org/10.1080/14640748608402242
O’Callaghan JP, Miller DB (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270(2):741–751
O’Keefe J, Nadel L (1979) Précis of O’Keefe & Nadel’s The hippocampus as a cognitive map. Behav Brain Sci 2:487–494. https://doi.org/10.1017/S0140525X00063949
Packard MG (2009) Exhumed from thought: basal ganglia and response learning in the plus-maze. Behav Brain Res 199(1):24–31. https://doi.org/10.1016/j.bbr.2008.12.013
Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE (2012) Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology 62(2):1119–1126. https://doi.org/10.1016/j.neuropharm.2011.11.003
Rondi-Reig L (2006) Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci 26(15):4071–4081. https://doi.org/10.1523/JNEUROSCI.3408-05.2006
Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Flynn NM, Henik A, Pfefferbaum A, Sullivan EV (2002) Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res 111(1):65–74. https://doi.org/10.1016/S0165-1781(02)00111-7
Schroder N, O’Dell SJ, Marshall JF (2003) Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse 49(2):89–96. https://doi.org/10.1002/syn.10210
Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N (2006) Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 63(1):90–100. https://doi.org/10.1001/archpsyc.63.1.90
Son J-H, Latimer C, Keefe KA (2011) Impaired formation of stimulus–response, but not action–outcome, associations in rats with methamphetamine-induced neurotoxicity. Neuropsychopharmacology 36(12):2441–2451. https://doi.org/10.1038/npp.2011.131
Sonsalla PK, Gibb JW, Hanson GR (1986) Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. J Pharmacol Exp Ther 238(3):932–937
Thierry AM, Gioanni Y, Dégénétais E, Glowinski J (2000) Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus 10(4):411–419. https://doi.org/10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A
Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN (2001) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158(3):377–382. https://doi.org/10.1176/appi.ajp.158.3.377
Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun A a, Amos-Kroohs R, Williams MT (2011) Comparison of (+)-methamphetamine, ±-methylenedioxymethamphetamine, (+)-amphetamine and ±-fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse 65(5):368–378. https://doi.org/10.1002/syn.20854
Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT (2008) Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci 26(6):599–610. https://doi.org/10.1016/j.ijdevneu.2008.04.002
Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT (2009) Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci 27(3):289–298. https://doi.org/10.1016/j.ijdevneu.2008.12.003
Vorhees CV, Williams MT (2016) Cincinnati water maze: a review of the development, methods, and evidence as a test of egocentric learning and memory. Neurotoxicol Teratol 57:1–19. https://doi.org/10.1016/j.ntt.2016.08.002
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858. https://doi.org/10.1038/nprot.2006.116.Morris
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2(6):699–703. https://doi.org/10.1038/nm0696-699
Yin HH, Knowlton BJ, Balleine BW (2004) Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 19(1):181–189. https://doi.org/10.1111/j.1460-9568.2004.03095.x
Zhong N, Jiang H, Du J, Zhao Y, Sun H, Xu D, Li C, Zhuang W, Li X, Hashimoto K, Zhao M (2016) The cognitive impairments and psychological wellbeing of methamphetamine dependent patients compared with health controls. Prog Neuro-Psychopharmacology Biol Psychiatry 69:31–37. https://doi.org/10.1016/j.pnpbp.2016.04.005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutierrez, A., Williams, M.T. & Vorhees, C.V. A Single High Dose of Methamphetamine Reduces Monoamines and Impairs Egocentric and Allocentric Learning and Memory in Adult Male Rats. Neurotox Res 33, 671–680 (2018). https://doi.org/10.1007/s12640-018-9871-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9871-9