Abstract
Rationale
Drugs that block fatty acid amide hydrolase (FAAH, which elevates anandamide [AEA]) and drugs which block monoacylglycerol (MAGL, which elevates 2-arachidonyl glycerol [2-AG]) have promise in treating both acute and anticipatory nausea in human patients.
Objective
This study aims to evaluate the relative effectiveness of dual MAGL/FAAH inhibition with either alone to reduce acute and anticipatory nausea in rat models.
Materials and methods
AM4302, a new dual MAGL/FAAH inhibitor, was compared with a new selective MAGL inhibitor, AM4301, and new selective FAAH inhibitor, AM4303, for their potential to reduce acute nausea (gaping in taste reactivity) and anticipatory nausea (contextually elicited conditioned gaping) in two rat models.
Results
Our in vitro studies indicate that AM4302 blocks human and rat FAAH: IC50 60 and 31 nM, respectively, with comparable potencies against human MAGL (IC50 41 nM) and rat MAGL (IC50 200 nM). AM4301 selectively blocks human and rat MAGL (IC50 8.9 and 36 nM, respectively), while AM4303 selectively inhibits human and rat FAAH (IC50 2 and 1.9 nM), respectively. Our in vivo studies show that the MAGL inhibitor, AM4301, suppressed acute nausea in a CB1-mediated manner, when delivered systemically or into the interoceptive insular cortex. Although the dual FAAH/MAGL inhibitor, AM4302, was equally effective as the FAAH inhibitor or MAGL inhibitor in reducing acute nausea, it was more effective than both in suppressing anticipatory nausea.
Conclusions
Dual FAAH and MAGL inhibition with AM4302 may be an especially effective treatment for the very difficult to treat symptom of anticipatory nausea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although animal models of vomiting have identified the neural mechanisms regulating the emetic reflex (e.g., Hornby 2001), much less is understood about nausea. Yet, clearly nausea is one of the most debilitating treatment-resistant side effects of chemotherapy among cancer patients; that is, current anti-emetic therapies are highly effective in reducing chemotherapy-induced vomiting, but they are much less effective in treating chemotherapy-induced nausea (Andrews and Horn 2006; Foubert and Vaessen 2005; Hickok et al. 2003; Hornby 2001; Morrow et al. 2002; Roscoe et al. 2000). Indeed, when nausea is untreated, cues of the clinic can become associated with this sensation resulting in classically conditioned anticipatory nausea. When anticipatory nausea occurs, currently available anti-emetic treatments are completely ineffective (Morrow et al. 2002).
The search for effective treatments for acute and anticipatory nausea has been limited by the lack of selective animal models of nausea. Most recently, however, the model of conditioned gaping in rats has been developed which selectively detects compounds that produce the side effect of nausea (see Parker 2014). Conditioned gaping is displayed when rats are exposed to a flavored solution that has previously been paired with an emetic agent. Indeed, if rats are pretreated with agents that reduce emesis in other species, the establishment of these conditioned gaping reactions can be prevented by reducing the acute nausea produced by an emetic drug (Parker 2014). Most recently, it has been shown that rats also display conditioned gaping reactions when re-exposed to a contextual cues previously paired with a nauseating treatment, such as LiCl; thus, contextually elicited gaping in rats is analogous to contextually elicited nausea experienced by chemotherapy patients (Limebeer et al. 2008; Rock et al. 2008). Interestingly, just as in human chemotherapy patients, when contextually elicited conditioned gaping is produced it is not treatable with standard 5-HT3 antiemetics (Limebeer et al. 2006; Rock et al. 2015), whereas cannabinoid compounds effectively reduce this behavior (Limebeer et al. 2014; Rock et al. 2008), likely by reducing the underlying nausea.
The endocannabinoid (eCB) system is intimately involved in the regulation of nausea and vomiting (Parker et al. 2011; Sharkey et al. 2014). Unlike most neurotransmitters, anandamide (AEA) and 2-arachidonyl glycerol (2-AG) are synthesized on-demand in the post-synaptic neuron and act on presynaptic cannabinoid (CB1) receptors within regions regulating nausea and vomiting. The action of AEA is limited by enzymatic hydrolysis via fatty acid amide hydrolase (FAAH) and 2-AG by monoacylglyceroal lipase (MAGL). Thus, blocking these enzymes prevents the rapid degradation of these endocannabinoids and may have great promise for treatment for acute and anticipatory nausea. We have previously demonstrated that systemic administration of the FAAH inhibitor, URB597 (0.3 mg/kg, but not 0.1 mg/kg), suppresses acute nausea (Cross-Mellor et al. 2007) and anticipatory nausea (Rock et al. 2008). Furthermore, the dual FAAH/MAGL inhibitor, JZL195 (10 mg/kg), also suppresses anticipatory nausea, primarily by elevating AEA in rat tissue (Limebeer et al. 2014). Finally, we have shown that systemic administration of the MAGL inhibitor, MJN110 (10–20 mg/kg) suppresses acute and anticipatory nausea in rats by a CB1 receptor-dependent action (Parker et al. 2014). The suppression of nausea by MJN110 is most likely mediated by the action of 2-AG within the region of the brain that mediates nausea (Tuerke et al. 2012), the visceral insular cortex (VIC), because administration of the agonist HU-210 (Limebeer et al. 2012), 2-AG (Sticht et al. 2015), and MJN110 (Sticht et al. 2012b) into this region suppressed acute nausea in rats. Furthermore, Sticht et al. (2015) have shown that systemic and intra-VIC administration of MJN110 elevates 2-AG, but not AEA in the VIC.
Although our previous work has shown that drugs that elevate 2-AG hold great promise as potential candidates for treating acute and anticipatory nausea, compounds that effectively suppress MAGL in rats have only recently been developed and limited compounds have been evaluated. In order to broaden the scope of pharmacological treatment options for preclinical candidate selection, we investigated a novel MAGL inhibitor in our model, AM4301, which is highly selective in rats. Similarly, novel FAAH inhibitor, AM4303, was compared with URB597 in our acute nausea model. Since we have previously shown that the MAGL inhibitor, MJN110, interferes with nausea-induced conditioned gaping when delivered to the VIC, we evaluated the effect of intra-VIC administration of AM4301 on acute nausea. As well, our prior study has demonstrated that the FAAH-selective inhibitor, URB597 (0.3 mg/kg), suppresses acute (Cross-Mellor et al. 2007) and anticipatory nausea (Rock et al. 2008), but does not completely abolish these symptoms in the rodent models. We are particularly interested in AM4303 because prior unpublished toxicity screening has identified the novel FAAH inhibitor as an advance candidate exhibiting low affinity for off target proteins (e.g., HERG [IC50 >15 μM], CPY450 isoforms [IC50 >15 μM]) and it was not cytotoxic at doses as high as 15 μM. Moreover, our previous investigation of JZL195 has also shown the dual FAAH/MAGL inhibitor was ineffective at blocking the activities of rat MAGL in whole brain analysis, since its effects were primarily due to FAAH inhibition (Limebeer et al. 2014), while the overall pharmacological profile of the compound was not entirely consistent with the anticipated result. The search for a dual FAAH/MAGL inhibitor that effectively suppresses MAGL in rats while also inhibiting FAAH in our model led to the evaluation of dual FAAH/MAGL inhibitor, AM4302, which has the potential to attenuate acute and anticipatory nausea. This study aims to gain a better understanding the effect of concomitant increases in AEA and 2-AG levels in regulating nausea in rats.
Methods
Animals
Animal procedures complied with the Canadian Council on Animal Care. The protocols were approved by the Institutional Animal Care Committee, which is accredited by the Canadian Council on Animal Care. Naïve male Sprague-Dawley rats, weighing between 262 and 373 g on the first day of conditioning, obtained from Charles River Laboratories (St Constant, Quebec) 1 week prior to manipulations were used for assessment of anti-nausea-like behavior. They were pair-housed in home cages in a colony room at an ambient temperature of 21 °C with a 12/12-h light-dark schedule (lights off at 8 am and on at 8 pm), with all experimental procedures completed in the dark cycle. The rats were maintained on food (Iams rodent chow, 18 % protein) and water ad libitum.
Drugs and materials
All injections were administered intraperitoneally (ip). A 0.15 M lithium chloride (LiCl; Sigma Aldrich) solution was prepared as a 0.15 M solution with sterile water and administered i.p. in a volume of 20 ml/kg (127.2 mg/kg). FAAH inhibitors, URB 597 (0.3 mg/kg, see Fegley et al. 2005) and AM4303 (5–20 mg/kg), MAGL inhibitor AM4301 (5–20 mg/kg), the dual FAAH/MAGL inhibitor, AM4302 (5–20 mg/kg), and SR141716 (2.5 mg/kg) were prepared in a vehicle (VEH) consisting of a 1:1:18 mixture of ethanol, Tween 80 (Sigma), and saline (SAL). The drugs were first dissolved in ethanol then Tween 80 was added to the solution and the ethanol was evaporated off with a nitrogen stream after which the saline was added. The final VEH consisted of 1:9 (Tween/saline).
In vitro experimental procedures
In vitro MAGL inhibition assay
Medium-throughput fluorimetric screening assays were employed to determine inhibitory potencies of AM4301-3 using recombinant human hMGL and rat rMGL according to established protocols (Zvonok et al. 2008a, b). Briefly described, recombinant human MAGL (hMAGL) or rat MAGL (rMAGL) were expressed in Escherichia coli cells and purified (Zvonok et al. 2008a, b). Various concentrations of inhibitor were incubated with purified hMAGL or rMAGL lysates in a 96-well plate for 15 min at room temperature. The fluorigenic MAGL substrate, 7-hydroxy-6-methoxy-4-methylcoumarin ester (AHMMCE), was added prior to reaction incubation at 25 °C for an additional 3 h. Fluorescence readings were taken every 15 min at 360 nm/460 nm (λexcitation/λemission) using a Synergy HT Plate Reader (BioTek, Winooski, VT). External standards were used to convert relative fluorescence units at the 3-h time point to the amount of coumarin formed. All MAGL assays were performed in triplicate for each inhibitor concentration, and IC50 values were determined using Prizm software (GraphPad Software, Inc., San Diego, CA).
In vitro hFAAH and rFAAH assay
Truncated rat FAAH (Rat ΔTM FAAH) was expressed in E. coli cells and purified (Patricelli et al. 1998). Similarly, truncated human FAAH, preparation developed at the CDD, was expressed in E. coli cells using pMALcE4 vector (New England Biolabs, Alapafuja et al. 2012). Various concentrations of test compounds (diluted in 50:50 DMSO/assay buffer (50 mM HEPES, 1 mM EDTA, 0.1 % BSA, pH 7.4)) and 15 μg total protein E. coli lysate were incubated at 25 °C for 15 min. Then the fluorigenic substrate N-arachidonoyl, 7-amino-4-methylcoumarin amide (AAMCA) was added to each well and incubation continued for additional 3 h (Ramarao et al. 2005). Kinetic fluorescence reading was performed every 20 min (λex = 360/λem = 460) on a BioTek Synergy HT Microplate Reader (BioTek Instruments, Winooski, VT). External standards were used to convert relative fluorescence units at the 3-h time point to the amount of coumarin formed. All MAGL assays were performed in triplicate for each inhibitor concentration, and IC50 values were determined using Prizm software (GraphPad Software, Inc., San Diego, CA).
Intraoral (IO) cannulation surgery
The intraoral cannulation surgery was conducted as described by Limebeer et al. (2012) under isoflurane gas anesthesia following an injection carprofen (0.1 mg/kg).
Stereotaxic surgery and histology
In experiment 3, the rats underwent stereotaxic surgery during which intracranial cannula were bilaterally implanted into the visceral insular cortex (VIC) as described by Sticht et al. (2012a, b). Stainless steel guide cannulae (22G, 6 mm below pedestal) were implanted bilaterally into the VIC (at 10° divergent angle) using the following coordinates (Contreras et al. 2007) relative to bregma: AP −0.5; LM +5.0; DV −4.5 from skull surface. They were then implanted with intraoral cannulae. Following the completion of behavioral testing, rats were sacrificed and perfused as described by Sticht et al. (2012a, b). Coronal sections (60 μm) of PRh were taken on a cryostat freezing microtome and mounted on glass slides. Following thionin staining, cannula placements were examined using bright-field microscopy. The n’s reported reflect the rats with proper cannulae placements.
Behavioral experimental procedures
Experiment 1: effect of MAGL inhibition by systemic AM4301 on acute nausea
Three days after intraoral cannula surgery, the rats received an adaptation trial in which they were placed in the taste reactivity chamber with their cannula attached to an infusion pump (Model KDS100, KD Scientific, Holliston, MA, USA) for fluid delivery. The taste reactivity chambers were made of clear Plexiglas (22.5 × 26 × 20 cm) that sat on a table with a clear glass top. A mirror beneath the chamber on a 45° angle facilitated viewing of the ventral surface of the rat to observe orofacial responses. Water was infused into their intraoral cannulae for 2 min at the rate of 1 ml min−1.
On the day following the adaptation trial, the rats received either one or two (separated by 72 h) taste reactivity conditioning trials in which they were administered a pretreatment injection of VEH or AM4301 (20 mg/kg). Two hours after the pretreatment injection, the rats were individually placed in the taste reactivity chamber and intraorally infused with 0.1 % saccharin solution for 2 min at the rate of 1 ml min−1 while the orofacial responses were video recorded from a mirror at a 45° angle beneath the chambers, with the feed from the video camera (Sony DCR-HC48, Henry’s Cameras, Waterloo, ON, Canada) fire-wired into a computer. Immediately after the saccharin infusion, all rats were injected with 20 ml kg−1 of 0.15 M LiCl and returned to their home cage. The groups (with random assignment) were as follows: one conditioning trial, VEH (n = 8) or AM4301 (n = 4), and two conditioning trial, VEH (n = 8) or AM4301 (n = 4).
Seventy-two hours following the final conditioning trial, the rats were given the taste reactivity test trial, drug-free. Rats were again intraorally infused with 0.1 % saccharin solution for 2 min at the rate of 1 ml min−1 while the orofacial responses were video recorded. Rats were then returned to their home cages. At 1600 hours on the day of the taste reactivity test trial, the rats were water restricted (water bottles removed from cage). Eighteen hours later on the following morning, they were given the conditioned taste avoidance test. Each rat was presented with a single graduated tube containing 0.1 % saccharin solution and consumption measures were taken at 30, 120, and 360 min.
The videotapes of the taste reactivity conditioning trial and the test trial were later scored (at ½ speed) by an experienced rater blind to the experimental conditions using “The Observer” (Noldus Information Technology Inc., Leesburg, VA, USA) for the number of occurrences of gaping (large openings of the mouth and jaw, with lower incisors exposed). The inter-rater reliability for the scoring of gaping is greater than r = 0.9 (Tuerke et al. 2012).
Experiment 2: effect of CB1 antagonism on suppression of nausea by systemic AM4301
To determine if the suppression of LiCl-induced conditioned gaping by the MAGL inhibitor is CB1 mediated, three groups were run: VEH-VEH (n = 9), AM4301 (20 mg/kg)-VEH (n = 9), and AM4301 (20 mg/kg)-SR141716 (2.5 mg/kg; n = 7). The experimental procedures were identical to experiment 1, except that the rats received either VEH or SR141716 ninety minutes following the initial pretreatment. They received a single conditioning trial.
Experiment 3: effect of intra-VIC administration of AM4301 on suppression of nausea
Since we have demonstrated that elevation of 2-AG in the VIC by the MAGL inhibitor MJN110 suppresses acute nausea (Sticht et al. 2012b), we evaluated the potential of delivery of AM4301 to the VIC to reduce acute nausea. Following surgery, rats were allowed a 2-week recovery period. This experiment consisted of adaptation, two conditioning trials (separated 72 h apart), and a drug-free test. One hour prior to the saccharin-LiCl pairing on each of the conditioning trials, the rats received bilateral VIC infusions of vehicle (n = 13 after histology) or 2 μg AM4301 (n = 8 after histology) at a rate of 0.5 μl/min for 2 min. Seventy-two hours following the second conditioning trial, the rats underwent a drug-free test to assess gaping.
Experiment 4: comparison of MAGL, FAAH, and dual MAGL/FAAH inhibition on acute nausea
The experimental procedures were similar to experiment 1, except that 2 h prior to a single conditioning trial, the rats were injected with the FAAH inhibitors, URB597 (0.3 mg/kg, see Fegley et al. 2005) or AM4303 (20 mg/kg), the MAGL inhibitor, AM4301 (20 mg/kg), or the dual FAAH/MAGL inhibitor, AM4302 (20 mg/kg).
Experiment 5: dose-response effectiveness of MAGL inhibitor (AM4301), FAAH inhibitor (AM4303), and dual inhibitor (AM4302) to suppress anticipatory nausea
In experiment 5, the rats received four conditioning trials, during which a distinctive contextual chamber was paired with 20 ml/kg of 0.15 M LiCl. On each conditioning trial, each rat was injected with LiCl and immediately placed in the distinctive context for a 30-min period. This procedure occurred on a total of four conditioning trials, with 72 h between each trial. The conditioning chambers were made of black opaque Plexiglas (22.5 × 26 × 20 cm) and sat on a table with a clear glass top in a dark room with two 25-W lights beside the chambers. A mirror beneath the chamber on a 45° angle facilitated viewing of the ventral surface of the rat to observe orofacial responses.
The test trial to assess anticipatory nausea occurred 72 h after the final conditioning trial. Two hours prior to placement in the conditioning chambers, the rats were injected with VEH (n = 8), AM4301 (5 [n = 7], 10 [n = 8], or 20 [n = 8] mg/kg), AM4303 (5 [n = 8], 10 [n = 8], or 20 [n = 8] mg/kg), or AM4302 (5 [n = 8], 10 [n = 8], or 20 [n = 8] mg/kg). The rats remained in the chamber for 5 min during which their behavior was videotaped. The videotapes from the test trial were later scored by a trained observer for the response of gaping (wide open mouth with lower incisors exposed).
Immediately following the 5-min test trial, rats were given a 15-min activity test to assess locomotor activity. The activity chamber was constructed of white Plexiglass with the dimensions of 60 cm × 25 cm × 25 cm and located in a different room than the conditioning chamber, illuminated with a red light. A video camera mounted on an extension pole captured the activity of the rat, which was sent to a computer for analysis of distance (cm) traveled using the Ethovision software program (Noldus, Inc, NL).
Immediately following the test for locomotor activity (approximately 2.5 h following drug treatment with the enzyme inhibitors), the rats in groups VEH, 20 mg/kg AM4301, 20 mg/kg AM4302, and 20 mg/kg AM4303 were euthanized by rapid decapitation (restrained in a decapicone-Braintree Scientific, MA, USA) for extraction of the VIC (Sticht 2015). After rapid decapitation, brains were removed and placed in a brain matrix. Using stereotaxic landmarks, the posterior aspect of the bilateral olfactory tubercle was identified and the first blade was inserted followed by a second blade 2 mm posterior to the first. The coronal section was removed and placed on a metal platform, set on dry ice, and the VIC was excised bilaterally by a slicing a section that follows along the lower, outer edge of the external capsule, one horizontal slice across to the rhinal fissure and one final horizontal slice 1 mm above. The tissue was frozen in dry ice-cooled isopentane and stored in a −80 °C freezer prior to shipment to Northeastern University in dry ice.
Experiment 6: potential of SR to reverse suppressive effect of AM4302 on AN
A dose of 20 mg/kg AM4302 was most effective in suppressing AN in experiment 5; therefore, the potential of the CB1 antagonist to reverse this effect was evaluated. Experiment 6 was conducted as experiment 5 except that the rats received the following treatments: VEH (n = 8), VEH-SR (2.5 mg/kg; n = 8), AM4302 (20 mg/kg; n = 8), AM4302 (20 mg/kg)-SR (2.5 mg/kg; n = 7). They were injected with VEH or AM4302 2 h prior to the AN test and those rats receiving SR were injected 90 min later.
Statistical analysis of behavioral studies
The mean number of gaping reactions was entered into an analysis of variance, with subsequent post-hoc comparisons using a Bonferroni t test as appropriate. In experiment 1, the number of gapes during the drug-free taste reactivity test was entered into a 2 (pretreatment) × 2 (number of conditioning trials) ANOVA. In experiments 2 and 4, the number of gapes during the taste reactivity test was entered into a one-way ANOVA. In experiment 3, a t test evaluated the difference in the number of gapes elicited by intra-VIC administration of AM4301 or VEH. In the tests for anticipatory nausea, in experiment 5, a single factor ANOVA was conducted across doses of the number of gapes elicited during the AN test following each pretreatment condition. As well, to directly compare across drugs, a 3 (pretreatment drug) × 4 (dose) ANOVA was conducted. Finally in experiment 6, a single factor ANOVA evaluated the number of gapes elicited following the various pretreatments. For all statistical tests, p < 0.05 was considered significant.
Endocannabinoid analysis
Standard curve preparation
Mixtures of the dried endocannabinoids and their deuterated analogs (NIDA, Bethesda MD; Cayman Chemical, Ann Arbor MI; Nu-Check Prep, Elysian MI and synthesized in-house by CDD, Boston MA) that had been stored at −80 °C were reconstituted in ethanol for further dilution in a 20 mg/ml solution of fatty acid-free bovine serum albumin (BSA) to simulate analyte-free tissue and in ethanol to make the calibration standards and quality control (QC) samples, as previously described (Williams et al. 2007). The calibration curves were constructed from the ratios of the peak areas of the analytes versus the respective deuterated internal standard.
Sample extraction
The extraction procedure for the calibration standards, BSA quality control samples, and tissue samples is a modified version of the Folch extraction (Folch et al. 1957; Williams et al. 2007). Frozen samples were weighed prior to homogenization in ice cold acetone/PBS, pH 7.4 (3:1), and internal standard followed by centrifugation at 20,000×g for 5 min at 4 °C. The resulting supernatant was dried under nitrogen until the acetone was removed. To the remaining supernatant, 100 μl PBS, one volume of methanol and two volumes of chloroform were added for liquid-liquid phase extraction of the lipids. The two phases were separated by centrifugation and the bottom organic layer was evaporated to dryness under nitrogen. Samples were reconstituted in ethanol, vortexed, sonicated briefly, and centrifuged prior to analysis for the endocannabinoids.
LC-MS analysis for endocannabinoids
Chromatographic separation was achieved using a Agilent Zorbax SB-CN column (2.1 × 50 mm, 5 mm) on a Finnigan TSQ Quantum Ultra triple quad mass spectrometer (Thermo Electron, San Jose CA) with an Agilent 1100 HPLC on the front end (Agilent Technologies, Wilmington DE) as previously described (Williams et al. 2007). The mobile phase consisted of 10 mM ammonium acetate, pH 7.3 (A), and methanol (B) in a flow rate of 0.5 ml/min; the autosampler was kept at 4 °C to prevent analyte degradation. Eluted peaks were ionized via atmospheric pressure chemical ionization (APCI) in MRM mode. Deuterated internal standards were used for each analyte’s standard curves and their levels per gram tissue were determined.
Results
In vitro procedures
Results of our in vitro evaluation show that AM4301 is a MAGL selective inhibitor exhibiting high potencies for both human and rat MAGL (IC50 values 8.9 and 36 nM, respectively) with low effectiveness in inhibition rat FAAH (IC50 of 4920 nM), and that AM4302 is a dual FAAH/MAGL inhibitor exhibiting potencies for both human and rat FAAH (IC50 values 60 and 31 nM, respectively) and with comparable effectiveness for human MAGL (IC50 41 nM) and rat MAGL (IC50 200 nM). Additionally, AM4303 is a FAAH selective inhibitor with high potencies for both human and rat FAAH (IC50 values 2 and 1.9 nM, respectively) and with low efficacy for human MAGL (IC50 of ≥10,000 nM).
Behavioral procedures
Experiment 1: effect of MAGL inhibition by AM4301 on acute nausea
Following one or two conditioning trials, AM4301 interfered with acute nausea. As is apparent in Fig. 1, rats pretreated with 20 mg/kg of AM4301 displayed fewer gapes during the TR test trial than rats pretreated with VEH. The 2 × 2 between-groups ANOVA revealed significant main effects of pretreatment, F(1, 20) = 21.8; p < 0.001, and of trials, F(1, 20) = 12.5; p = 0.002.
Experiment 2: suppression of nausea by AM4301 is CB1 mediated
As is evident in Fig. 2, SR141716 reversed the suppressive effect of AM4301 on acute nausea. The one-way ANOVA of the gaping data revealed a significant pretreatment effect, F(2, 22) = 5.4; p = 0.013; subsequent Bonferroni t tests revealed that group VEH-VEH displayed significantly more gaping than AM4301-VEH (p < 0.01), but not AM4301-SR.
Mean (+SEM) number of gapes elicited by a LiCl-paired flavor in the drug-free TR test trial following one conditioning trial. During the conditioning trial, rats were injected with VEH or AM4301 (20 mg/kg, ip) 90 min prior to an injection of VEH or SR141716 (2.5 mg/kg) and 30 min later received a saccharin-LiCl pairing. **p < 0.01 different from VEH
Experiment 3: suppression of nausea by intra-VIC AM4301
As seen in Fig. 3a, when bilaterally infused directly into the VIC, AM4301 suppressed LiCl-induced nausea. Representative cannulation placements are shown in Fig. 3b. An independent t test revealed that on the taste reactivity test which followed two conditioning trials, the group pretreated with AM4301 displayed significantly fewer gapes than did the group pretreated with VEH, t(19) = 2.8; p = 0.012.
Experiment 4: comparison of MAGL, FAAH, and dual MAGL/FAAH inhibition on acute nausea
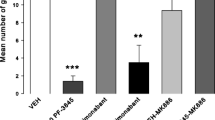
Inhibition of MAGL, FAAH, or both suppressed acute nausea, with similar potency. Figure 4 presents the mean number of gapes displayed by the various pretreatment conditions. The single factor ANOVA revealed a significant pretreatment effect, F(4, 38) = 7.3; p < 0.001; subsequent Bonferroni tests indicated that group VEH displayed significantly more gaping than any other group (p values <0.01).
Mean (+SEM) number of gapes elicited by a LiCl-paired flavor in the drug-free TR test trial following one conditioning trial. During the conditioning trial, rats were injected with VEH, the selective MAGL inhibitor, AM4301 (20 mg/kg), the dual FAAH/MAGL inhibitor AM4302 (20 mg/kg), or the FAAH inhibitors AM4303 (20 mg/kg) or URB597 (0.3 mg/kg) 2 h prior to a saccharin-LiCl pairing. **p < 0.01 different from VEH
Experiment 5: dose-response effectiveness of MAGL inhibitor, FAAH inhibitor, and dual inhibitor on anticipatory nausea
All compounds interfered with anticipatory nausea elicited by a LiCl-paired context, but the dual MAGL/FAAH inhibitor, AM4302, was more effective than the FAAH inhibitor, AM4303, and the MAGL inhibitor, AM4301. Figure 5 displays the mean number of gapes elicited by the LiCl-paired context following pretreatment with 0.0, 5, 10, and 20 mg/kg of AM4301 (top section), AM4303 (middle section), and AM4302 (bottom section). Separate one-way ANOVAs across doses for each compound revealed significant main effects (AM4301: F(3, 27) = 4.9; p = 0.008; AM4303: F(3, 28) = 3.2; p = 0.042; AM4302: F(3, 28) = 22.3; p < 0.001). For each of the compounds, a dose of 20 mg/kg suppressed contextually elicited conditioned gaping relative to vehicle controls (p values <0.05). For both AM4301 (p < 0.05) and AM4302 (p < 0.001), but not AM4303, a dose of 10 mg/kg also suppressed gaping relative to controls. Finally, at a dose of 5 mg/kg, only those rats pretreated with AM4302 displayed suppressed gaping reactions (p < 0.001) relative to VEH controls. An overall 3 (drug) by 4 (dose) between-groups ANOVA revealed only main effects of drug, F(2, 83) = 4.4; p = 0.015, and dose, F(3, 83) = 20.2; p < 0.01. For the drug effect, subsequent Bonferroni pairwise comparisons revealed that rats pretreated with AM4302 displayed fewer conditioned gaping reactions overall than rats pretreated with AM4303 (p values <0.01). Rats pretreated with AM4301 did not differ overall from those pretreated with AM4303 or AM4302, suggesting an intermediate effect.
Mean (+SEM) number of gapes elicited by a LiCl-paired context in an anticipatory nausea test following pretreatment with various doses of AM4301 (top section), AM4303 (middle section), or AM4302 (bottom section). Different from VEH: *p < 0.05; **p < 0.01; ***p < 0.001; different from a dose of 5 mg/kg of AM4302; #p < 0.05; ##p < 0.01; n’s = 7–8/group
In each of the 15-min tests of locomotor activity that followed the AN test trial, none of the compounds modified general activity. The one-way ANOVA across doses for each compound revealed no significant effects, F values (3, 28) <2.2.
Experiment 6: potential of AM251 to reverse suppressive effect of AM4302 on AN
The suppressive effect of AM4302 (20 mg/kg, ip) on contextually elicited conditioned gaping was reversed by treatment with the CB1 antagonist, SR-141716 (2.5 mg/kg). SR did not have an effect on its own on contextually elicited gaping. Figure 6 presents the mean number of gapes elicited by a LiCl-paired chamber following VEH, VEH-SR (2.5 mg/kg), AM4302 (20 mg/kg), or AM4302 (20 mg/kg)-SR (2.5 mg/kg). The single factor ANOVA revealed a significant effect of pretreatment, F(3, 27) = 5.8; p = 0.004. Subsequent Bonferroni pairwise comparison tests showed that rats pretreated AM4302 gaped significantly (p values <0.001) less than all other groups.
Mean (+SEM) number of gapes elicited by the LiCl-paired context in the anticipatory nausea test trial among rats pretreated with VEH, VEH-SR (2.5 mg/kg), 20 mg/kg AM430, or AM4302-SR.; n’s = 7–8/group. The asterisks (***p < 0.001; **p < 0.01) depicts a significant difference from VEH and AM4302 + SR. The pound sign (#p < 0.05)
Endocannabinoid analysis
Standard curves for each endocannabinoid was linear with a regression value of >0.99. The extraction efficiencies for the quality controls in BSA compared to those in ethanol were greater than 90 %. Figure 7 presents the mean nanogram/gram levels of 2-AG and AEA from the VIC samples collected immediately following behavioral testing in experiment 5. Because of the violation of homogeneity of variance assessed by Levene’s test for both AEA (p < 0.001) and 2-AG (p < 0.001), the data were log transformed and analyzed as a single factor analysis of variance which revealed a significant difference among the groups for 2-AG, F(3, 27) = 22.0; p < 0.001, and for AEA, F(3, 27) = 10.4; p < 0.001. As seen in Fig. 7, relative to VEH, AM4301 elevated 2-AG (p < 0.025), but not AEA and AM 4303 elevated AEA (p < 0.01), but not 2-AG. Relative to VEH, AM4302 elevated both 2-AG (p < 0.001) and AEA (p < 0.001). Finally, it is interesting to note that AM4302 elevated 2-AG relative to AM4301 (p < 0.01), but did not differ from AM4303 in elevation of AEA.
Discussion
These experiments evaluated the potential of three newly developed endocannabinoid enzyme inhibitors to interfere with acute and anticipatory nausea in the rat gaping models. The in vitro studies revealed the following: (1) AM4301 selectively inhibits MAGL; (2) AM4303 selectively inhibits FAAH; (3) AM4302 inhibits both MAGL and FAAH. Indeed, the selectivity of these compounds was evident in the current endocannabinoid analysis conducted 2.5 h following administration of each of these compounds in which 2-AG and AEA were selectively increased by AM4301 and AM4303, respectively, while AM4302 increased both endocannabinoids. Systemic administration of each of these drugs suppressed both acute and anticipatory nausea in the rodent models of conditioned gaping. When administered prior to conditioning, each of the compounds equally suppressed acute LiCl-induced nausea as revealed by reduced conditioned gaping reactions displayed during the drug-free tests. As well, when directly administered to the VIC, the selective MAGL inhibitor, AM4301, suppressed acute nausea as we have shown with intra-VIC administration of 2-AG and the MAGL inhibitor MJN110 (Sticht et al. 2015). When administered prior to a test for contextually elicited conditioned gaping, the dual inhibitor, AM4302, was more effective than the FAAH inhibitors, AM4303, or the MAGL inhibitor AM4301; also the MAGL inhibitor, AM4301, was more effective than the FAAH inhibitor. This suggests that MAGL inhibition may be more effective than FAAH inhibition in suppressing anticipatory nausea (see also Parker et al. 2014). This is especially accentuated when considering the significantly higher overall efficacy of AM4303 with (Ki = 1.9 nM) when compared to the corresponding efficacy of AM4301 (Ki = 36 nM). Indeed, AM4302 was highly effective in suppressing anticipatory nausea at a dose as low as 5 mg/kg, whereas AM4301 required a dose of 10 mg/kg to suppress AN and the FAAH inhibitor 4303 was marginally effective even at a dose of 20 mg/kg. In the acute nausea paradigm, none of the compounds completely suppressed nausea as we have previously reported (Cross-Mellor et al. 2007; Parker et al. 2014).
The effect of systemic administration of the MAGL inhibition with AM4301 on both acute and anticipatory nausea in the current study was CB1 dependent because it was reversed by the CB1 antagonist, rimonabant, at a dose (2.5 mg/kg) that did not enhance acute or contextually elicited nausea. As well, the suppression of anticipatory nausea by AM4302 was also reversed by pretreatment with rimonabant.
We have previously reported that the dual FAAH/MAGL inhibitor, JZL195, also attenuates anticipatory nausea in rats (Limebeer et al. 2014). Although we did not measure AEA and 2-AG levels in the VIC following treatment with JZL195, we found that in rat whole brain tissue, treatment with JZL195 elevated AEA, but not 2-AG. However, whole brain levels of AEA and 2-AG measured by Limebeer et al. (2014) may not reflect localized concentrations of these endogenous cannabinoids at key CB1 receptors in the VIC that have been shown to play a critical role in nausea (Sticht et al. 2015) and may reflect the lower overall efficacy as a MAGL inhibitor. Moreover, Wiskerke et al. (2012) found that JZL195 elevated both 2AG and AEA levels in a localized brain area (nucleus accumbens) by about 50 and 100 %, respectively. Therefore, it is likely that JZL195 may elevate both AEA and 2-AG in the VIC as did AM4302 in the present study.
These results provide additional support for the regulation of nausea by treatments that elevate endocannabinoids. Moreover, the current study demonstrates that AM4302 is a highly effective dual MAGL/FAAH inhibitor that elevates both 2-AG and anandamide in rat tissue and is a tool for pharmacological investigation of behavioral effects following prolonged increases in endocannabinoid. Anticipatory nausea is a distressing disorder that can become so severe that as many as 20 % of patients discontinue their treatment even when vomiting is pharmacologically controlled (Foubert and Vaessen 2005; Hickok et al. 2003; Morrow et al. 2002; Roscoe et al. 2000). These results suggest that dual FAAH/MAGL inhibition may be a new direction for the development of treatments for this distressing and difficult to control side effect of chemotherapy.
References
Alapafuja SO, Nikas SP, Bharathan IT, Shukla VG, Nasr ML, Bowman AL et al (2012) Sulfonyl fluoride inhibitors of fatty acid amide hydrolase. J Med Chem 55:10074–10089
Andrews PL, Horn CC (2006) Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci 125:100–115
Contreras M, Ceric F, Torrealba F (2007) Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318:655–658
Cross-Mellor SK, Ossenkopp KP, Piomelli D, Parker LA (2007) Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacol 190:135–143
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358
Foubert J, Vaessen G (2005) Nausea: the neglected symptom? Eur J Oncol Nurs 9:21–32
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR (2003) Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: a University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer 97:2880–2886
Hornby PJ (2001) Central neurocircuitry associated with emesis. Am J Med 11(Suppl 8A):106S–112S
Limebeer CL, Abdullah R, Rock EM, Imhof E, Wang K, Lichtman AH, Parker LA (2014) Attenuation of anticipatory nausea in a rat model of contextually-elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacol 231:603–612
Limebeer CL, Hall G, Parker LA (2006) Exposure to a lithium-paired context elicits gaping in rats: a model of anticipatory nausea. Physiol Behav 88:398–403
Limebeer CL, Krohn JP, Cross-Mellor S, Litt DE, Ossenkopp KP, Parker LA (2008) Exposure to a context previously associated with nausea elicits conditioned gaping in rats: a model of anticipatory nausea. Behav Brain Res 187:33–40
Limebeer CL, Rock EM, Mechoulam R, Parker LA (2012) The anti-nausea effects of CB1 agonists are mediated by an action at the visceral insular cortex. Br J Pharmacol 167:1126–36
Morrow GR, Roscoe JA, Hickok JT, Andrews PR, Matteson S (2002) Nausea and emesis: evidence for a biobehavioral perspective. Support Care Cancer 10:96–105
Parker LA (2014) Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Eur J Pharmacol 722:122–133
Parker LA, Niphakis R, Downey R, Limebeer CL, Rock EM, Sticht MA, Morris H, Abdullah R, Lichtman A, Cravatt B (2014) A new MAGL inhibitor, MJN110, reduces acute and anticipatory nausea in rats and vomiting in Suncus murinus. Psychopharmacology 232:583–93
Parker LA, Rock EM, Limebeer CL (2011) Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol 163:1411–1422
Patricelli MP, Lashuel HA, Giang DK, Kelly JW, Cravatt BF (1998) Comparative characterization of a wild type and transmembrane domai-deleted fatty acid amide hydrolase: identification of the transmembrane domain as a site for oligomerization. Biochemistry 37:15177–87
Ramarao MK, Murphy EA, Shen MW, Wang Y, Bushell KN, Huang N et al (2005) A fluorescence-based assay for fatty acid amide hydrolase compatible with high-throughput screening. Anal Biochem 343:143–151
Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA (2008) The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacol 196:389–395
Rock EM, Limebeer CL, Ward JM, Cohen A, Grove K, Niphakis MJ, Cravatt BF, Parker LA (2015) Interference with acute nausea and anticipatory nausea in rats by fatty acid amide hydrolase (FAAH) inhibition through a PPARα and CB1 receptor mechanisms respectively in a double dissociation. Psychopharmacol 232:3841–48
Roscoe JA, Morrow GR, Hickok JT, Stern RM (2000) Nausea and vomiting remain a significant clinical problem: trends over time in controlling chemotherapy-induced nausea and vomiting in 1413 patients treated in community clinical practices. J Pain Symptom Manage 20:113–121
Sharkey KA, Darmani NA, Parker LA (2014) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146
Sticht MA, Limebeer CL, Abdullah RA, Poklis JL, Niphakis M, Rafla BF, Ho W, Cravatt BF, Sharkey KA, Lichtman AH, Parker LA (2012a) Effects of exogenous endocannabinoid administration and pharmacological inhibition of endocannabinoid catabolic enzymes in the visceral insular cortex on acute nausea-induced conditioned gaping in rats. Neuropharmacology 102:92–102
Sticht MA, Limebeer CL, Rafla BR, Parker LA (2015) Intra-visceral cortex 2-arachidonoylglycerol, but not N-arachidonoylethanolamide suppresses acute nausea-indcued conditioned gaping in rats. Neuroscience 286:338–44
Sticht MA, Long JZ, Rock EM, Limebeer CL, Mechoulam R, Cravatt BF, Parker LA (2012b) The MAGL inhibitor, JZL184, attenuates LiCl-induced vomiting in the Suncus murinus and 2-AG attenuates LiCl-induced nausea-like behaviour in rats. Br J Pharmacol 165:2425–2435
Tuerke KJ, Limebeer CL, Fletcher PJ, Parker LA (2012) Double dissociation between regulation of conditioned disgust and taste avoidance by serotonin availability at the 5-HT3 receptor in the posterior and anterior insular cortex. J Neurosci 32:13709–13717
Williams J, Wood J, Pandarinathan L, Karanian DA, Bahr BA, Vouros P et al (2007) Quantitative method for the profiling of the endocannabinoid metabolome by LC-atmospheric pressure chemical ionization-MS. Anal Chem 79:5582–5593
Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, Pattij T, Parsons LH (2012) Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS Chem Neurosci 3:407–417
Zvonok N, Pandarinathan L, Williams J, Johnston M, Karageorgos I, Janero DR et al (2008a) Covalent inhibitors of human monoacylglycerol lipase: ligand-assisted characterization of the catalytic site by mass spectrometry and mutational analysis. Chem Biol 15:854–862
Zvonok N, Williams J, Johnston M, Pandarinathan L, Janero DR, Li J et al (2008b) Full mass spectrometric characterization of human monoacylglycerol lipase generated by large-scale expression and single-step purification. J Proteome Res 7:2158–2164
Acknowledgments
This research was supported by a grant from the Natural Sciences and Engineering Research Council (92056) to LAP, Canadian Institute of Health Research (137122) to LAP, and National Institute of Health to AM and MAK Scientific LLS (2R44DA023737) to S0A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parker, L.A., Limebeer, C.L., Rock, E.M. et al. A comparison of novel, selective fatty acid amide hydrolase (FAAH), monoacyglycerol lipase (MAGL) or dual FAAH/MAGL inhibitors to suppress acute and anticipatory nausea in rat models. Psychopharmacology 233, 2265–2275 (2016). https://doi.org/10.1007/s00213-016-4277-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4277-y