Abstract
The burden of myocardial ischemia/reperfusion (IR) injury is 2–3-folds higher in diabetic patients, so protecting diabetic hearts is clinically important. Here, we investigated the effect of combinational therapy with vildagliptin and ischemic postconditioning (IPostC) on cardioprotection and the expression of genes regulating autophagy and mitochondrial function in diabetic hearts with IR injury. Type 2 diabetes was induced through high-fat diet and streptozotocin protocol in Wistar rats. Vildagliptin was orally administered to diabetic rats 5 weeks before IR injury. Myocardial-IR injury was modeled by ligation of left the coronary artery for 30 min followed by 60-min reperfusion, on a Langendorff-perfusion system. IPostC was applied at early reperfusion as 6 alternative cycles of 10-s reperfusion/ischemia. Creatine-kinase levels were measured spectrometrically, and infarct size was evaluated by TTC staining method. Left ventricles were harvested for assessing the expression levels of autophagy and mitochondrial-related genes using real-time PCR. Induction of diabetes significantly increased creatine-kinase release in comparison to healthy rats, and all treatments significantly reduced the release of enzyme toward control levels (P < 0.05). Only the combination therapy (IPostC + vildagliptin) could significantly reduce the infarct size of diabetic hearts as compared to untreated diabetic-IR group (P < 0.01). The levels of autophagy genes LC3 and p62 were significantly higher in diabetic groups than healthy ones. Induction of IR injury in diabetic hearts enhanced mitochondrial fission (drp-1) and reduced mitochondrial fusion (mfn1 and mfn2) genes. IPostC alone had no significant effect on the gene expression and vildagliptin alone could only affect LC3-II and mfn2 expressions. Nevertheless, administration of combination therapy significantly reduced the expression of both autophagy genes and increased both LC3-II/I and mfn2/1 ratios as compared with diabetic-IR hearts (P < 0.01–0.05). Application of this combination therapy could overcome the diabetes-induced failure of cardioprotection by individual treatments and improve mitochondrial dynamic and autophagy flux.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease and myocardial infarction are still at the forefront of diseases with high mortality rates, worldwide (Finegold et al. 2013). Myocardial ischemia/reperfusion (IR) injury is an unavoidable condition in cardiac procedures such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) surgery and heart transplantation. The size of infarction is the core determinant in the prognosis of myocardial IR injury. Reperfusion is the first and effective therapy for ischemic injury, but despite its necessity, reperfusion itself is also detrimental and can increase the infarct size severely and even cause death (Luc et al. 2018; Saeid et al. 2018). Type 2 diabetes is a global pandemic that increases the risk of cardiovascular mortality and makes the heart prone to IR insults (Saeid et al. 2018). This high risk of ischemic injuries in diabetic patients needs the multifactorial preventive and therapeutic approaches.
Conditioning treatments, applied before and/or after ischemia, can greatly reduce the infarct size and preserve cardiac function (Qiming et al. 2018; Zhao et al. 2003). In the IR heart without risk factors and comorbidities, application of ischemic postconditioning (IPostC), as the repetitive opening and closing of coronary artery, at the onset of reperfusion protects the cardiomyocytes against IR damages through a number of signaling pathways (Zhao et al. 2003). However, previous studies have shown that the protective effects of these interventions are abolished in the presence of diabetes (Bayrami et al. 2018). One possible explanation for this interaction is that the diabetes negatively alters the activity of intracellular survival effectors and thereby reduces the therapeutic power of interventions. One approach to overcome this challenge can be the combinational therapies.
Autophagy plays an important role in adapting cells to stress conditions by eliminating non-functional protein aggregates and defective organelles in cells and providing a “fast source of energy” for maintaining cell survival. However, an unnecessary increase in autophagy can destroy normal cell components and lead to cell death (Mizushima and Komatsu 2011). Growing evidence suggests that autophagy is over-activated during myocardial ischemic injuries (Ma et al. 2015), and on the other hand, its normal activity is impaired in diabetic circumstances (Munasinghe and Katare 2016). Further, the mitochondrial health and its normal function substantially depend on the normal expression of factors promoting normal autophagy (LC3B and p62), mitophagy, and mitochondrial fission and fusion (dynamic-related protein-1; drp1, mitofusins 1 and 2; and mfn1/2, respectively) (Maneechote et al. 2017; Kim and Yi 2018). The normal mitochondrial function can optimize the activity of autophagy regulators, and in turn, the optimized autophagy destroys defective mitochondria and improves its function. Thus, with the participation of these two cellular elements, cell homeostasis is maintained considerably. Therefore, modification of autophagy and mitochondrial dynamic can be considered as one of the effective tools to reduce the myocardial IR damages in diabetes.
Dipeptidyl peptidase-4 inhibitors (DPP-4, gliptins) are anti-diabetic drugs that have positive cardiovascular effects in addition to their hypoglycemic roles (Apaijai et al. 2013). It has been observed in a previous study that vildagliptin can improve the contractile activity and hemodynamic function of the IR heart by modifying the inflammatory and oxidative responses (Bayrami et al. 2018). It has also been reported that this drug affects the signaling pathways of cellular autophagy responses (Murase et al. 2015). However, its role in regulating autophagy/mitophagy and related mitochondrial processes in myocardial IR injury and diabetes is still unclear. Molecular changes in autophagy/mitophagy signaling can play an important role in determining the effectiveness of cardioprotective interventions in diabetic heart (Kim and Yi 2018). The normal function of both mitochondria and autophagy is impaired in diabetes as well as in cardiac ischemic injuries, and correcting this impairment by the therapies targeting these two main homeostatic mechanisms can be a good clinical approach. Therefore, with regard to the promising potentials of vildagliptin and postconditioning, the aim of this study was to investigate the effect of combinational therapy with vildagliptin and IPostC on cardioprotection and the expression of genes involved in autophagy, and mitochondrial fission and fusion in diabetic heart with IR injury.
Materials and methods
Animals
In this study, male Wistar rats weighing 200–250 g were used. Animals were kept in transparent polyethylene cages (4 rats in each) on wood shavings in the university animal house with a temperature of 22 ± 2 °C and a cycle of 12:12 h of dark and light. They had free access to water and normal food. All animal procedures and experimental interventions were carried out in accordance with the ethical guidelines and approved by the local Ethical Committee for Animal Research.
Animal grouping and study design
First, the animals were randomly allocated to healthy and diabetic groups. Healthy rats received standard diet and diabetic ones received high-fat diet with streptozotocin (STZ) injection (see the next section). Then, healthy rats were divided into two subgroups and diabetic rats were divided into five diabetic subgroups (n = 6/each), as following (Fig. 1):
-
Healthy sham (H-sham) group without IR injury: healthy rats were kept untreated for 12 weeks, and then, their beating hearts were isolated and mounted on a Langendorff perfusion system and received normal perfusion solution for 105 min. Myocardial IR injury was not induced in this group.
-
Healthy IR (H-IR) group: healthy rats were kept untreated for 12 weeks, and then, their beating hearts were isolated and mounted on a Langendorff perfusion system, in which after stabilization period (15 min), they experienced regional ischemia, through coronary artery ligation, for 30 min and then reperfusion for 60 min (as IR insult).
-
Diabetic ham (D-sham) group without IR injury: after induction of type 2 diabetes and completing the 12-week period (next section), the beating hearts of diabetic rats were isolated and mounted on a Langendorff perfusion system and received normal perfusion solution for 105 min. Myocardial IR injury was not induced in this group.
-
Diabetic IR (D-IR) group: after completing the 12-week diabetic period, the beating hearts of diabetic rats were isolated and mounted on a Langendorff perfusion system, in which after stabilization period (15 min), they experienced regional ischemia for 30 min and then reperfusion for 60 min (as IR insult).
-
Ischemic postconditioning (D-IPostC) group: the condition was similar to the D-IR group except that the isolated hearts received 6 alternative cycles of 10-s reperfusion and 10-s ischemia (as IPostC protocol), immediately at the beginning of main reperfusion.
-
Vildagliptin (D-Vilda) group: the condition was similar to the D-IR group except that before myocardial IR injury induction on a Langendorff system, the diabetic rats were pretreated with vildagliptin orally for 5 weeks (weeks 8–12).
-
Vildagliptin-IPostC (D-Vilda-IPostC) group: the condition was similar to the D-IR group except that before myocardial IR injury induction on a Langendorff system, the diabetic rats were first pretreated with vildagliptin orally for 5 weeks, and during IR protocol, the isolated beating hearts received six alternative cycles of 10-s reperfusion and 10-s ischemia (as IPostC protocol), at the onset of main reperfusion.
Rats in two diabetic groups received vildagliptin 6 mg/kg/day orally for 5 weeks from the beginning of the 8-week until the end of 12-week diabetic period (Bayrami et al. 2018). Each day at a certain time (4 pm), the required amount of vildagliptin was dissolved in distilled water according to the body weight of rats and was given to each rat by a gavage syringe. Normal saline was gavaged to the rats in other groups.
Induction of diabetes
Type 2 diabetes mellitus was induced in rats using a high-fat diet and low-dose STZ protocol (Bayrami et al. 2018). After 1 week of adaptation period, rats were fed with a high-fat saturated diet containing 35% normal pellet (Behparvar Co., Iran), 30% lard (local market source), 24% casein (Kazeinat Co., Iran), 4% sucrose, 1% cholesterol, and 0.3% dl-methionine (62% calories from fat) (all from Merck, Germany). Then, at the beginning of seventh week, 35 mg/kg of STZ (dissolved in citrate buffer pH 4.5) was injected intraperitoneally after 8 h of night fasting. One week after STZ injection, a glucometry was performed for testing fasting blood glucose (FBS) and oral glucose tolerance (OGT). Rats with FBS above 250 mg/dL and impaired OGTT (indicative of the initial phase of type 2 diabetes) were allocated to diabetic groups. The diabetes period was extended for the next 6 weeks (total period was 12 weeks). Rats with lower FBS were excluded from the study. Body weight and food intake of animals were recorded weekly.
Langendorff perfusion system and IR injury protocol
After completing 12 weeks, a constant-pressure mode of the Langendorff perfusion system was used to study myocardial injury ex vivo (Badalzadeh et al. 2017a). Following animal anesthesia with a mixture of ketamine (60 mg/kg) and xylasine (10 mg/kg) and heparinization with 500 IU of heparin sodium, the hearts of rats were isolated and rapidly mounted on the Langendorff system and then perfused with a perfusion solution containing (in mM/L): NaCl 118; KCl 4.7; NaHCO3 25; KH2PO4 1.2; MgSO4 1.2; CaCl2 2.5; and glucose 11.1 (all from Merck co, Germany) and bubbled with 95% O2, 5% CO2 at 37 °C and pH 7.4. After 15 min of stabilization period, the hearts in all groups except the two sham groups received regional ischemia for 30 min and then reperfusion for 60 min. Regional ischemia was applied by occluding the left anterior descending (LAD) coronary artery and reperfusion was achieved by reopening of it. The LAD occlusion was done using a temporary ligation of LAD with a 5–0 silk suture placed around the LAD. The effective coronary blockage and reperfusion were confirmed by a rapid drop in coronary flow and its recovery at the onset of reperfusion, respectively.
Measurement of infarct size
For the measurement of infarct sizes, the 2,3,5-triphenylte-trazolium chloride (TTC) staining method and a computerized planimetry was employed (Bayrami et al. 2018). Briefly, the LAD coronary artery was re-occluded at the end of reperfusion and then 2 mL Evans blue dye (0.25%) (Sigma, Germany) was administered to the hearts through aortic cannula. Thereafter, the hearts were frozen and cut into 2-mm slices; the slices were immersed in 1% TTC (Sigma, Germany) in phosphate-buffered solution (pH 7.4) for 20 min. Then, the ventricular volumes, areas at risk, and infarct sizes of the hearts were calculated, using the ImageJ software.
Measurement of creatine kinase
The myocardial release of enzyme creatine kinase-myocardial band (CK-mB) was evaluated to compare the level of heart damage among groups. For this purpose, the coronary fluid released from the heart was collected during reperfusion phase. The enzyme was then measured in the mixed coronary effluent samples by the spectrophotometric method according to the kits instructions (Pars Azmoon Co., Iran). The absorbance of the samples was read at 525 nm, and the levels of CK-mB enzyme were reported in units per liter.
RNA extraction and real-time PCR
Approximately 100 mg of areas at risk of left ventricles were obtained and lysed on ice in 1 mL of lysis buffer solution containing protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO) at pH 7.4. The resultant sample solution was used for Real-Time PCR experiments. Total RNA of the samples was extracted using miRCURYTM RNA isolation kit, based on the protocol provided by the manufacturer (Exiqon, Vedbaek, Denmark). A nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, 19810, USA) was used to measure the RNA content and purity.
The expression profiles of mRNAs were performed on total RNA extracted by using the universal cDNA synthesis kit (Yavari et al. 2016). Briefly, total RNA was poly adenylated and cDNA was synthesized using a poly (T) primer with a 30 degenerate anchor and a 50 universal tag (Exiqon, Vedbaek, Denmark). Revert Aid First Strand cDNA Synthesis Kit (FermentasGmBH, Leon-Rot, Germany) with random hexamer primers and MMLV reverse transcriptase (as a complete system for efficient synthesis of first strand cDNA from mRNA or total RNA templates) were used for determination of mRNAs expression levels. Real-time PCR reactions were performed on a Bio-Rad iQ5 detection System (Bio-Rad, Richmond, CA, USA). Forward and reverse primers for genes are shown in Table 1. Relative expressions of mRNAs were normalized to GAPDH expression levels. The 2−(ΔΔCt) method was used to determine the relative-quantitative levels of individual mRNAs. The results were expressed as the fold change to the relevant controls.
Statistical analysis
All values were expressed as mean ± standard deviations. The parametric variables between groups were analyzed using one-way ANOVA followed by Tukey’s post hoc test. The statistical significance of differences was considered as P < 0.05.
Results
Basic characteristics
One week after STZ administration (at the end of 7th week), diabetic rats had significantly higher blood glucose level and impaired OGT test values as compared with those of healthy rats (Table 2). At the end of 12th week, the ANOVA analysis showed the higher heart weight and the ratio of heart weight to body weight in diabetic versus healthy rats. In addition, vildagliptin pretreatment in diabetic rats could significantly diminish hyperglycemia (P < 0.01), and reduce heart and body weights (P < 0.05), comparing to the untreated diabetic rats (Table 2).
Level of CK-mB enzyme released from I/R hearts
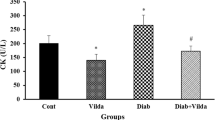
The content of CK-mB release from the diabetic hearts with or without IR injury was significantly higher than those of healthy non-diabetic groups (P < 0.05) (Fig. 2). Induction of IR injury could not further increase the CK-mB levels as compared to the D-sham group. Application of IPostC to the hearts and pretreatment of rats with vildagliptin similarly reduced the levels of CK-mB, in comparison to the D-IR group (P < 0.05). Additionally, combinational therapy with vildagliptin and IPostC had greater impact on the reduction of this enzyme (P < 0.01 vs. D-IR group).
Myocardial infarct size
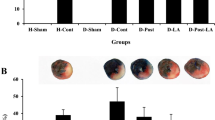
Induction of myocardial IR injury in healthy and diabetic rats provided almost similar infarct sizes (42 ± 2.4% and 38 ± 1.6%, respectively) (Fig. 3). The infarct sizes were reduced in the D-post and D-vild groups in comparison to the D-IR group, but these reductions were not statistically significant. However, concomitant application of treatments in diabetic rats had significant infarct reducing effect as compared with those of the D-IR group (P < 0.01; Fig. 3).
The expression levels of autophagy genes
Changes in the expression levels of LC3-I, LC3-II, and p62 genes were evaluated as the main determinants of autophagic activity in cardiomyocytes (Fig. 4). Induction of type 2 diabetes significantly increased the expression levels of both autophagy genes (P < 0.05 D-sham vs. H-sham). LC3 levels were significantly reduced after IR injury in diabetic rats (P < 0.05), but the level of p62 remained unchanged (D-IR vs. D-sham). In addition, the ratio of LC3-II/LC3-I was also reduced by IR injury in diabetic hearts (P < 0.05). IPostC could not significantly affect both genes in diabetic IR hearts. While vildagliptin alone significantly lowered only the level of LC3-II expression, the combination of IPostC and vildagliptin significantly reduced the expression levels of both autophagy genes LC3 and p62 in comparison to the D-IR group (P < 0.05 for LC3 and P < 0.01 for p62). Moreover, LC3-II/LC3-I ratio tended to increase after combined therapy.
Expression levels of autophagy genes, LC3-I, LC3-II, LC3-II/LC3-I ratio, and p62, in cardiomyocytes of experimental groups.*P < 0.05 as compared with the H-sham group; $P < 0.05 as compared with the D-sham group; #P < 0.05 and ##P < 0.01 as compared with the D-IR group. Mean ± SD. n = 6. (H, healthy; IR, ischemia/reperfusion; D, diabetic; post, ischemic postconditioning; vild, vildagliptin)
The expression levels of mitochondrial fission gene
Changes in the gene expression levels of drp-1 were evaluated as the main indicator of mitochondrial fission in cardiomyocytes (Fig. 5). Induction of IR injury in both series of healthy and diabetic rats significantly increased the expression level of drp-1 gene, compared with the corresponding sham groups (P < 0.01 and P < 0.05, respectively). IPostC alone or in combination with vildagliptin could significantly inhibit the sharp increase in drp-1 gene expression in comparison to the D-IR group (P < 0.05, both), while vildagliptin alone could not have a significant effect (Fig. 5).
Expression levels of mitochondrial fission gene, Drp-1, in cardiomyocytes of the experimental groups. **P < 0.01 as compared with the H-sham group; $P < 0.05 as compared with the D-sham group; #P < 0.05 as compared with the D-IR group. Mean ± SD. n = 6. (H, healthy; IR, ischemia/reperfusion; D, diabetic; post, ischemic postconditioning; vild, vildagliptin)
The expression levels of mitochondrial fusion genes
Changes in the gene expression levels of mfn-1 and mfn-2 and the ratio of mfn2/mfn1 were analyzed as the main indicators of mitochondrial fusion in IR-experienced hearts. Following myocardial IR injury in both healthy and diabetic rats, the expression of both genes tended to decrease, so that this decrease was more considerable in healthy rats (Fig. 6). Using IPostC alone in diabetic hearts had no significant effect on any of the mitochondrial fusion genes and the ratio of mfn2/mfn1, and vildgliptin-pretreatment could increase only the expression level of mfn-2 (P < 0.05). However, following the administration of both therapeutic interventions simultaneously, the expression levels of both genes as well as their ratio were significantly increased compared with those of the D-IR group (P < 0.05 for mfn-1 and ratio, and P < 0.01 for mfn-2).
Expression levels of mitochondrial fusion genes, mfn-1, mfn-2, and mfn2/1 ratio, in cardiomyocytes of the experimental groups. *P < 0.05 and **P < 0.01 as compared with the H-sham group; #P < 0.05 and ##P < 0.01 as compared with the D-IR group. Mean ± SD. n = 6. (H, healthy; IR, ischemia/reperfusion; D, diabetic; post, ischemic postconditioning; vild, vildagliptin)
Discussion
According to the results of the present study, administration of any therapeutic interventions individually was associated with some limited positive effects on gene expression and cardioprotection in IR injury of rats with type 2 diabetes. Postconditioning with ischemia (IPostC) alone could only decrease the levels of CK-mB release and drp-1 expression. Similarly, preconditioning or pretreatment with vildagliptin only decreased the levels of CK-mB and LC3-II expression and increased mfn-2 expression. Nevertheless, administration of both therapies (vildagliptin and IPostC) concomitantly had more considerable and consistent effects on all parameters and significantly protracted the diabetic hearts against IR insult. This combinational therapy decreased the amount of CK-mB and infarct size and reduced the expression levels of autophagy genes LC3-II/LC3-I and p62 and mitochondrial fission marker drp-1 and increased the mitochondrial fusion markers mfn1/2 in diabetic IR hearts. In this study, we focused on two important cellular homeostatic processes, namely autophagy and mitochondrial dynamic (fission and fusion), which are functionally interconnected. Both processes are negatively implicated in myocardial IR injury and diabetes circumstances (Munasinghe and Katare 2016; Maneechote et al. 2017; Kim and Yi 2018; Apaijai et al. 2013). Therefore, using treatments that can correct negative changes in these two cellular phenomena may have promising cardioprotective outcomes against IR hearts, especially during diabetic settings.
The precise role of autophagy in cardiac complications of diabetes is still unclear. Some studies have reported that autophagy’s activity in myocardial IR injury increases dramatically, and this increase can exacerbate the cardioprotection (Matsui et al. 2007). It has also been shown that the level of autophagy decreases during diabetes (Munasinghe and Katare 2016). Nevertheless, there are quite opposite reports, as well (Xie et al. 2016). The normal autophagy flux is due to the enhancement of the activity of autophagic promoters and the clearance of autophagosomes by the lysosomes. LC3-II is a main autophagy promoter and its expression is associated with the increased levels of autophagic activity. On the other hand, p62 is a selective substrate of autophagy and subjected to autophagic degradation by the lysosome, so a reduction in its level as well as an augmentation in LC3-II/LC3-I ratio indicate the autophagic flux enhancement (Matsui et al. 2007; Gottlieb and Mentzer Jr 2010; Kadowaki and Karim 2009). In our study, the expression levels of both markers, LC3 and p62, were upregulated in diabetic hearts in comparison to the healthy ones, and induction of myocardial IR injury reduced the expression of LC3-II and LC3-II/I ratio with no considerable effect on p62 level. These findings are in agreement with the previous reports and indicate an impairment in the autophagy pathway and autophagy flux in diabetic hearts with IR injury (Munasinghe and Katare 2016; Matsui et al. 2007). Combination therapy with IPostC and vildagliptin significantly reduced the expression levels of both genes and increased LC3-II/I ratio. These indicate that combination therapy in diabetic IR hearts could modulate the upregulated authophagic activity and enhanced the autophagy flux. These changes can provide an encouraging intracellular condition for producing a better cardioprotection by combination therapy.
On the other hand, the mitochondrial fission and fusion are two necessary processes for the maintenance of normal mitochondrial function. Overproduction of reactive oxygen species (ROS) and an intracellular calcium overload during IR injury can contribute to the mitochondrial fragmentation through increasing the expression and activity of fission factor, drp-1 (Ong et al. 2010; Orcid et al. 2018). This condition is also seen during diabetes (Rovira-Llopis et al. 2017). When activated, drp-1 translocates on outer mitochondrial membrane and divides the mitochondria (Maneechote et al. 2017). However, the fusion process can create an interconnected mitochondrial network and make better cell homeostasis by improved communication with other intracellular organelles, such as the endoplasmic reticulum (Maneechote et al. 2017). It has been reported that reduced mitochondrial fusion during myocardial IR injury can also lead to the fragmentation and dysfunction of mitochondria (Vasquez-Trincado et al. 2016). Mfn1/2 and OPA-1 mediate mitochondrial fusion through acting on its outer and inner membranes, respectively (Maneechote et al. 2017; Vasquez-Trincado et al. 2016). In the present study in line with previous studies, even though diabetes itself did not have any significant effect on mitochondrial dynamic markers, induction of IR injury increased drp-1 and reduced mfn1/2 expressions in diabetic hearts. Combined treatment significantly modified both fission and fusion indicators, while the effect of individual treatments on these parameters was not completely reliable. Thus, it can be understood that diabetes can exacerbate the activity of intracellular survival effectors and the application of monotherapy does not overcome these diabetes-induced intracellular changes to produce its protective effect. However, by combining two therapeutic interventions together, their individual effects are strengthened and full cardioprotection achieved. It seems that their additive effects are through acting on both common and parallel survival pathways. Choosing the true elements in the combination therapy is very important. In this case, both IPostC and vildagliptin have been shown to have protective effects in non-diabetic hearts (Apaijai et al. 2013; Badalzadeh et al. 2017a). Additionally, both have the potential to influence on mitochondrial function and autophagic activity in different tissues (Bayrami et al. 2018; Apaijai et al. 2013). So, it can be reasoned that their combined positive effects are amplified if administered concomitantly.
We previously reported in a study that concomitant administration of cyclosporine-A and IPostC was able to overcome the failure of individual IPostC and to protect significantly the IR hearts of type 1 diabetic rats (Badalzadeh et al. 2017b). In addition, it has recently been observed that the use of vildagliptin and metformin has had better additive effects on the IR hearts in obese mice (Apaijai et al. 2014). Also, combining metformin and fenofibrate yields cardioprotection in IR hearts of rats with type 2 diabetes (Oidor-Chan et al. 2016). These findings are in consistent with our study and may represent a novel combined treatment approach to overcome the interfering effects of diabetes with cardioprotection. Our findings confirm the previous hypothesis that diabetes can cause many metabolic and intracellular changes, all negatively affect the activity of survival mediators, and therefore, administration of any of the therapeutic interventions individually cannot have a considerable potency to activate cellular protective pathways. Recent experimental studies have proposed that DPP-4 inhibitors as a monotherapy or in combination with other agents have potentially beneficial effects on cardiovascular outcomes in hypertension, heart failure, and myocardial infarction (Home 2019; Park et al. 2016). Although different DPP-4 inhibitors act on the human body in similar ways, there are some pharmacological differences among these drugs with regard to different cardiovascular endpoints (Home 2019). However, the reasons for the different effects of DPP-4 inhibitors on cardiovascular disease are unclear yet.
Comprehensive analysis of the effect of vildagliptin on the different substrates in IR hearts will help to identify the underlying protection mechanisms. Many humoral factors in circulation and within the myocardium may activate cardiomyocyte signaling pathways, which eventually convey the cardioprotective signal to the end effectors, particularly the mitochondria. Platelets, inflammatory cytokines/chemokines, nitric oxide, growth factors and adenosine can mediate the effect of the vildagliptin in increasing the power of postconditioning response (Saeid et al. 2018; Zhao et al. 2003). These factors also have preconditioning properties. Diabetes likely rises the platelet capability to increase microvascular thrombosis and inflammatory response (Maiocchi et al. 2018). In patients with type 2 diabetes who are suffering from coronary artery disease, combining vildagliptin to metformin has led to a significant suppression of the levels of IL-1ß, TNF-α, C-reactive protein, and hemoglobin-A1C during follow up (Younis et al. 2017). Thus, a change in cytokine expression by vildagliptin may contribute to the improving of IPostC potency.
Furthermore, some protective actions of vildagliptin can be attributed to its improving effects on the metabolic alterations induced by diabetes. It has been approved that this drug can improve lipid profile and hyperglycemia during diabetes (Bayrami et al. 2018). Increased fatty acid in diabetes triggers opening of mitochondrial permeability transition at reperfusion, leading to the worsening of mitochondrial function and dysregulation of ROS, apoptosis and autophagy process (Bayrami et al. 2018). Moreover, vildagliptin effects can be mediated through increasing the incretin hormone, glucagon-like peptide-1 (GLP-1), as a result of DPP-4 inhibition (Ravassa et al. 2012). Nonetheless, we did not explore the direct effect of vildagliptin on GLP-1 in this study, as a limitation. Myocardial GLP-1 receptor activation during IR injury can potentiate the insulin signaling, augment the myocardial intake of glucose, and thereby promote energy metabolism (Ravassa et al. 2012). GLP-1 was also reported to activate the cAMP-dependent protein kinase A (PKA) by which it can boost ionic flow (such as l-type Ca2+current) and the activity of reperfusion-induced salvage kinase signaling, specifically AMP kinase and Akt-dependent intracellular pathways in cardiomyocytes (Saeid et al. 2018; Home 2019; Ravassa et al. 2012).
Another possibility is that increased GLP-1 levels by vildagliptin directly influences mitochondrial preservation (Home 2019), since GLP-1 has directly targeted mitochondria in hepatocytes, exerting insulin-like actions via modifying oxidative-phosphorylation and attenuating oxidative stress (Tomas et al. 2011). Finally, vildagliptin-mediated survival and cardiac preservation after IR injury may also be attributed to a reduced activation of inflammatory responses, phosphorylation of endothelial nitric oxide synthase, direct vascular actions, and improvement of myocardial microcirculation and endothelial function, through the non-enzymatic GLP-1-independent pathway (Younis et al. 2017; Miyoshi et al. 2014). Deciphering the exact role of DPP-4 in the heart will shed light on the novel functions of DPP-4 inhibitors in cardiovascular medicine.
In conclusion, one of the best approaches to reduce the impact of IR injuries in diabetic conditions is to use suitable combination therapies to modulate the diabetes-induced structural and functional changes in two important intracellular arms of cardioprotection, namely autophagy and mitochondrial function. Concomitant administration of both IPostC and vildagliptin had greater cardioprotective effects and improved the low effectiveness of each treatment on infarct size and gene expressions. Modification of autophagy and autophagic flux, inhibition of mitochondrial fission, and improvement of mitochondrial fusion can contribute to the cardioprotection of combination therapy. Our finding indicated the potential role of vildagliptin combination therapy on the early cardioprotection, but long-term effects after reperfusion injury need further investigation. There are many gaps in the knowledge that modulation of autophagy and mitochondrial dynamic can protect the diabetic heart. This modulation requires future intensive research before clinical application is applied.
References
Apaijai N, Pintana H, Chattipakorn SC, Chattipakorn N (2013) Effects of vildagliptin versus sitagliptin, on cardiac function, heart rate variability and mitochondrial function in obese insulin-resistant rats. Br J Pharmacol 169(5):1048–1057. https://doi.org/10.1111/bph.12176
Apaijai N1, Chinda K, Palee S, Chattipakorn S, Chattipakorn N (2014) Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia-reperfusion injury in obese-insulin resistant rats. PLoS One 9(7):e102374. https://doi.org/10.1371/journal.pone.0102374
Badalzadeh R, Azimi A, Alihemmati A, Yousefi B (2017a) Chronic type-I diabetes could not impede the anti-inflammatory and antiapoptotic effects of combined postconditioning with ischemia and cyclosporine a in myocardial reperfusion injury. J Physiol Biochem 73(1):111–120. https://doi.org/10.1007/s13105-016-0530-4
Badalzadeh R, Tabatabaei SM, Mohammadi M, Khaki A, Mohammadnezhad D (2017b) Combined postconditioning with ischemia and cyclosporine-A restore oxidative stress and histopathological changes in reperfusion injury of diabetic myocardium. Iran J Basic Med Sci 20(10):1079–1087. https://doi.org/10.22038/IJBMS.2017.9444
Bayrami G, Karimi P, Agha-Hosseini F, Feyzizadeh S, Badalzadeh R (2018) Effect of ischemic postconditioning on myocardial function and infarct size following reperfusion injury in diabetic rats pretreated with vildagliptin. J Cardiovasc Pharmacol Ther 23(2):174–183. https://doi.org/10.1177/1074248417729881
Finegold JA, Asaria P, Francis DP (2013) Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int J Cardiol 168(2):934–945. https://doi.org/10.1016/j.ijcard.2012.10.046
Gottlieb RA, Mentzer RM Jr (2010) Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol 72:45–59. https://doi.org/10.1146/annurev-physiol-021909-135757
Home P (2019) Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia. 62(3):357–369. https://doi.org/10.1007/s00125-018-4801-1
Kadowaki M, Karim MR (2009) Cytosolic LC3 ratio as a quantitative index of macroautophagy. Methods Enzymol 452:199–213. https://doi.org/10.1016/S0076-6879(08)03613-6
Kim JS, Yi HK (2018) Schisandrin C enhances mitochondrial biogenesis and autophagy in C2C12 skeletal muscle cells: potential involvement of anti-oxidative mechanisms. Naunyn Schmiedeberg's Arch Pharmacol 391(2):197–206. https://doi.org/10.1007/s00210-017-1449-1
Luc JGY, Choi JH, Rizvi SA, Phan K, MonchoEscrivà E, Patel S, Reeves GR, Boyle AJ, Entwistle JW, Morris RJ, Massey HT, Tchantchaleishvili V (2018) Percutaneous coronary intervention versus coronary artery bypass grafting in heart transplant recipients with coronary allograft vasculopathy: a systematic review and meta-analysis of 1,520 patients. Ann Cardiothorac Surg 7(1):19–30. https://doi.org/10.21037/acs.2018.01.10
Ma S, Wang Y, Chen Y, Cao F (2015) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim. Biophys Acta Mol Basis Dis 1852(2):271–276. https://doi.org/10.1016/j.bbadis.2014.05.010
Maiocchi S, Alwis I, Wu MCL, Yuan Y, Jackson SP (2018) Thromboinflammatory functions of platelets in ischemia-reperfusion injury and its dysregulation in diabetes. Semin Thromb Hemost 44(2):102–113. https://doi.org/10.1055/s-0037-1613694
Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N (2017) Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med 21(11):2643–2653. https://doi.org/10.1111/jcmm.13330
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion. Circ Res 100(6):914–922. https://doi.org/10.1161/01.RES.0000261924.76669.36
Miyoshi T, Nakamura K, Yoshida M, Miura D, Oe H, Akagi S, Sugiyama H, Akazawa K, Yonezawa T, Wada J, Ito H (2014) Effect of vildagliptin, a dipeptidyl peptidase 4 inhibitor, on cardiac hypertrophy induced by chronic beta-adrenergic stimulation in rats. Cardiovasc Diabetol 13:43. https://doi.org/10.1186/1475-2840-13-43
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell. 147(4):728–741. https://doi.org/10.1016/j.cell.2011.10.026
Munasinghe PE, Katare R (2016) Maladaptive autophagy in diabetic heart disease. Int J ClinExpPhysiol 3:155–165 http://www.ijcep.org/text.asp?2016/3/4/155/196893
Murase H, Kuno A, Miki T, Tanno M, Yano T, Kouzu H, Ishikawa S, Tobisawa T, Ogasawara M, Nishizawa K, Miura T (2015) Inhibition of DPP-4 reduces acute mortality after myocardial infarction with restoration of autophagic response in type 2 diabetic rats. Cardiovasc Diabetol 11(14):103. https://doi.org/10.1186/s12933-015-0264-6
Oidor-Chan VH, Hong E, Pérez-Severiano F, Montes S, Torres-Narváez JC, Del Valle-Mondragón L, Pastelín-Hernández G, Sánchez-Mendoza A (2016) Fenofibrate plus metformin produces cardioprotection in a type 2 diabetes and acute myocardial infarction model. PPAR Res 8237264:1–14. https://doi.org/10.1155/2016/8237264
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 121(18):2012–2022. https://doi.org/10.1161/CIRCULATIONAHA.109.906610
Orcid J, Cooper KF, Strich R (2018) Reactive oxygen species and mitochondrial dynamics: the yin and Yang of mitochondrial dysfunction and cancer progression. Antioxidants 7(1):13. https://doi.org/10.3390/antiox7010013
Park SH, Nam JY, Han E, Lee YH, Lee BW, Kim BS, Cha BS, Kim CS, Kang ES (2016) Efficacy of different dipeptidyl peptidase-4 (DPP-4) inhibitors on metabolic parameters in patients with type 2 diabetes undergoing dialysis. Medicine(Baltimore) 95(32):e4543. https://doi.org/10.1097/MD.0000000000004543
Qiming D, Yunhui X, Pauline M, Jennifer K, Eric M, Sandrine P (2018) Preconditioning and postconditioning by cardiac glycosides in the mouse heart. J Cardiovasc Pharmacol 71(2):95–103. https://doi.org/10.1097/FJC.0000000000000549
Ravassa S, Zudaire A, Díez J (2012) GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res 94(2):316–323. https://doi.org/10.1093/cvr/cvs123
Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM (2017) Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol 11:637–645. https://doi.org/10.1016/j.redox.2017.01.013
Saeid F, Aniseh J, Reza B, Manouchehr VS (2018) Signaling mediators modulated by cardioprotective interventions in healthy and diabetic myocardium with ischaemia-reperfusion injury. Eur J Prev Cardiol 25(14):1463–1481. https://doi.org/10.1177/2047487318756420
Tomas E, Stanojevic V, Habener JF (2011) GLP-1-derived nonapeptide GLP-1(28-36) amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul Pept 11(167):177–184. https://doi.org/10.1016/j.regpep.2011.01.003
Vasquez-Trincado C, Garcia-Carvajal I, Pennanen C (2016) Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol 594(3):509–525. https://doi.org/10.1113/JP271301
Xie J, Cui K, Hao H, Zhang Y, Lin H, Chen Z (2016) Acute hyperglycemia suppresses left ventricular diastolic function and inhibits autophagic flux in mice under prohypertrophic stimulation. Cardiovasc Diabetol 15(1):136. https://doi.org/10.1186/s12933-016-0452-z
Yavari R, Badalzadeh R, Alipour MR, Tabatabaei SM (2016) Modulation of hippocampal gene expression of microRNA-146a/microRNA-155-nuclear factor-kappa B inflammatory signaling by troxerutin in healthy and diabetic rats. Indian J Pharm 48(6):675–680. https://doi.org/10.4103/0253-7613.194847
Younis A, Eskenazi D, Goldkorn R, Leor J, Naftali-Shani N, Fisman EZ, Tenenbaum A, Goldenberg I, Klempfner R (2017) The addition of vildagliptin to metformin prevents the elevation of interleukin 1ß in patients with type 2 diabetes and coronary artery disease: a prospective, randomized, open-label study. Cardiovasc Diabetol 16(1):69. https://doi.org/10.1186/s12933-017-0551-5
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285(2):H579–H588. https://doi.org/10.1152/ajpheart.01064.2002
Acknowledgments
The authors thank the Clinical Research Development Unit, Shohada Hospital and Molecular Medicine Research Center, Tabriz University of Medical Sciences for their kind supports.
Funding
This study was supported by a grant from National Elites Foundation, Tehran—Iran, and Molecular Medicine Research Center, Tabriz University of Medical Sciences, Tabriz—Iran.
Author information
Authors and Affiliations
Contributions
VB and RB conceived and designed research. LP and RB conducted the experiments. LP, VB, and NP analyzed the data. LP, RB, and NP wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
All animal procedures and experimental interventions were carried out in accordance with the ethical guidelines and approved by the local Ethical Committee for Animal Research.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pirzeh, L., Babapour, V., Badalzadeh, R. et al. Pretreatment with vildagliptin boosts ischemic-postconditioning effects on cardioprotection and expression profile of genes regulating autophagy and mitochondrial fission/fusion in diabetic heart with reperfusion injury. Naunyn-Schmiedeberg's Arch Pharmacol 392, 1371–1382 (2019). https://doi.org/10.1007/s00210-019-01660-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01660-z