Abstract
The molecular study of muscles is needed to overcome chronic inflammation and maintenance of muscles in the human body. Schisandrin C is a pharmacological compound derived from the fruit of Schisandra chinensis and has many characteristics including anti-inflammation, anti-tumor, and anti-oxidation. However, the cellular and molecular mechanisms of Schisandrin C are still not well understood especially in skeletal muscle. Therefore, the present study was evaluated whether the properties of Schisandrin C in C2C12 skeletal muscle cells involved maintenance of cellular homeostasis and protection against oxidative damage. Differentiated C2C12 cells were exposed to H2O2 to induce oxidative stress. The characteristics of anti-oxidants, anti-inflammation, autophagy, and mitochondrial biogenesis were tested by Western blotting. Confocal microscopy was also used to observe mitochondrial activity. Schisandrin C inhibited inflammatory molecules with enhancing anti-oxidant activity and reducing reactive oxygen species (ROS) even in the presence of H2O2. The dual anti-inflammation and anti-oxidant roles of Schisandrin C regulated the translocation of nuclear factor kappa B (NF-κB) and nuclear factor erythroid 2-related factor-2 (Nrf-2) to nucleus followed by inhibition of the mitogen-activated protein kinase (MAPK) pathway. Schisandrin C promoted the expression of autophagy and mitochondrial biogenesis molecules. Furthermore, the effect of Schisandrin C increased the mitochondrial activity against oxidative stress. Consequently, the action of Schisandrin C enhanced the regulation of autophagy and mitochondrial biogenesis with potential involvement of anti-oxidative mechanisms including the MAPKs/Nrf-2/heme oxygenase-1 signaling pathway in C2C12 skeletal muscle cells exposed to oxidative stress. Therefore, Schisandrin C may be considered as a beneficial compound for several muscle inflammations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is the underlying cause of many human diseases (Guo et al. 2008). Chronic inflammation of skeletal muscle is characterized by the invasion of mononuclear immune cells, myositis, and the destruction of muscle tissue caused by exercising too much or performing extreme exercises (Guo et al. 2008; Oh et al. 2013). Therefore, the study of molecular pathway to protect muscles is needed to overcome chronic inflammation and facilitate the repair and maintenance of muscles in the human body.
The inflammatory responses are mediated by various inflammatory cytokines and molecules, which are indices of inflammatory activity (Oh et al. 2010; Chun et al. 2014; Liu et al. 2015). NF-κB is a key regulator known to exacerbate inflammatory diseases. Pro-inflammatory genes, matrix metalloproteinase-2, metalloproteinase-9 (MMP-2/9), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) are modulated by NF-κB translocation (Rahman and Fazal 2011). Heme oxygenase-1 (HO-1) plays an important role in mitochondrial biogenesis and downregulation of chronic inflammatory molecules, intracellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) (Zukor et al. 2009). In addition, HO-1 promotes mitochondrial macroautophagy and reduces redox-activity in age-related diseases (Zukor et al. 2009; Lee et al. 2015). The stimulation of autophagy is critical for maintaining muscle mass and mitochondrial biogenesis in skeletal muscle (Masiero et al. 2009; Lesmana et al. 2016). It has been known that HO-1 expression is continuously regulated by PI3K/Akt, MAPKs, NF-κB, and Nrf-2 signaling pathways, which induce anti-oxidative and anti-inflammatory responses (Surh et al. 2008; Furukawa et al. 2010; Paine et al. 2010; Park et al. 2011).
The anti-inflammatory effects of medicinal plants have been exploited in prior studies to repair damaged muscles (Oh et al. 2013; Kang et al. 2014). Particularly, Schisandrin C is a pharmacological compound extract from the fruit of Schisandra chinensis existing anti-inflammatory properties (Oh et al. 2010; Park et al. 2013). The beneficial therapeutic effects of Schisandrin C are associated with anti-oxidants and anti-inflammation characteristics, which exert protective effects in various tissues (Panossian and Wikman 2008; Park et al. 2011, 2013; Chun et al. 2014). Some studies reports that Schisandrin C-mediated anti-inflammatory activity is correlated with the inhibition of pro-inflammatory cytokine expression through blocking of NF-κB translocation followed by the inhibition of the p38 and stress-activated protein kinase/Jun N-terminal kinase (SAPK/JNK) pathway (Guo et al. 2008; Park et al. 2013; Chun et al. 2014). However, the molecular signaling pathway by which Schisandrin C exerts its influence on HO-1-mediated authophagy and mitochondrial biogenesis has not been explored. These pathways are intimately associated with the effects maintenance of cellular homeostasis and protection by Schisandrin C against oxidative damage in skeletal muscle cells.
Therefore, in this study, we examined whether anti-oxidant and anti-inflammatory activities of Schisandrin C exerted an effect on H2O2-stimulated C2C12 skeletal muscle cells and we endeavored to identify any associated molecular signaling pathway. Furthermore, this study evaluated the regulation of cellular homeostasis by examined autophagy and mitochondrial biogenesis.
Materials and methods
Reagents
Schisandrin C (PubChem CID: 443027; SMB00323) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies to Mn-SOD (sc-130345; MW: 25 kDa), CuZn-SOD (sc-101523; MW: 19 kDa), IL-1β (sc-7884; MW: 31 kDa), Nrf-2 (sc-722; MW: 57 kDa), NF-κB (p65) (sc-109; MW: 65 kDa), PGC-1α (sc-13067; MW: 90 kDa), HO-1 (sc136960; MW: 32 kDa), VCAM-1 (sc-8304; MW: 110 kDa), MMP-9 (sc-10,737; MW: 92 kDa), and SIRT-1 (sc-74465; MW: 120 kDa) were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody to p62 (ab91526; MW: 47 kDa) was acquired from Abcam (Boston, MA, USA). The antibodies to ATG-5 (BZ01274; MW: 56 kDa), Beclin-1 (BZ01799; MW: 60 kDa), MMP-2 (BS1236; MW: 72 kDa), NRF-1 (BS7179; MW: 53 kDa), iNOS (BZ01465; MW: 130 kDa), phosphorylated AMPK (BS5003; MW: 62 kDa), and Lamin B1 (BS3547; MW: 68 kDa) were acquired from Bioworld Technology (Louis Park, MN, USA). Antibodies to LC3A/B (#4108; MW: 14–16 kDa), TNF-α (#3707; MW: 25 kDa), COX-2 (#4842; MW: 74 kDa), phosphorylated ERK1/2 (#4377 and #9102; MW: 42–44 kDa), p38 (#9215 and #9212; MW: 43 kDa), JNK (#9251 and #9252; MW: 46–54 kDa), and AKT (#9271; MW: 60 kDa) were supplied by Cell Signaling (Beverly, MA, USA), and the antibody to actin (A2066; MW: 42 kDa) was purchased from Sigma-Aldrich. The Muse™ oxidative stress kit was obtained from Merck KGaA, Darmstadt, Germany.
Cell culture and oxidative stress with H2O2 stimulation
C2C12 skeletal muscle cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured as previously described in the literature (Singh et al. 2007). Briefly, the cells were maintained at 37 °C in a humidified 5% CO2 atmosphere in DMEM (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum and 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin and subculture in a 1:4 ratio. C2C12 myoblasts at approximately 80–90% confluence were differentiated into myotubes upon further incubation in 2% horse serum for 5 days. The differentiation medium used to generate myotubes was replaced every 48 h with fresh medium. Differentiated C2C12 cells were incubated with serum-free DMEM for 12 h and then treated with different concentrations of Schisandrin C for specified times. We then exposed the treated cells for at the indicated times to hydrogen peroxide (H2O2, 200 μM; Sigma-Aldrich, St. Louis, MO, USA) for oxidative stress. After the oxidative stress, the cells were rinsed with phosphate-buffered saline (PBS, pH 7.4) and given a fresh medium for indicated days. Unless otherwise specified, all other reagents were purchased from Sigma-Aldrich.
MTT assay
Briefly, after 48 h of incubation with different concentrations of Schisandrin C in 24-well plates, cells were washed twice with phosphate-buffered saline (PBS). MTT (100/100 ml of PBS) was added to each well. The cells were incubated at 37 °C for 3 h, and dimethyl sulfoxide (500 μl) was added to dissolve the formazan crystals. The absorbance was measured at 570 nm with an ELISA reader (Bio-TeK, Winooski, VT, USA). The relative percentage of survival was calculated by dividing the absorbance of treated cells by that of the control for each well.
ROS generation
ROS generation by C2C12 cells was measured by the Muse™ Oxidative Stress Kit using the Muse cell analyzer (Merck Millipore, Germany) and performing fluorescence-based analysis. The manufacturer’s protocol was followed without deviation for the assay. Briefly, C2C12 cells were treated with 20 μM of Schisandrin C for 1 h prior to H2O2 (200 μM/ml) treatment and incubated for 24 h. Samples (1 × 107 cells/ml) were prepared in 1× assay buffer and treated with oxidative stress reagent, based on dihydroethidium (DHE) used to detect ROS that is oxidized with superoxide anion to form the DNA-binding fluorophore ethidium bromide which intercalates with DNA resulting in red fluorescence.
Confocal imaging analysis for mitochondrial activity
Cells were cultured on collagen-coated coverslips and incubated with 100 nM MitoTracker Red CMXRos (Invitrogen, Carlsbad, CA, USA) for 30 min according to the manufacturer’s instructions. The DAPI—4′,6-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich)—counterstain was applied to localize merged images. The imaging analysis was performed using a confocal laser scanning microscope (model LSM510, Carl Zeiss, Ostalbkreis, Germany). The fluorescence condition was created using an emission and excitation wavelength of 405 and 543 nm, respectively.
Preparation of cytosolic and nuclear protein
The cytosolic and nuclear proteins were prepared by slight modification of a previous method (Lee et al. 2013). Briefly, the cells were washed with ice-cold PBS and scraped into buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 10 mM ethylenediaminetetraacetic acid [EDTA], 100 mM dithiothreitol, 10% IGEPAL (Rhodia Operations, Aubervilliers, France), 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). The cells were disrupted with a pipette and centrifuged at 15,000×g for 10 min at 4 °C. The cytosolic supernatant was removed, and the pellet containing the nuclear fraction was re-suspended in buffer B (20 mM HEPES pH 7.9, 400 mM NaCl, 1 mM EDTA, 100 mM dithiothreitol, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) on ice for 30 min. After centrifugation at 15,000×g for 10 min at 4 °C, the supernatant was collected as the nuclear protein. The resulting supernatants were used as the nuclear and cytosolic proteins for the analysis of NF-κB and Nrf-2.
Western blot analysis
Western blot analysis was performed as previously described (Lee et al. 2013). The samples were separated by 8–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under denaturing conditions and electroblotted onto nitrocellulose membranes. The membranes were incubated with a blocking buffer; 5% non-fat dry milk in Tris-buffered saline Tween-20 buffer (25 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20) and incubated with the primary antibody. The membranes were washed with PBS and incubated with the horseradish peroxidase-conjugated secondary antibody. The signals were visualized by chemiluminescent detection according to the manufacturer’s protocol (Amersham Pharmacia Biotech, London, UK). The membranes were reprobed with anti-actin antibody to confirm an equal protein loading. The signals were analyzed by densitometry scanning (LAS-3000; FujiFilm, Tokyo, Japan).
Statistical analysis
Results were expressed as the mean ± standard deviation. Statistical significance between groups was assessed by the ANOVA and Duncan’s test; p values of < 0.05 were considered statistically significant. At least, three independent experiments were carried out.
Results
Effects of Schisandrin C on cellular viability and anti-oxidant activity of oxidative stress-induced C2C12 cells
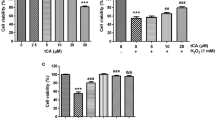
The differentiated C2C12 skeletal muscle cells were treated with indicated Schisandrin C concentrations to verify the effect on cell viability and removal of ROS. The cytotoxicity of Schisandrin C or H2O2 was checked by the MTT assay, and H2O2 was used to induce oxidative stress in C2C12 cells. Schisandrin C did not show cell toxicity below a concentration of 40 μM. Maximum cell viability was observed at a Schisandrin C concentration of 20 μM compared with control cells (Mock) in the absence or presence of H2O2 (p < 0.05; Fig. 1a, b). In addition, removal of ROS by Schisandrin C was measured by the Muse™ oxidative stress kit using the Muse cell analyzer (Fig. 1c, d). The C2C12 cells exposed to oxidative stress indicated the upregulation of ROS generation compared with Mock. However, the anti-oxidant effect of Schisandrin C inhibited ROS generation (p < 0.05; Fig. 1c, d).
Cell viability and ROS formation of Schisandrin C in differentiated C2C12 skeletal muscle cells. a The cell viability was determined by the MTT assay. The differentiated C2C12 cells were treated with Schisandrin C (5–40 μM) for 48 h. b The C2C12 cells were treated with indicated Schisandrin C concentration, and then cells were exposed to H2O2 at a concentration of 200 μM for 48 h. c, d The level of ROS formation was analyzed by a Muse oxidative stress assay, and then C2C12 cells were treated with 20 μM of Schisandrin C for 1 h prior to H2O2 (200 μM/ml) treatment and incubated for 24 h. The figure represented the population and ROS profile of the cell. Data was presented as a percentage of mean Mock group values. Each value carried out at least three independent experiments. The symbol asterisk indicated a significantly different between H2O2 and Schisandrin C + H2O2 (p < 0.05).
Anti-oxidant and anti-inflammatory activity of Schisandrin C in oxidative stress-induced C2C12 cells
The anti-oxidant activity of Schisandrin C was measured with Cu/Zn and Mn-SOD activity by western blotting in the C2C12 cells exposed to oxidative stress. The cells with oxidative stress by H2O2 were shown downregulated SOD enzymes, but Schisandrin C-treated cells were gradually increased SOD enzymes even with H2O2 stimulation (p < 0.05; Fig. 2a, b). In addition, Schisandrin C significantly decreased the inflammatory molecules including TNF-α, IL-1β, COX-2, MMP-2, MMP-9, and VCAM-1 (p < 0.05; Fig. 2c, d).
Anti-oxidant and anti-inflammatory effects of Schisandrin C in the C2C12 cells exposed to oxidative stress. a–d The anti-oxidant and anti-inflammatory effect of Schisandrin C was measured Cu/Zn-SOD, Mn-SOD, TNF-α, IL-1β, COX-2, VCAM-1, and MMP-2 and MMP-9 protein levels in H2O2-stimulated C2C12 cells. C2C12 cells were treated with Schisandrin C for indicated hours. Data was presented as a percentage of mean Mock group values. Representative blots were shown. Each value carried out at least three independent experiments. The symbol asterisk indicated a significantly different between H2O2-48 h and Schisandrin C-48 h + H2O2 (p < 0.05).
Schisandrin C inhibits the activation of MAPK signaling pathway and NF-κB translocation in oxidative stress-induced C2C12 cells
To clarify the mechanism of Schisandrin C about above dual effect, MAPK signaling and NF-κB translocation were examined in the C2C12 cells exposed to oxidative stress. The C2C12 cells with oxidative stress were activated all MAPKs such as p-ERK, p-p38, and p-JNK, but Schisandrin C inhibited the H2O2-induced MAPKs signaling (p < 0.05; Fig. 3a, b). Furthermore, Schisandrin C blocked the NF-κB translocation from the cytosol to the nucleus (p < 0.05; Fig. 3c, d). This result implies that the character of anti-inflammatory and anti-oxidant of Schisandrin C involves the inhibition of NF-κB translocation to nucleus through the MAPK signaling pathway.
The effects of Schisandrin C on the MAPK signaling pathway, NF-κB, and Nrf-2 translocation in the C2C12 cells exposed to oxidative stress. The activation of NF-κB translocation and MAPK pathways including ERK, JNK, and p38 was determined. a, b Cells were treated with 20 μM of Schisandrin C. Total protein was analyzed by western blotting for p-ERK, p-JNK, and p-p38. c–f The translocation of NF-κB and Nrf-2 by Schisandrin C was determined. Cytosolic and nuclear fractions were used for experiments. Data were presented as a percentage of mean Mock group values. Representative blots were shown. Each value carried out at least three independent experiments. The symbol asterisk indicated a significantly different between H2O2-24 h and Schisandrin C-24 h + H2O2 (p < 0.05).
Combination of Schisandrin C and H2O2 induces autophagy and mitochondrial biogenesis via Nrf-2 translocation
Schisandrin C induced the cytosol-to-nuclear translocation of a major transcription factor, Nrf-2. This transcription factor is associated with mitochondrial biogenesis (Fig. 3e, f). Furthermore, autophagy has been known to protect cells against various external stresses as well as to maintain mitochondrial numbers in skeletal muscle (Masiero et al. 2009; Lesmana et al. 2016). Therefore, whether the effect of Schisandrin C on autophagy and mitochondrial biogenesis capacity were examined in the C2C12 cells exposed to oxidative stress, autophagy molecules were present at negligible levels at the onset of oxidative stress (ATG-5, Beclin-1, and LC3-II) but accumulated in the presence of Schisandrin C. However, p62 level showed the opposite result (Fig. 4a, b).
Schisandrin C induces the molecules of autophagy and mitochondrial biogenesis. a–d The effect of Schisandrin C on autophagy and mitochondrial biogenesis was determined in the C2C12 cells with H2O2 stimulation. e Mitochondria activity was observed with confocal microscopy using Mitotracker stain (red). Nuclei were stained with DAPI (blue), and the images were merged. Data were presented as a percentage of mean Mock group values. Representative blots and images were shown. Each value carried out at least three independent experiments. The symbol asterisk indicated a significantly different between H2O2-48 h and Schisandrin C-48 h + H2O2 (p < 0.05) (color figure online)
The same pattern of accumulation of compounds associated with mitochondrial biogenesis (HO-1, PGC-1α, SIRT 1 and NRF-1) was also observed in the presence of Schisandrin C (Fig. 4c, d). In addition, Schisandrin C affected the activation of both AKT and AMPK (Fig. 4c, d). As shown by these comprehensive results, Schisandrin C maintained helped to maintain the activity of mitochondria in C2C12 cells, even in the presence of oxidative stress (Fig. 4e). So, these results indicated that Schisandrin C was elevated the activity of mitochondria via the expression of factors associated with autophagy and mitochondrial biogenesis (Fig. 4a–e).
Schisandrin C promotes the autophagy and mitochondrial biogenesis activation via HO-1-dependent pathway
On the basis of the abovementioned results, mitochondrial biogenesis promoted by Schisandrin C was hypothesized to involvement of HO-1. Levels of expressed iNOS and p62 were increased in C2C12 cells with the HO-1 inhibitor (ZnPP-treated cells) (p < 0.05; Fig. 5a–c). On the contrary, the expression of autophagy (ATG-5, Beclin-1 and LC3-II), mitochondrial biogenesis (HO-1 and PGC-1α), and mitochondria activity (Fig. 5d) was inhibited by the HO-1 inhibitor in C2C12 cells (p < 0.05; Fig. 5a–d).
Schisandrin C promotes the autophagy and mitochondrial biogenesis activation via an HO-1-dependent pathway. a, c, d The effects of Schisandrin C on the anti-inflammation, autophagy, and mitochondrial biogenesis were determined by ZnPP, HO-1 activity inhibitor in the C2C12 cells. b Mitochondria activity was observed with confocal microscopy using Mitotracker stain (red). Nuclei were stained with DAPI (blue), and the images were merged. Data were presented as a percentage of mean Mock group values. Representative blots were shown. Each value carried at least three independent experiments. The symbol asterisk indicated a significantly different between ZnPP and ZnPP + Schisandrin C (p < 0.05). The symbol number sign indicated a significantly different between Mock and ZnPP (p < 0.05) (color figure online)
However, Schisandrin C-treated C2C12 cells even with the HO-1 inhibitor, the expression of autophagy, and mitochondrial biogenesis factors were significantly increased with mitochondria activity (p < 0.05; Fig. 5a–d). These results suggested that Schisandrin C enhanced mitochondrial biogenesis effects by regulating anti-inflammatory activities and autophagy mediated via HO-1.
Discussion
The major compounds of Schisandra chinensis are Schisandrins (Schisandrin A, B, C) and Gomisins (Gomisin A, N, J), and these compounds have a wide array of pharmacological and biological activities (Park et al. 2009; Oh et al. 2010; Chun et al. 2014). Among these, Schisandrin C has several biological properties such as anti-tumor, hepatoprotective, anti-oxidant, and anti-inflammatory effects (Kim et al. 2010; Oh et al. 2010; Lin et al. 2011; Park et al. 2013). According to previous studies, Schisandrin C shows its anti-inflammatory activity in LPS-induced macrophage (Oh et al. 2010). The results of previous studies with its anti-inflammatory and anti-oxidant activities were in accordance with results of this study such as downregulation of the pro-inflammatory molecules in C2C12 skeletal muscle cells. Also, the anti-inflammatory mechanism is associated with down-activation of mitogen-activated protein kinase (MAPK) pathways (Guo et al. 2008; Oh et al. 2010; Paudel et al. 2014). Thus, MAPK signaling is a very important therapeutic target for the treatment of inflammatory disease (Seo et al. 2010). Previous study reports that Schisandrin inhibits the pathway of ERK, JNK, and p38 in a dose-dependent manner (Oh et al. 2010; Park et al. 2011). NF-κB, as a transcription factor, is also involved in inflammatory reactions and is controlled by the MAPK signaling pathway (Tak and Firestein 2001; Guo et al. 2008). In this study, Schisandrin C inhibited the three types of MAPKs; p-ERK1/2, p-JNK, and p-38. Also, Schisandrin C blocked the translocation of NF-κB from cytosol to nucleus even with exposed to oxidative stress. Therefore, it can be clearly define that the anti-inflammatory mechanism of Schisandrin C blocked the translocation of NF-κB following the inhibition of MAPK signaling pathway. Also, Schisandrin C had a character to remove reactive oxidants, which was predicted by increasing anti-oxidants enzymes. These findings strongly supported that the specific role of Schisandrin C suppressed the production of pro-inflammatory molecules, and it may have therapeutic perspective for the regulation of anti-inflammatory or tissue-protective signaling pathways.
Autophagy protects cells from the cellular stress of inflammation, immune responses, and control cell death (Saitoh and Akira 2010; Marino et al. 2014). In particular, autophagy also plays a crucial role in the regulation of inflammation and homeostatic process (Lee et al. 2008; Levine et al. 2011). In the recently study, Schisandrin B moderately increases the beclin-1 expression among an autophagy molecules for protective against oxidative stress-induced inflammation (Giridharan et al. 2015). Also, it suggests that the hepatoprotective mechanisms of Schisandrin A may involve in autophagy flux activation (Lu et al. 2014). In this study, it has been demonstrated for the first time that Schisandrin C increased the autophagy molecules such as ATG-5, LC3-II, and Beclin-1 in C2C12 skeletal muscle cells. So, these results predicted that Schisandrin C like as other Schisandrins may be able to activate of autophagy for enhancement of skeletal muscle homeostasis. In addition, p62 is an adapter molecule for the formation of autophagosome and essential molecule to maintain cellular homeostasis and muscle mass (Fujita et al. 2008; Masiero et al. 2009). The p62 protein generally increases under oxidative stress, and p62 accumulation inhibits the autophagy activity such as Beclin-1 expression and LC3B-I to LC3B-II conversion (Zheng et al. 2009; Park et al. 2017). Thus, p62 level reduction indicates to increase of autophagic flux (Bjørkøy et al. 2009; Lesmana et al. 2016). In this study, the oxidative stress to C2C12 cells increased p62 level but Schisandrin C downregulated its expression, which meant that Schisandrin C activated autophagic flux under oxidative stress. Interestingly, the inhibition of autophagy expression leads to increase the production of oxidative reactive species (ROS) and mitochondrial damage (Mortensen et al. 2010). In addition, the meaning of autophagy balance is critical for maintaining appropriate function and number of mitochondria in skeletal muscle (Lesmana et al. 2016). Therefore, it is consider that the regulation of autophagy by Schisandrin C is essential for the mitochondrial biogenesis and activity in C2C12 cells exposed to oxidative stress.
HO-1 is recommended to a potential therapeutic target molecule for various inflammatory diseases (Kirkby and Adin 2006; Lee et al. 2013). HO-1 increases the expression of mitochondrial biogenesis related molecules such as peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) through Nrf-2 translocation (MacGarvey et al. 2012). In this study, Schisandrin C activated the regulators of mitochondrial biogenesis such as high expression of PGC-1α and Nrf-2 followed by HO-1 expression in C2C12 skeletal muscle cells. The vital role of Schisandrin C in accordance with mitochondrial activity was confirmed by the confocal assay. In addition, previous studies suggest that the expression of PGC-1α, Sirtuin-1 (SIRT-1), and phosphorylated-AMP-activated protein kinase (p-AMPK) enhances the mitochondrial biogenesis in skeletal muscles (Winder et al. 2000; Rodgers et al. 2005; Lagouge et al. 2006; Sasaki et al. 2014). In particular, the PGC-1α induces mitochondrial biogenesis by activating different transcription factors, including nuclear respiratory factor-1 (NRF-1) (Jornayvaz and Shulman 2010; Lesmana et al. 2016). In this study, Schisandrin C induced the expression of PGC-1α, SIRT-1, p-AMPK, and NRF-1. According to these results, the anti-inflammatory mechanism of Schisandrin C played an important role in the regulation of mitochondrial homeostasis in C2C12 skeletal muscle cells exposed to oxidative stress.
In this study, Schisandrin C also promoted AKT activation and inhibition of MAPK pathways. These results implicated that mechanism of mitochondrial biogenesis by Schisandrin C may be regulated by PI3K/AKT, MAPKs, and HO-1. Park et al. (2011) reported that the expression of HO-1 is regulated by p-AKT, MAPK, and transcription factors (NF-κB and Nrf-2 signaling pathways). It has known that HO-1 is regulated by PI3K/AKT and MAPK signaling pathways (Furukawa et al. 2010; Park et al. 2011, 2013). From this point, the HO-1 expression was inhibited by ZnPP, and then the molecules of autophagy and mitochondrial biogenesis were checked. Previous study suggests that the important role of HO-1 regulates autophagy activity against inflammatory damage (Carchman et al. 2011; Park et al. 2011). In this study, the HO-1 inhibition significantly decreased above molecules, but Schisandrin C improved autophagy and mitochondrial biogenesis even under HO-1 inhibition by inhibitor and oxidative stress. These finding suggested that the main event of Schisandrin C was to increase HO-1 expression which was involved in the regulation of anti-oxidation and anti-inflammation by the enhancement of autophagy and mitochondrial biogenesis.
In summary, this study confirms that the oxidative stress has an adverse effect on inflammation and mitochondrial biogenesis to act muscle homeostasis, but Schisandrin C could minimize side effects of oxidative stress. Therefore, Schisandrin C might provide one of the additional supporting compounds for enhancement of skeletal muscle homeostasis against diseases associated with oxidative stress.
References
Bjørkøy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T (2009) Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 452:181–197. https://doi.org/10.1016/S0076-6879(08)03612-4
Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS (2011) Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 53(6):2053–2062. https://doi.org/10.1002/hep.24324
Chun JN, Kim SY, Park EJ, Kwon EJ, Bae DJ, Kim IS, Kim HK, Park JK, Lee SW, Park HH, So IS, Jeon JH (2014) Schisandrin B suppresses TGF1-induced stress fiber formation by inhibiting myosin light chain phosphorylation. J Ethnopharmacol 52(2):364–371
Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T (2008) The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Bio Cell 19(5):2092–2100. https://doi.org/10.1091/mbc.E07-12-1257
Furukawa Y, Urano T, Minamimura M, Nakajima M, Okuyama S, Furukawa S (2010) 4-Methylcatechol-induced heme oxygenase-1 exerts a protective effect against oxidative stress in cultured neural stem/progenitor cells via PI3 kinase/Akt pathway. Biomed Res 31(1):45–52. https://doi.org/10.2220/biomedres.31.45
Giridharan VV, Thandavarayan RA, Arumugam S, Mizuno M, Nawa H, Suzuki K, Ko KM, Krishnamurthy P, Watanabe K, Konishi T (2015) Schisandrin B ameliorates ICV-infused amyloid β induced oxidative stress and neuronal dysfunction through inhibiting RAGE/NF-κB/MAPK and up-regulating HSP/Beclin expression. PLoS One 10(11):e0142483. https://doi.org/10.1371/journal.pone.0142483
Guo LY, Hung TM, Bae KH, Shin EM, Zhou HY, Hong YN (2008) Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur J Pharmacol 591(1–3):293–299. https://doi.org/10.1016/j.ejphar.2008.06.074
Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84. https://doi.org/10.1042/bse0470069
Kang JS, Han MH, Kim GY, Kim CM, Kim BM, Hwang HJ, Choi YH (2014) Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients 6(12):5667–5678. https://doi.org/10.3390/nu6125667
Kim SJ, Min HY, Lee EJ, Kim YS, Bae K, Kang SS, Lee SK (2010) Growth inhibition and cell cycle arrest in the G0/G1 by schizandrin, a dibenzocyclooctadiene lignan isolated from Schisandra chinensis, on T47D human breast cancer cells. Phytother Res 24(2):193–197. https://doi.org/10.1002/ptr.2907
Kirkby KA, Adin CA (2006) Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol 290(3):563–571
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127(6):1109–1122. https://doi.org/10.1016/j.cell.2006.11.013
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105(9):3374–3379. https://doi.org/10.1073/pnas.0712145105
Lee YH, Lee NH, Bhattarai G, Kim GE, Lee IK, Yun BS, Hwang PH, Yi HK (2013) Anti-inflammatory effect of pachymic acid promotes odontoblastic differentiation via HO-1 in dental pulp cells. Oral Dis 19(2):193–199. https://doi.org/10.1111/j.1601-0825.2012.01970
Lee YH, Lee HY, Kim TG, Lee NH, MK Y, Yi HK (2015) PPARγ maintains homeostasis through autophagy regulation in dental pulp. J Dent Res 94(5):729–737. https://doi.org/10.1177/0022034515573833
Lesmana R, Sinha RA, Singh BK, Zhou J, Ohba K, Wu Y, Yau WW, Bay BH, Yen PM (2016) Thyroid hormone stimulation of autophagy is essential for mitochondrial biogenesis and activity in skeletal muscle. Endocrinology 157(1):23–38. https://doi.org/10.1210/en.2015-1632
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469(7330):323–335. https://doi.org/10.1038/nature09782
Lin RD, Mao YW, LeuSJ HCY, Lee MH (2011) The Immuno-regulatory effects of Schisandra chinensis and its constituents on human monocytic leukemia cells. Molecules 16(12):4836–4849. https://doi.org/10.3390/molecules16064836
Liu X, Wu G, Shi D, Zhu R, Zeng H, Cao B, Huang M, Liao H (2015) Effects of nitric oxide on notexin-induced muscle inflammatory responses. Int J Biol Sci 11(2):156–167. https://doi.org/10.7150/ijbs.10283
Lu Y, Wang WJ, Song YZ, Liang ZQ (2014) The protective mechanism of schisandrin A in d-galactosamine-induced acute liver injury through activation of autophagy. Pharm Biol 52(10):1302–1307. https://doi.org/10.3109/13880209.2014.890232
MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, Piantadosi CA (2012) Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med 185(8):851–861. https://doi.org/10.1164/rccm.201106-1152OC
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15(2):81–94. https://doi.org/10.1038/nrm3735
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy is required to maintain muscle mass. Cell Metab 10(6):507–515. https://doi.org/10.1016/j.cmet.2009.10.008
Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK (2010) Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A 107(2):832–837. https://doi.org/10.1073/pnas.0913170107
Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim CY (2010) Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci Biotechnol Biochem 74(2):285–291. https://doi.org/10.1271/bbb.90597
Oh SL, Chang H, Kim HJ, Kim YA, Kim DS, Ho SH, Kim SH, Song W (2013) Effect of HX108-CS supplementation on exercise capacity and lactate accumulation after high-intensity exercise. J Int Soc Sports Nutr 10(1):21. https://doi.org/10.1186/1550-2783-10-21
Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S (2010) Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol 80(12):1895–1903. https://doi.org/10.1016/j.bcp.2010.07.014
Panossian A, Wikman G (2008) Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol 118(2):183–212. https://doi.org/10.1016/j.jep.2008.04.020
Park C, Choi YW, Hyun SK, Kwon HJ, Hwang HJ, Kim GY, Choi BT, Kim BW, Choi IW, Moon SK, Kim WJ, Choi YH (2009) Induction of G1 arrest and apoptosis by schisandrin C isolated from Schizandra chinensis Baill in human leukemia U937 cells. Int J Mol Med 24(4):495–502
Park SY, Park DJ, Kim YH, Kim Y, Kim SG, Shon KJ (2011) Upregulation of heme oxygenase-1 via PI3K/Akt and Nrf-2 signaling pathways mediates the anti-inflammatory activity of Schisandrin in Porphyromonas gingivalis LPS-stimulated macrophages. Immunol Lett 139(1–2):93–101. https://doi.org/10.1016/j.imlet.2011.05.007
Park SY, Park SJ, Park TG, Rajasekar S, Lee SJ, Choi YW (2013) Schizandrin C exerts anti-neuroinflammatory effects by upregulating phase II detoxifying/antioxidant enzymes in microglia. Int Immunopharmacol 17(2):415–426. https://doi.org/10.1016/j.intimp.2013.06.032
Park SY, Park MY, Park HG, Lee KJ, Kook MS, Kim WJ, Jung JY (2017) Nitric oxide-induced autophagy and the activation of activated protein kinase pathway protect against apoptosis in human dental pulp cells. Int Endod J 50(3):260–270. https://doi.org/10.1111/iej.12616
Paudel U, Lee YH, Kwon TH, Park NH, Yun BS, Hwang PH, Yi HK (2014) Eckols reduce dental pulp inflammation through the ERK1/2 pathway independent of COX-2 inhibition. Oral Dis 20(8):827–832. https://doi.org/10.1111/odi.12266
Rahman A, Fazal F (2011) Blocking NF-κB: an inflammatory issue. Proc AmThorac Soc 8:497–503
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434(7029):113–118. https://doi.org/10.1038/nature03354
Saitoh T, Akira S (2010) Regulation of innate immune responses by autophagy-related proteins. J Cell Biol 189:925–935
Sasaki T, Nakata R, Inoue H, Shimizu M, Inoue J, Sato R (2014) Role of AMPK and PPARγ1 in exercise-induced lipoprotein lipase in skeletal muscle. Am J Physiol Endocrinol Metab 306(9):1085–1092
Seo SW, Lee D, Minematsu H, Kim AD, Shin M, Cho SK, Kim DW, Yang J, Lee FY (2010) Targeting extracellular signal-regulated kinase (ERK) signaling has therapeutic implications for inflammatory osteolysis. Bone 46(3):695–702. https://doi.org/10.1016/j.bone.2009.10.032
Singh J, Verma NK, Kansagra SM, Kate BN, Dey CS (2007) Altered PPARr expression inhibits myogenic differentiation in C2C12 skeletal muscle cells. Mol Cell Biochem 294(1-2):163–171. https://doi.org/10.1007/s11010-006-9256-x
Surh YJ, Kundu JK, Na HK (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74(13):1526–1539. https://doi.org/10.1055/s-0028-1088302
Tak PP, Firestein GS (2001) NF-kappa B: a key role in inflammatory diseases. J Clin Invest 107(1):7–11. https://doi.org/10.1172/JCI11830
Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO (2000) Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88(6):2219–2226
Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH (2009) The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol 183(9):5909–5916. https://doi.org/10.4049/jimmunol.0900441
Zukor H, Song W, Liberman A, Mui J, Vali H, Fillebeen C, Pantopoulos K, TD W, Guerquin-Kern JL, Schipper HM (2009) HO-1-mediated macroautophagy: a mechanism for unregulated iron deposition in aging and degenerating neural tissues. J Neurochem 109(3):776–791. https://doi.org/10.1111/j.1471-4159.2009.06007.x
Funding
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01199003)” Rural Development Administration, Republic of Korea. In addition, this work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014S1A5B5A07042382)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PPTX 32145 kb)
Rights and permissions
About this article
Cite this article
Kim, JS., Yi, HK. Schisandrin C enhances mitochondrial biogenesis and autophagy in C2C12 skeletal muscle cells: potential involvement of anti-oxidative mechanisms. Naunyn-Schmiedeberg's Arch Pharmacol 391, 197–206 (2018). https://doi.org/10.1007/s00210-017-1449-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1449-1