Abstract
Background
Investigating the interaction of diabetes with ischemic postconditioning (IPostC)-associated cardioprotection in myocardial ischemia/reperfusion (I/R) damage is of great clinical importance. The present work was designed to determine the possible synergistic effects of alpha-lipoic acid (LA) preconditioning and IPostC on myocardial I/R damage in type-II diabetic rats through modulating autophagy, and the involvement of mitochondrial function.
Methods
High-fat diet/low dose of streptozotocin-induced type-II diabetic model with duration of 12 weeks was used in this study. LA (100 mg/kg/day) was administered orally in diabetic rats for 5 weeks before I/R. Myocardial I/R was established on Langendorff apparatus through the ligation of left anterior descending coronary artery for 35 min, then reperfusion for 60 min. IPostC was carried out immediately at the beginning of the reperfusion. At the end of the experiment, myocardial infarct size (IS), autophagy markers at both gene and protein levels, and mitochondrial ROS production and membrane potential were assessed.
Results
Combined conditioning with LA and ischemia significantly decreased the IS of diabetic hearts (P < 0.05), however, single therapies had no significant effects. LA in combination with IPostC more significantly decreased LC3 and p62 mRNA levels (P < 0.01), and LC3II/LC3I and p62 protein levels (P < 0.01). Also, this combined therapy decreased mitochondrial ROS generation and membrane depolarization (P < 0.01).
Conclusions
Pretreatment with LA in diabetic rats notably restored cardioprotection by IPostC via modulating autophagy and restoring mitochondrial function. This combined conditioning might be an effective strategy to diminish I/R damage in diabetic hearts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid restoration of blood flow to the myocardial ischemic area is an optimal therapeutic procedure in which decreases the mortality rate, but also has the potential to induce ischemia/reperfusion (I/R) injury [1, 2]. The risk of myocardial I/R development in diabetic patients is higher than non-diabetic ones. Recently, we and others demonstrated that diabetes sensitizes the myocardium to I/R damage, exacerbates myocardial damage, interacts with cardioprotective mechanisms, and leads to poor prognosis through multiple mechanisms that are largely unknown [3,4,5,6,7], but including overproduction of free radicals and pro-inflammatory cytokines, impaired production of nitric oxide, mitochondrial dysfunction, impaired activation of survival protein kinases, increased apoptosis, and dysregulated autophagy [5, 8].
Autophagy has house-keeping role in preserving cellular homeostasis via degrading misfolded and long-lived proteins, and dysfunctional organelles such as mitochondria [9]. The dual role of autophagy in the regulation of cell survival and death has been shown in preclinical studies, but the protective versus detrimental function of altered autophagy in myocardial I/R injury, particularly in the presence of cardiovascular risk factors such as diabetes still requires further investigations [10, 11]. It has been suggested that by inducing well-controlled autophagy at a proper level and timing, and inhibiting inappropriate autophagy, the myocardium can be protected from I/R damage [12]. Given that autophagy has an important role in preserving mitochondrial normal structure and performance in the heart, there is a potential association between altered autophagy and dysfunctional mitochondria [13]. Mitochondrial dysfunction is involved in the poor prognosis of ischemic heart disease (IHD) under diabetic condition. Since diabetes impairs mitochondrial biogenesis, and decreases the function and number of mitochondria in the heart [14], so reduction of mitochondrial oxidative stress and improving its biogenesis have emerged as potential targets for reducing cardiac I/R damage under diabetes [15].
Studies have revealed that in the prolonged myocardial ischemia, episodes of brief ischemia at the beginning of the reperfusion can elicit a significant reduction in infarct size and induce cardioprotection, which is called postconditioning (IPostC). Therapeutic strategies such as IPostC which have shown positive effects against myocardial I/R damage in non-diabetic experimental models, have failed to exert same protection under diabetes. Diabetes eliminates the beneficial effects of IPostC against myocardial I/R damage via interference with the cell survival signaling pathways [5]. Since diabetic heart is more resistant to common cardioprotections, emerging data suggests that optimal cardioprotection may be achieved by combination therapy. Thus, for enhancing the potency of IPostC in diabetic myocardium, and restoring diabetes-associated loss of cardioprotection, its combination with appropriate pharmacological agents has been proposed in several studies [3, 4].
Alpha-lipoic acid (LA) is a potent natural antioxidant and free radical scavenger [16, 17] which has beneficial effects in preventing cardiovascular diseases [18]. LA exerts anti-inflammatory and antioxidative effects, and has the ability to increase cellular defense and the resistance to ROS-induced myocardial damage, diminish myocardial injury, and preserve heart function during I/R [19]. There are reports that LA is a protective agent against oxidative stress in several experimental or clinical setting, including I/R damage and diabetes [20, 21]. In addition, LA has a wide range of pharmacological capabilities in diabetic patients including glucose and lipid-lowering effect, insulin-sensitizing, and anti-oxidative, anti-apoptotic, and anti-inflammatory properties [22].
It has been argued that presence of chronic diabetes may enhance the susceptibility of the heart to I/R damage, and interfere with and attenuate the cardioprotective efficacy of IPostC [23]. Considering the therapeutic potentials of LA in cardiovascular diseases and diabetes, we have designed this work to explore whether combination therapy with LA preconditioning and IPostC is able to protect the diabetic heart from I/R damage, and to identify the role of cardiomyocyte autophagy and mitochondrial function in this setting.
Materials and methods
Ethics and animals
All procedures of this work were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (8th Edition, NRC 2011), and were approved by the ethical committee of Tabriz University of Medical Sciences (ethical code: IR.TBZMED.VCR.REC.1400.154), and Iran’s National Committee for Ethics in Biomedical Research (ethical code: IR.NIMAD.REC.1396.029). Male Wistar rats (84 rats at 8 weeks of age, 200–250 g) were supplied by the animal center of Tabriz University of Medical Sciences. The rats were kept under 12-h light/dark cycles in the individual cages in the humidity- and temperature-controlled animal room, and received humane care with unlimited access to standard diet and water ad libitum.
Induction of type-II diabetes
In order to induce type-II diabetes with insulin resistance, high-fat diet-fed and low-dose streptozotocin (STZ) (Sigma, St. Louis, MO, USA) method was used in accordance with the previous studies [24]. For simulation of chronic diabetes, the duration of 12 weeks was considered. Following the adaptation of animals for 1 week, diabetic rats received ad libitum with a high-fat diet (0.3% DL-Methionine, 1% cholesterol, 4% sucrose, 24% casein, 30% lard, and 35% normal pellet) for 6 weeks (total caloric value ≈ 4.6 kcal/g; 62% of which was from fat). After 6 weeks, STZ at the dose of 35 mg/kg in citrate buffer (pH 4) was injected intraperitoneally (i.p) in diabetic rats following 8 h fasting. Non-diabetic rats received typical food and an equal volume of citrate buffer. For confirmation of diabetes, blood samples were collected 72 h following the injection of STZ, and the levels of blood glucose were determined by means of a glucometer device. The animals with blood glucose levels beyond 250 mg/dl were enrolled to the further experiment.
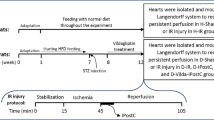
Preparation of I/R injury model and IPostC in Langendorff-perfused rat hearts
All rats were heparinized (500 IU/kg) to avoid blood clotting during the surgery, and anesthetized by i.p injection of ketamine/xylazine (60/10 mg/kg, respectively). Next, by using the Langendorff perfusion apparatus (ML176-V; AD Instruments, New South Wales, Australia) at a constant pressure of 80 mmHg, the isolated hearts were retrogradely perfused through the aorta with a Krebs–Henseleit (K-H) buffer gassed with carbogen (5% CO2 and 95% O2) at 37 °C ± 0.5 °C, and pH of 7.4, of the following composition (in mmol/L): KCl 4.7; NaCl 118; KH2PO4 1.2; MgSO4 1.2; CaCl2 2.5; NaHCO3 25; and glucose 11.1 [25]. Following a stabilization period for 15 min with 12–14 ml/min coronary flow rate, the isolated hearts underwent left anterior descending (LAD) coronary artery blocking for 35 min to induce regional ischemia, then LAD re-opening for 60 min to induce reperfusion. The Sham-operated groups underwent the above procedure, without LAD ligation. Successful I/R cycle was indicated by a decline of at least 25% in coronary flow in the obstruction period, and the subsequent recovery of the coronary flow during reperfusion. IPostC in corresponding groups was carried out at the beginning of the reperfusion by 6 cycles of 10 s ischemia/10 s reperfusion.
Study design
The isolated hearts were randomly assigned to one of these groups (n = 12/each): H-Sham (non-diabetic hearts receiving 110 min full perfusion); H-Cont (non-diabetic hearts receiving 35 min ischemia plus 60 min reperfusion); D-Sham (diabetic hearts receiving 110 min full perfusion); D-Cont (diabetic hearts receiving 35 min ischemia plus 60 min reperfusion); D-Post (diabetic hearts receiving 35 min ischemia plus 6 cycles of 10 s ischemia/10 sec reperfusion at the beginning of 60 min reperfusion); D-LA (the hearts of LA-receiving diabetic rats undergoing 35 min ischemia plus 60 min reperfusion); and D-Post-LA (the hearts of LA-receiving diabetic rats undergoing 35 min ischemia plus 6 cycles of 10 s ischemia/10 sec reperfusion at the beginning of 60 min reperfusion). LA (Sigma-Aldrich, St. Louis, USA) at the dose of 100 mg/kg/day, dissolved in DMSO (0.1%), was administered in D-LA and D-LA-Post groups through feeding gavage in the last 5 weeks. Body weight recording was performed weekly, with the final body weight recording before isolation of the heart. The ratio of heart/body weight (%) was measured as an index of diabetic cardiomyopathy.
Assessment of HOMA1-IR index
The levels of fasting blood glucose and plasma insulin were evaluated for confirmation of insulin resistance and type-II diabetes. Before isolation of the hearts, blood samples were collected from the hearts, and immediately centrifuged for separation of plasma (3500 × g, 10 min, 4 °C). Then, plasma levels of insulin were determined using a rat-specific enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chem., Ann Arbor, Michigan) based on the manufacturer’s protocols. The levels of fasting blood glucose were determined using a glucometer device. Homeostasis model assessment of insulin resistance (HOMA1-IR) index was employed in order to identify insulin resistance based on the following formula: fasting blood glucose (mmol/l) × fasting insulin (µu/l)/22.5 [26].
Infarct size estimation
At the end of 60 min reperfusion in a separate grouping of rats, the LAD was tied again, and 2 ml Evans blue dye (0.25%) was infused into the coronary system. Next, the heart samples were stored at − 20 °C. After 24 h, sections of frozen heart were prepared and placed in 2,3,5-triphenylte-trazolium chloride (TTC, 1%) in phosphate-buffered solution (pH 7.4) for 15–20 min at 37 °C. Then, the slices were fixed in 10% formalin for 24–48 h to improve the contrast of the staining. A computerized planimetry and ImageJ software (NIH, Bethesda, USA) were utilized for calculation of area at risk (AAR) and infarct size (IS) of each slice, which were defined as a percentage of left ventricle (AAR/LV × 100), and a percentage of AAR (IS/AAR × 100), respectively.
Real-time PCR
The mRNA levels of microtubule-associated proteins 1 A/1B light chain 3 (LC3) and p62 were determined by Real-time PCR with the following steps: denaturation (94–97 °C), annealing (55–60 °C), and extension (≈ 72 °C). Total RNA was extracted from fresh samples of LVs using Trizol Reagent (Invitrogen Company, San Diego, CA, USA) on ice, in accordance to the manufacturer’s guidelines. In order to detect the RNA yield and purity, NanoDrop spectrophotometer (NanoDrop ND-2000 C, Thermo Fisher Scientific, USA) at a wavelength of 260/280 nm was employed. Utilizing an Exiqon cDNA Synthesis Kit, RNA series were converted to cDNA. By means of a LightCycler-96 Roche device, the assessment of the gene expressions of LC3 and p62 were performed. Primers used in this study are listed in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was the internal control for this study. 2−∆∆CT method was employed for determining the relative-quantitative levels of autophagy genes. The results were reported as fold-change difference to the relevant controls.
Western blot assay
At 60 min following reperfusion, the expression of LC3II/LC3I and p62 were determined in the LVs using western blot assay. After dissecting and homogenizing fresh-frozen samples in lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) based on the manufacturer’s guidelines, the resulting solutions were centrifuged at 10,000 RCF for 10 min at 4 °C to collect the supernatants. BCA Protein Assay Kit (Pierce, Rockford, IL, USA) was carried out to determine protein concentration.
After the separating equal amounts of proteins (50 µg) in 12% SDS-PAGE gel electrophoresis, separated soluble proteins were transferred to a polyvinylidene difluoride (PVDF, Sigma-Aldrich, St. Louis, MO, USA) membrane. After blocking non-specific bindings in 5% non-fat dry milk solution for 2 h, the membranes were then probed overnight with primary antibodies against LC3B (1:1000, Cell Signaling), p62/SQSTM1 (1:500, Santa Cruz), and β-actin (1:500, Cell Signaling) overnight at 4 °C on a shaker. Membranes were washed 3 times and then incubated with anti-rabbit secondary antibody (1:7000, Cell Signaling) for 1 h on a shaker at room temperature. Using an enhanced chemiluminescence (ECL) detection kit (Amersham Pharmacia Biotech, England), the immunoreactivities were visualized based on the manufacturer’s protocols, then exposed to a film, quantified by densitometric analysis, and normalized with the intensity of β-actin bands as loading control. The measured values were presented in arbitrary unit.
Evaluation of mitochondrial function
Isolation of cardiac mitochondria was performed based on our previous works [8, 25]. The generation of mitochondrial ROS in the heart was evaluated by dichlorohydro-fluorescein diacetate (DCFDA) dye using a fluorometric technique. Mitochondrial ROS generation levels in the heart were expressed as the fluorescence intensity per mg protein of samples. Mitochondrial membrane potential alterations in the heart were evaluated utilizing the 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) by an isolated mitochondrial staining kit (Sigma, Germany), based on the manufacturer’s instructions. Reduction of red/green fluorescence intensity ratio was considered as mitochondrial membrane depolarization [25].
Statistical analysis
All quantitative data were expressed as means ± standard errors of the mean (SEM), and analysed with one-way ANOVA followed by Tukey post-hoc test. Statistical analysis of data was carried out using the SPSS software 25 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Characteristics of the animals
The statistical analyses showed significant increases in the levels of fasting blood glucose and plasma insulin, HOMA1-IR index, heart weight (HW), and the ratio of heart/body weight (HW/BW) as compared to the non-diabetic rats (P < 0.01 for all). Pretreatment of diabetic rats with LA significantly decreased hyperglycemia (P < 0.01), increased the plasma levels of insulin (P < 0.05), and reduced HOMA-IR index (P < 0.05), HW (P < 0.05), and HW/BW ratio (P < 0.05) as compared to the diabetic rats (Table 2).
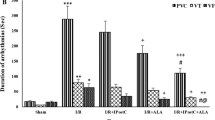
Myocardial AAR and IS
As shown in Fig. 1A, AAR volumes were similar between all of the hearts subjected to 35 min ischemia and 60 min reperfusion. As shown in Fig. 1B, induction of 35 min ischemia and 60 min reperfusion in non-diabetic rats caused the development of IS to 39 ± 3.2%, and this value in the diabetic rats was 47 ± 8.1%. Postconditioning with ischemia alone or preconditioning with LA alone could not significantly reduce the IS in comparison to those of non-treated diabetic hearts. However, combined therapy with LA and IPostC caused a significant decrease in the IS compared with D-Cont group (P < 0.05).
The expression of LC3 and p62 genes
As shown in Fig. 2A and B, induction of I/R in non-diabetic hearts increased the mRNA levels of LC3 to some extent, however, this difference was not significant, and increased the mRNA levels of p62 to a significant level (P < 0.05) when compared with H-Sham group. In diabetic hearts, induction of I/R significantly increased the mRNA levels of LC3 (P < 0.05), as well as increased the mRNA levels of p62 to some extent when compared with D-Sham group. Application of IPostC alone significantly decreased the expression of LC3 gene in diabetic hearts (P < 0.05), however, had no significant influence on the expression of p62 gene as compared with D-Cont group. Preconditioning with LA significantly decreased the mRNA levels of LC3 and p62 as compared with D-Cont group (P < 0.01 and P < 0.05, respectively). LA preconditioning in combination with IPostC significantly and more potently decreased LC3 and p62 mRNA levels when compared with D-Cont group (P < 0.01 for both).
The expression of LC3 (A), and p62 (B) genes in experimental groups (n = 6 for each group). The data were expressed as mean ± SEM. (*P < 0.05 and **P < 0.01 vs. H-Sham group, $P < 0.05 vs. D-Sham group, #P < 0.05 and ##P < 0.01 vs. D-Cont group, +P < 0.05 vs. D-Post group). H Healthy, Cont control, D diabetic, Post ischemic postconditioning, LA alpha-lipoic acid
The expression of LC3II/LC3I and p62 proteins
As shown in Fig. 3B and C, LC3II/LC3I and p62 protein levels in H-Cont group tended to increase to some extent in comparison with H-Sham group, but these differences were not significant. Furthermore, protein levels of LC3II/LC3I and p62 in D-Cont group were significantly higher than D-Sham group (P < 0.05 for both). Postconditioning with ischemia significantly decreased LC3II/LC3I expression (P < 0.05), however, could not significantly decrease p62 protein expression compared with D-Cont group. The protein levels of LC3II/LC3I and p62 were significantly decreased in the group receiving LA preconditioning when compared with I/R group (P < 0.01 and P < 0.05, respectively). Concomitant application of both LA and IPostC significantly and more effectively decreased LC3II/LC3I and p62 protein levels when compared with D-Cont group (P < 0.01 for both).
Representative immunoblots (A), changes of LC3II/LC3I ratio (B), and p62 expression (C) in experimental groups (n = 6 for each group). The data were expressed as mean ± SEM. (*P < 0.05 and **P < 0.01 vs. H-Sham group, $P < 0.05 vs. D-Sham group, #P < 0.05 and ##P < 0.01 vs. D-Cont group, +P < 0.05 vs. D-Post group). H Healthy, Cont control, D diabetic, Post ischemic postconditioning, LA alpha-lipoic acid
Mitochondrial function
Figure 4A and B shows the levels of mitochondrial ROS production and the alterations of mitochondrial membrane potential in the heart, which were reported as fluorescence intensity of DCF, and red/green fluorescence intensity ratio of JC-1 staining respectively. Lack of unwanted depolarization or normal membrane potential associates with greater red/green ratios. As shown, induction of I/R in non-diabetic hearts led to the increased levels of ROS production and decreased ratio of red/green fluorescence intensity when compared with H-Sham hearts (P < 0.05 and P < 0.01, respectively). In diabetic hearts, induction of I/R led to the enhanced levels of ROS production, and decreased ratio of red/green fluorescence intensity as compared with D-Sham group (P < 0.05 and P < 0.01, respectively). Interestingly, the levels of mitochondrial ROS production were significantly decreased in D-Post (P < 0.01), D-LA (P < 0.05), and D-Post-LA (P < 0.01) groups, and the ratio of red/green fluorescence intensity were significantly increased in D-LA and D-Post-LA (P < 0.01 for both) groups, as compared with D-Cont group.
Mitochondrial ROS production in the heart assessed by DCFDA dye (A), and mitochondrial membrane potential alterations in the heart assessed by JC-1 dye (B). (n = 6 for each group). The data were expressed as mean ± SEM. (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. H-Sham group, $P < 0.05 and $$P < 0.01 vs. D-Sham group, #P < 0.05 and ##P < 0.01 vs. D-Cont group, +P < 0.05 and ++P < 0.01 vs. D-Post group). H Healthy, Cont control, D diabetic, Post ischemic postconditioning, LA alpha-lipoic acid
Discussion
The present experimental study showed the effectiveness of combined therapy with LA preconditioning and IPostC in type-II diabetic hearts injured by I/R by investigating autophagic flux and mitochondrial function. Pretreatment of type-II diabetic rats with LA restored the cardioprotective effects of IPostC following I/R injury, as evidenced by decreased myocardial infarct size. The reduction of myocardial infarct size by combined conditioning was partly mediated by its influence on autophagic flux and mitochondrial function following reperfusion injury in diabetic hearts. Our data documented that this combined therapy decreased the expression of genes and proteins involved in autophagic flux (LC3II and p62), as well as improved mitochondrial function by decreasing mitochondrial ROS generation and membrane depolarization.
Studies have shown that diabetes mellitus leads to the aggravation of heart function after I/R injury, and reduces the efficacy of cardioprotective strategies [27]. The characteristics of type-II diabetes include glucose intolerance, hyperglycemia, hyperinsulinemia, insulin resistance, and hyperlipidemia [10]. In this study, using high-fat diet/streptozotocin (HFD/STZ) in rats resulted in hyperglycemia, hyperinsulinemia, insulin resistance, and elevated HW/BW ratio. In details, at the end of 7th week, the diabetic rats showed glucose intolerance, and at the end of 12th week showed high fasting blood glucose, hyperinsulinemia, and increased HOMA1-IR. Accordingly, these results confirmed that the rats underwent type-II diabetes and insulin resistance. Surprisingly, preconditioning with LA decreased blood glucose levels to the normal state, elevated insulin sensitivity, and reduced HW/BW ratio in diabetic animals. In agreement with our findings, a previous experimental study showed that LA has positive effects against diabetic cardiomyopathy development by inhibition of mitochondrial oxidative injury through decreasing cardiomyocyte apoptosis and increasing the levels of glutathione (GSH) and the activity of manganese superoxide diamutase (Mn-SOD) [19]. Several previous studies have suggested that diabetes can increase cardiac susceptibility to I/R damage, leading to the increased infarct size compared with non-diabetic hearts [6, 28]. Inconsistent, this model of diabetes in our study could not significantly increase the infarct size. The impacts of diabetes on cardiac I/R damage and infarct size in experimental setting are controversial. The discrepancy of the results can be described by the possible ability of diabetes to increase cardiac resistance to I/R injury due to its possible preconditioning impacts [7, 23].
It has been revealed that diabetic heart leads to the excessive generation of mitochondrial ROS, reduction of NO levels, endothelial dysfunction, myocardial inflammation, apoptosis, dysregulated autophagy, and consequently contractile dysfunction [29, 30]. Autophagy is critical in degradation of old damaged organelles and proteins in the cell, and has an important role in physiological function of the heart through maintaining cellular homeostasis [31]. Under myocardial I/R injury, autophagy plays both destructive and beneficial roles, which depend on the level of autophagy activity [12, 14]. Thus, maladaptive or adaptive function of autophagy under I/R condition is still under discussion. It has been demonstrated that increase or decrease in autophagy activity induces cell death via excessive degradation of vital organelles and proteins, or accumulation of injured organelles and proteins, respectively [12, 32]. Several preclinical studies reported that induction of adaptive autophagy in the heart may be protective under I/R injury. On the other hand, there are preclinical studies demonstrating that reduction of autophagy activity in the heart may be protective under I/R injury (for more details see Mokhtari and Badalzadeh) [12]. Impaired autophagy in the heart of animals with HFD-induced insulin resistance and type-II diabetes, as well as ischemia could be occurred [33]. Under diabetic condition, metabolic abnormalities such as hyperglycemia, insulin resistance, dyslipidemia, and excessive ROS can inhibit or induce autophagy process in the myocardium, thus lead to the diabetic cardiomyopathy development [10]. In the present work, we used western blotting to monitor changes in LC3II/LC3I and p62, and found that I/R injury increased LC3II and p62 in the heart at both gene and protein levels under diabetic condition. According to the related data of this study, it can be indirectly said that impaired autophagic flux following I/R in diabetic hearts occurred, and combined therapy restored the autophagic flux by decreasing the expression of genes and proteins involved in autophagic flux (LC3 and p62). It should be noted that because of some limitations, the whole “autophagic flux” was not assessed in our study. So it will be necessary to perform further studies to explore the effect of this combined therapy on autophagic flux in the future researches.
Mitochondrial dysfunction has been associated with insulin resistance, and is considered as a major target of oxidative damage in diabetic heart injury. Excessive production of ROS from injured mitochondria which causes cardiomyocyte death through releasing pro-death factors, and subsequent autophagosome accumulation, are commonly observed in diabetic myocardium. Autophagy has a key role in the reduction of diabetic heart injury through elimination of dysfunctional or damaged mitochondria, and prevention of ROS generation [34,35,36]. Similarly, our study showed that I/R injury led to the increased levels of ROS production in the heart under normal and diabetic conditions. In addition, reduction of mitochondrial membrane potential which indicates more depolarization was observed in non-diabetic and diabetic hearts with I/R injury. These findings may reflect the fact that targeting novel strategies to prevent excessive ROS generation by removing dysfunctional mitochondria via restoration of autophagic flux in appropriate level and timing would be useful in the diabetic myocardium with I/R injury. Accordingly, in the current work, application of LA alone caused significant reduction of LC3II and p62 at both gene and protein levels in one hand, and decreased mitochondrial ROS production and improved mitochondrial membrane potential on the other hand. Also, application of IPostC alone significantly reduced LC3 gene expression, LC3II/LC3I protein levels, and mitochondrial ROS generation. However, preconditioning with LA alone, or postconditioning with ischemia alone could not significantly decrease myocardial infarct size. It can be probably explained with this hypothesis that other protective mechanisms may not be influenced by single therapy with LA or IPostC in diabetic hearts with I/R injury. Nevertheless, additional investigations are needed to evaluate this suggestion. The current work showed that combined therapy with LA and IPostC decreased the infarct size of diabetic hearts. This positive effect of combined therapy on infarct size was in consistent with its effects on restoring autophagic flux and improving mitochondrial function. Of note, the effects of combined therapy with LA and IPostC on autophagic flux and mitochondrial function were more effective and potent as compared to the single therapy with them. In other words, addition of LA with IPostC elevated the efficacy of IPostC to modulate autophagic flux and improve mitochondrial function in diabetic I/R hearts. Taken together, it seems that diabetes interferes with cardioprotective effects of IPostC, and possibly reduces its efficacy against myocardial I/R damage. It seems that application of LA raises the potency and efficacy of IPostC in diabetic hearts likely via normalization of some diabetes-associated cellular alterations. This finding suggests that combined conditioning may possibly lead to the effective co-activation of multiple mechanisms of cardioprotection, which requires further clarification. In agreement with the present results, our previous works also confirmed that IPostC alone was not able to decrease infarct size and improve cardiac function following I/R damage under diabetic condition. However, additioning an appropriate therapeutic agent (vildagliptin) with IPostC caused more cardioprotective effects via restoration of autophagic flux and improving mitochondrial function in diabetic hearts injured with I/R [3, 8].
Conclusions
Combined therapy with LA and IPostC induced more effective cardioprotection in type-II diabetic hearts injured by I/R through modulation of autophagic flux and improving mitochondrial function in risk areas of I/R hearts. Even though, further investigations are required to clarify the role of different mechanisms and signaling pathways affected by this combined conditioning to protect the diabetic myocardium, particularly in the in vivo models of I/R injury.
Data availability
The datasets used during the present work are available from the corresponding author on reasonable request.
Abbreviations
- I/R:
-
Ischemia/reperfusion
- IHD:
-
Ischemic heart disease
- IPostC:
-
Ischemic postconditioning
- LA:
-
Alpha-lipoic acid
- HOMA1-IR:
-
Homeostasis model assessment of insulin resistance
- LC3:
-
Microtubule-associated proteins 1A/1B light chain 3
- HFD/STZ:
-
High-fat diet/streptozotocin
References
Mokhtari B et al (2020) Human amniotic membrane mesenchymal stem cells-conditioned medium attenuates myocardial ischemia-reperfusion injury in rats by targeting oxidative stress. Iran J Basic Med Sci 23(11):1453–1461
Naseroleslami M et al (2020) Nesfatin-1 attenuates injury in a rat model of myocardial infarction by targeting autophagy, inflammation, and apoptosis. Arch Physiol Biochem. https://doi.org/10.1080/13813455.2020.1802486
Bayrami G et al (2017) Effect of ischemic postconditioning on myocardial function and infarct size following reperfusion injury in diabetic rats pretreated with vildagliptin. J Cardiovasc Pharmacol Ther. https://doi.org/10.1177/1074248417729881
Badalzadeh R, Azimi A, Alihemmati A, Yousefi B (2017) Chronic type-I diabetes could not impede the anti-inflammatory and anti-apoptotic effects of combined postconditioning with ischemia and cyclosporine A in myocardial reperfusion injury. J Physiol Biochem. https://doi.org/10.1007/s13105-016-0530-4
Penna C et al (2020) Effect of hyperglycaemia and diabetes on acute myocardial ischaemia–reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br J Pharmacol 177(23):5312–5335
Ferdinandy P, Schulz R, Baxter GF (2007) Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59(4):418–458
Ferdinandy P et al (2014) Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66(4):1142–1174
Bayrami G et al (2018) Combination of vildagliptin and ischemic postconditioning in diabetic hearts as a working strategy to reduce myocardial reperfusion injury by restoring mitochondrial function and autophagic activity. Adv Pharm Bull 8(2):319–329
Mokhtari B, Badalzadeh R, Aboutaleb N (2021) Modulation of autophagy as the target of mesenchymal stem cells-derived conditioned medium in rat model of myocardial ischemia/reperfusion injury. Mol Biol Rep 48(4):3337–3348
Ouyang C, You J, Xie Z (2014) The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol 71:71–80
Dewanjee S et al (2021) Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev 68:101338
Mokhtari B, Badalzadeh R (2021) The potentials of distinct functions of autophagy to be targeted for attenuation of myocardial ischemia/reperfusion injury in preclinical studies: an up-to-date review. J Physiol Biochem 77(3):377–404
Schiattarella GG, Hill JA (2016) Therapeutic targeting of autophagy in cardiovascular disease. J Mol Cell Cardiol 95:86–93
Takagi H, Matsui Y, Sadoshima J (2007) The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal 9(9):1373–1382
Yu L et al (2017) Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1α-SIRT3 signaling. Sci Rep 7(1):1–13
Ajami M et al (2011) Expression of Bcl-2 and Bax after hippocampal ischemia in DHA+ EPA treated rats. Neurol Sci 32(5):811–818
Ajami M et al (2013) Effect of DHA+ EPA on oxidative stress and apoptosis induced by ischemia-reperfusion in rat kidneys. Fund Clin Pharmacol 27(6):593–602
Ghibu S et al (2009) Antioxidant properties of an endogenous thiol: alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J Cardiovasc Pharmacol 54(5):391–398
Li C-J et al (2009) Attenuation of myocardial apoptosis by alpha-lipoic acid through suppression of mitochondrial oxidative stress to reduce diabetic cardiomyopathy. Chin Med J 122(21):2580–2586
Ding Y et al (2021) Effects of lipoic acid on ischemia-reperfusion injury. Oxid Med Cell Longev. https://doi.org/10.1155/2021/5093216
Cao X et al (2013) Alpha-lipoic acid protects cardiomyocytes against hypoxia/reoxygenation injury by inhibiting autophagy. Biochem Biophys Res Commun 441(4):935–940
Ghelani H, Razmovski-Naumovski V, Nammi S (2017) Chronic treatment of (R)‐α‐lipoic acid reduces blood glucose and lipid levels in high‐fat diet and low‐dose streptozotocin‐induced metabolic syndrome and type 2 diabetes in Sprague‐Dawley rats. Pharmacol Res Perspect. https://doi.org/10.1002/prp2.306
Miki T et al (2012) Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovas Diabetol 11(1):1–13
Srinivasan K et al (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52(4):313–320
Hosseini L, Vafaee MS, Badalzadeh R (2020) Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats. J Cardiovasc Pharmacol Ther 25(3):240–250
Geloneze B et al (2009) HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq Bras Endocrinol Metab 53(2):281–287
Zhao D, Yang J, Yang L (2017) Insights for oxidative stress and mTOR signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev. https://doi.org/10.1155/2017/6437467
Alegria JR et al (2007) Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J 154(4):743–750
Hsu H-C et al (2016) High-fat diet induces cardiomyocyte apoptosis via the inhibition of autophagy. Eur J Nutr 55(7):2245–2254
Hayat SA et al (2004) Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci 107(6):539–557
Xuan F et al (2017) 17-methoxyl-7-hydroxy-benzene-furanchalcone ameliorates myocardial ischemia/reperfusion injury in rat by inhibiting apoptosis and autophagy via the PI3K–Akt signal pathway. Cardiovasc Toxicol 17(1):79–87
Aghaei M et al (2019) Targeting autophagy in cardiac ischemia/reperfusion injury: a novel therapeutic strategy. J Cell Physiol 234(10):16768–16778
Sciarretta S et al (2015) Boosting autophagy in the diabetic heart: a translational perspective. Cardiovasc Diagn Ther 5(5):394–402
Gottlieb RA, Mentzer RM Jr (2010) Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol 72:45–59
Kobayashi S, Liang Q (2015) Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta Mol Basis Dis 1852(2):252–261
Gonzalez CD et al (2011) The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy 7(1):2–11
Acknowledgements
The authors appreciate the National Institute for Medical Research Development (NIMAD) for their supports, also the help of Dr. Nasrin Abolhasanpour in providing information about some parts of the initial draft of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by a grant from National Institute for Medical Research Development, Iran (NIMAD; grant No. 957279), and direct contribution of Molecular Medicine Research Center, Tabriz University of Medical Sciences, Iran (No: 66545).
Author information
Authors and Affiliations
Contributions
BM and MA performed the experimental tests, and gathered and analyzed the data. BM wrote the manuscript. AA and AJ contributed in interpretation of the results. RB did the study design, supervised the whole project, and contributed in interpretation of the results. RB and BM critically revised the manuscript and finalized the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All experimental protocols and procedures were approved by the Institutional Animal Ethical Committee at the Faculty of Medicine of Tabriz University of Medical Sciences (Ethical code: IR.NIMAD.REC.1396.029).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mokhtari, B., Abdoli-Shadbad, M., Alihemmati, A. et al. Alpha-lipoic acid preconditioning plus ischemic postconditioning provides additional protection against myocardial reperfusion injury of diabetic rats: modulation of autophagy and mitochondrial function. Mol Biol Rep 49, 1773–1782 (2022). https://doi.org/10.1007/s11033-021-06987-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06987-6