Abstract
Purpose
Fatal arrhythmias are one of the main manifestations of ischemic heart disease in diabetic patients. Here, we investigated the effect of pretreatment with vildagliptin on myocardial arrhythmias, inflammatory responses, and expression of genes regulating mitochondrial biogenesis following cardiac ischemic injury in type II diabetic male Wistar rats.
Methods
Chronic diabetes was modeled by a high-fat diet and low-dose streptozotocin method and lasted for 12 weeks. Vildagliptin (6 mg/dl) was orally administered during the last 4 weeks of the diabetic period. Then, rats’ hearts (n = 8/each group) were immediately isolated and transferred to the Langendorff apparatus, in which left anterior descending coronary artery was tightened for 35 min to induce regional ischemia. Electrocardiography was continuously recorded and myocardial arrhythmias were interpreted according to the Lambeth Convention. Inflammatory cytokines in left ventricular samples were measured using ELISA kits, and gene expression was assayed using real-time PCR.
Results
Diabetic groups showed increased incidence and duration of ventricular fibrillation (VF) than controls (P < 0.05). Pretreatment of diabetic rats with vildagliptin resulted in a significant decrease in number, duration, and severity of premature ventricular complexes (PVC), tachycardia (VT), and VF during ischemia, compared to non-treated diabetic group (P < 0.05). Additionally, vildagliptin significantly increased the expression of genes PGC-1α, SIRT-1, and NRF-2 and reduced the levels of myeloperoxidase, creatine kinase release, and myocardial content of TNF-α and IL-1β in nondiabetic and diabetic rats as compared to corresponding controls (P < 0.01–0.05).
Conclusion
Vildagliptin preconditioning reduced the occurrence and severity of fatal ventricular arrhythmias induced by myocardial ischemia in type II diabetic rats through increased activity of mitochondrial biogenesis-regulating genes and reduction of inflammatory reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The prevalence of type II diabetes, as a chronic metabolic disorder, is increasing today [1]. Diabetes has been considered as an independent risk factor for ischemic heart disease and myocardial infarction. Due to the complex pathophysiology and poor prognosis, the mortality and morbidity of diabetic patients following ischemic heart disease are at least twice as high as nondiabetic patients [1, 2]. Myocardial ischemia and subsequent coronary artery disease develop due to the lack of blood supply or stenosis of one or more coronary arteries, leading to the sufferance for cardiomyocytes, and it may occur during several clinical conditions [3]. Myocardial ischemic injury causes more severe disorders such as cardiac hyper-contracture and stunning, contractile dysfunction, and fatal arrhythmias [4]. Therefore, reducing the severity of myocardial ischemic injuries and returning normal electromechanical activity of the heart in diabetic patients has high clinical significance.
Myocardial arrhythmia is one of the main consequences of ischemic insults and a major problem in clinical practice [3, 4]. Several mechanisms such as increased intracellular calcium load, inflammatory responses and cytokines, generation of reactive oxygen species (ROS), and oxidative stress contribute to the pathogenesis of myocardial arrhythmias [4]. Diabetes has also a causative role in inducing these intracellular abnormalities [2, 3]. The increased production of inflammatory cytokines during ischemic injury has a central involvement in the occurrence of arrhythmias [5]. Ischemia triggers inflammatory cascades to activate nuclear factor kappa-B (NF-κB) signaling and production of pro-inflammatory cytokines tumor necrotic factor-alpha (TNF-α), interleukin-1beta (IL-1β), and IL-6. Besides, it has been proved that myeloperoxidase (MPO) is also involved in myocardial remodeling following myocardial ischemic insults [6]. Circulatory levels of MPO are linked to the occurrence of ventricular arrhythmias in cardiac patients, and it causally leads to the myocardial arrhythmias in mice [6]. In the presence of diabetes, the accumulation of leukocytes and activation of adhesion molecules and cytokines are accelerated, and this situation can exaggerate the inflammatory response in the diabetic-ischemic heart [5].
The health of mitochondria in cardiomyocytes is crucial in determining the fate of ischemic injury. Disrupting the function and biogenesis of mitochondria following cardiac ischemia can aggravate inflammatory responses and oxidative stress, leading to cardiac arrhythmias and necrosis [7]. The PPAR-γ coactivator (PGC)-1α transcriptional factor is a powerful regulator of mitochondrial biogenesis in the myocardium [8]. Sirtuin (SIRT)-1 can increase the deacetylase activity of this coactivator to enhance the transcriptional control of mitochondrial protection [9]. On the other hand, the nuclear factor erythroid 2-related factor (NRF)-2 is also targeted by PGC-1α and plays a vital role in maintaining mitochondrial respiratory activity and regulating antioxidant gene expression [10]. It seems that the PGC-1α/SIRT-1/NRF-2 signaling pathway acts as a coordinated network that controls mitochondrial dynamic and biogenesis. Since the mitochondrial biogenesis signaling is impaired in cardiomyocytes during diabetes and metabolic syndrome, modification of these mitochondrial elements can be considered as an effective approach to reducing the outcomes of myocardial arrhythmias and inflammatory responses in these circumstances.
One of the best strategies to reduce the severity of ischemic injuries in the diabetic hearts would be the administration of drugs owning cardioprotective potentials besides their antidiabetic effects. Vildagliptin, as an inhibitor of enzyme dipeptidyl peptidase-4, has beneficial impacts on reducing blood glucose and lipid profiles in diabetes and also has shown anti-inflammatory, anti-oxidative, and anti-apoptotic features [11,12,13,14], and therefore it can be a good candidate for cardioprotection. Furthermore, it has been recently reported that this drug had improving effects on the autophagy process and mitochondrial function in diabetic heart and reduced the size of infarction [15], but its antiarrhythmogenic capability is yet to be clarified. Considering these significant potentials as well as critical roles of ventricular arrhythmias in cardioprotective management of diabetic heart, the present study aimed to determine whether vildagliptin has the antiarrhythmic effects in the myocardial ischemic injury of rats with type II diabetes and that whether the anti-inflammatory and mitochondrial biogenesis-activating effects are involved in this process.
2 Materials and methods
2.1 Animals and materials
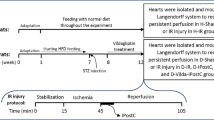
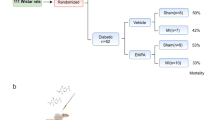
Thirty male Wistar rats with weight range of 220–250 g were purchased from the animal center of the iuniversity and transferred to the experimental animal room. Four rats were allocated in each cage; all had free access to food and water under a 12-h lightness/darkness cycle at the humidity of 50 ± 10% and temperature of 22 ± 3°C. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution and approved by local Animal Care Committee under the ethical number of 93.5–4.8. The materials for high-fat diet were purchased from Merck (Germany), Sigma (Germany), or local suppliers with the highest quality available. Vildagliptin was obtained from Novartis (Swiss), and streptozotocin (STZ) was bought from Sigma (Germany). The sources of commercial kits specific for biochemical and molecular parameters have been reported in corresponding sections.
2.2 Type II diabetes induction
Type II diabetes mellitus was induced in rats by the method of high-fat diet and low-dose STZ [16]. After an acclimatization period of a week, rats were fed a high-fat diet containing 35% normal pellet, 30% lard, 24% casein, 4% sucrose, 1% cholesterol, and 0.3% DL-methionine. The total calorie of this regimen was calculated to be 4.6 kcal/g, 62% of which was from fat. At the beginning of the 7th week of feeding, 35 mg/kg of STZ (in citrate buffer, pH 4) were injected intraperitoneally to rats. At the end of the 7th week, a drop of blood was sampled from the tail vein of rats for measuring the blood glucose levels using a glucometer. The animals with blood glucose levels less than 250 mg/dl were excluded from the study and above this value were considered as diabetic rats. The period of experimental diabetes was continued for 12 weeks to simulate the chronic status of the disease.
2.3 Experimental protocols
Thirty-two rats were randomly divided into the following groups (n = 8/each group):
- Control group (Cont) – nondiabetic healthy rats fed by normal diet and with no pretreatment. Their hearts were subjected to 35 min of regional ischemia.
- Vildagliptin group (Vilda) – control rats were pretreated with vildagliptin orally for a month, and then their hearts were subjected to 35 min of regional ischemia.
- Diabetic group (Diab) – diabetic rats with no pretreatment. Their hearts were subjected to 35 min of regional ischemia.
- Diabetic + vildagliptin group (Diab+Vilda) – diabetic rats were pretreated with vildagliptin orally for a month, and then their hearts were subjected to 35 min of regional ischemia.
Vildagliptin (6 mg/kg) was dissolved in distilled water and administered by oral gavage for 4 weeks, in the last month of the diabetic period [15]. Gavage handling was implemented in the evenings once a day. In groups without pretreatment, distilled water was gavaged, and in control rats, citrate buffer was injected instead of STZ, to minimize the effect of stress caused by gavage and needling, respectively.
2.4 Induction of myocardial ischemia in Langendorff perfusion system
At the end of the 12th week, the rats were heparinized with 500 IU sodium heparin to prevent clots during surgery and then anesthetized with a mixture of ketamine and xylazine (60 and 10 mg/dl, respectively). After that, their chest was opened, and the heart was immediately and carefully isolated from the body and transferred to the Langendorff perfusion system. In this system, the hearts were nourished with a Krebs-Henseleit perfusion solution through the aortic cannula. The ingredients of solution (in mmol/L at pH 7.4 and 37°C) was 118 NaCl, 4.8 KCl, 1.2 MgSO4, 1.0 KH2PO4, 27.2 NaHCO3, 10 glucose, and 1.25 CaCl2. In addition, a gas with 95% O2 and 5% CO2 was bubbled through the solution, and the perfusion pressure was set constantly at 75 mmHg throughout the experiment. The isolated pacing hearts in the Langendorff system were firstly experienced 20 min of the stabilization period and then subjected to 35 min of regional ischemia through ligation of left anterior descending (LAD) coronary artery by a 4/0 silk thread. The confirmation of regional ischemia was made by the reduction of coronary flow to 30–40% of its baseline values [15].
2.5 Electrocardiogram recording and arrhythmias interpretation
To record electrocardiographic (ECG) tracing and spontaneous arrhythmias, three gold surface electrodes were attached to three parts of the heart surface, and electrical changes were transferred to a data acquisition system (Power Lab, AD Instruments, Australia) using a related bio-amplifier. The ECG recording was continued throughout the experiment and displayed and stored on a monitor and then analyzed using the Software Chart v7.7 for Windows (AD Instruments). Lambeth Convention was used to classify the spontaneous arrhythmias; determine the number, duration, and severity of them; as well as interpret their changes among groups (Table 1). During ischemia, the number and duration of arrhythmias including premature ventricular contraction (PVC), ventricular tachycardia (VT) and ventricular fibrillation (VF), and the severity of arrhythmias in each heart were calculated. A single ventricular premature beat (VPB), ventricular bigeminy (VB), and ventricular salvos (VS) were all known as PVC. VT was considered as a run of four or more consecutive VPBs, and VF was considered as the irregular morphology of QRS. The severity of arrhythmias was scored based on a 5-degree algorithm as follows: grade 0 = no arrhythmia, grade 1 = VPB, grade 2 = VB or VS, grade 3 = VT, and grade 4 = F (Table 2). The highest degree of arrhythmia was reported in each sampling (5).
2.6 Measurement of myeloperoxidase levels
In vildagliptin-treated and untreated control and diabetic rats, myeloperoxidase (MPO) levels in the plasma were determined by a colorimetric method. Briefly, plasma samples were obtained at the end of the treatment period and then immersed in 50 mM potassium-phosphate buffer solution (PBS) containing 0.5% hexa-decyltrimethyl-ammonium bromide and immediately homogenized at pH 6. The resultant solution was undergone with 20 min centrifugation at 4500 rpm at 4°C. Thereafter, 0.1 mL of supernatant or prepared standard solution (Sigma, Germany) was added to a 2.9 mL PBS buffer (pH 6) containing o-dianisidine hydrochloride (0.167 mg/mL) and H2O2 (0.0005%). The reaction was stopped by adding 0.1 mL of 1.2 M hydrochloric acid after 5 min. The absorbance of the solution for MPO was measured spectrophotometrically at 460 nm.
2.7 Measurement of creatine kinase
As an index for myocardial cellular damage, creatine kinase (CK) level was measured from the coronary effluent at the end of ischemic period by a colorimetric method, using a commercial kit (Roche Diagnostics, Germany) and an auto-analyzer device (Alcyon, Santa Clara, USA), according to the manufacturer’s instructions. The absorbance of the solution was read at 340 nm, and the recorded values were reported in U/l.
2.8 Measurement of pro-inflammatory cytokines
Enzyme-linked immunosorbent assay (ELISA) kits (BenderMed systems, Austria) were used to measure the levels of inflammatory cytokines (IL-1β and TNF-α), according to the manufacturer’s instructions. Briefly, at the end of the ischemic phase, the hearts were removed from the Langendorff apparatus and the ischemic zones of the left ventricles (~100 mg) were immediately isolated, homogenized in lysis buffer, and centrifuged at 10000 g for 10 min at 4°C. The resultant supernatants were separated from the rest of the solution. Then, 50 ml supernatants and standards were added to the related wells of the plate, and the polyclonal antibodies were then added to all wells. After incubation for 2 h at room temperature, the wells were washed by washing buffer, received the streptavidin-horseradish peroxidase enzyme, and incubated again for 1 h. Finally, after adding TMB substrate solution to all wells to form a colored product, the stop solution was added to each well to stop enzyme reaction, and the rates of absorbance of cytokines in wells were determined at 450 nm, using an ELISA reader. The resultant values were normalized according to the protein contents of each sample and reported as pg/mg of tissue protein.
2.9 Measurement of PGC-1α, SIRT-1, and NRF-2 genes expression levels: real-time PCR
Total RNA extraction: Extracting total RNA was performed by the Plus-RNX kit (Sinaclon, Iran), according to the manufacturer instructions. Briefly, the first 1 ml of Plus RNX solution was added to the tissue precipitates, and this was followed by adding 200 μl of chloroform. After mixing, the vial was incubated at room temperature for 5 min and then centrifuged at 4°C with 12,000 rpm for 15 min. The supernatant was discarded and 1 ml of 75% ethanol was added to the precipitates. The resultant solution was centrifuged again at 4°C with 7500 rpm for 8 min. Then, 50–30 ml of DEPC water was added to the tube. The samples were subjected to determination of RNA concentration via NanoDrop and then used for the synthesis of cDNA.
cDNA synthesis: For cDNA synthesis, the AccuPower cycle Script- RT Premix kit was used based on the instructions of the kit. After adding 1 μg of RNA and bringing the final volume to 20 μl, the resulting mixture was entered to the temperature-time cycles in a thermocycler device for the synthesis of cDNA. After completing cDNA synthesis, its concentration was measured by Picodrop. In addition, the primers for genes PGC-1α, SIRT-1, and NRF-2 and GAPDH (as housekeeping control) were designed using a Gene Runner software and shown in Table 3.
Real-time and PCR reactions: First, 10 μl Master Mix, 2 μL forward and reverse primers, 4 μl cDNA, and 4 μl deionized water were added on each other to reach a final volume of 20 μl. The resultant mixture was transferred to a real-time PCR thermocycler device (Light Cycler 96), with a certain temperature-time program. After reactions, the Ct difference of target gene to reference gene was calculated as ΔCt for each sample, and then the 2-ΔΔCt was obtained. The ratio of the resultant numbers for PGC-1α, SIRT-1, and NRF-2 to GAPDH was reported.
2.10 Statistical analysis
All statistical data were presented as mean ± SEM. The Kruskal–Wallis test was employed to analyze the data for myocardial arrhythmias among experimental groups. Other parameters were analyzed using one-way ANOVA followed by Tukey post hoc test. A p value of less than 0.05 was accepted as statistically significant.
3 Results
3.1 General information about experimental groups
The body weights in diabetic rats (302.92 ± 4.47 g) did not differ from those of control rats (297.75 ± 1.67 g), but vildagliptin significantly reduced the body weight (268.92 ± 4.51) in comparison to diabetic group (P < 0.01). In addition, the induction of chronic diabetes significantly increased the blood glucose and insulin levels compared to control group (glucose, 514.80 ± 8.98 vs. 95 ± 3.22 mg/dl, and insulin, 7.3 ± 0.2 vs. 4.6 ± 0.3 μU/ml) (P < 0.01). Pretreatment of diabetic rats with vildagliptin significantly reduced the blood glucose of diabetic rats to 361.20 ± 4.62 mg/dl (P < 0.01) and increased insulin level to 9.5 ± 0.2 μU/ml. Furthermore, the heart weight to body weight in the diabetic group was significantly greater than controls (0.54 ± 0.00 vs. 0.44 ± 0.01) (P < 0.01), and vildagliptin had no significant effect on this parameter (0.53 ± 0.01) as compared to diabetic group.
3.2 Myocardial CK release
The levels of CK release was measured as an indicator of myocardial damage. The level of CK release in diabetic hearts was significantly greater than that of control hearts (P < 0.05). One month pretreatment of diabetic and nondiabetic rats with vildagliptin could significantly reduce CK release from the myocardium as compared with those of corresponding control groups (both P < 0.05) (Fig. 1).
3.3 Myocardial arrhythmias
The findings of the number and duration and incidences of ventricular arrhythmias including PVC, VT, and VF as well as arrhythmias score during ischemic injury of the myocardium in experimental groups are shown in Fig. 2 a–d. Induction of diabetes for 12 weeks significantly increased some of arrhythmias episodes including VF number (P < 0.05), VF duration (P < 0.01), and incidence of VF episodes in comparison with control rats (Fig. 2a–c). Pretreatment of nondiabetic control rats with vildagliptin significantly reduced the VT duration (P < 0.05), VF number (P < 0.05), and VF incidence (P < 0.01) in comparison to the untreated control group (Fig. 2a–c). In addition, administration of vildagliptin to diabetic rats significantly reduced both number and duration of PVC episodes, VT episodes, and VF episodes (Fig. 2a, b) and incidence of VF (Fig. 2c) as compared with those of untreated diabetic group (P < 0.05 and P < 0.01). Following vildagliptin administration in diabetic rats, the number, duration, and incidence of VF episodes reached zero. Finally, arrhythmia severity scoring was significantly reduced in vildagliptin-receiving diabetic rats compared to the untreated diabetic group (P < 0.05; Fig. 2d).
The number (a) and duration (b) of PVC, VT, and VF and arrhythmias incidence (c) and scoring (d) in hearts of experimental groups during ischemic period. (*P < 0.05, **P < 0.01 vs. Cont group, and #P < 0.05, ##P < 0.01 vs. Diab group), n = 8/each group. Cont, control; Diab, diabetic; Vilda, vildagliptin
3.4 Plasma MPO levels
The plasma levels of MPO as a peroxidase and an inflammatory factor were measured in experimental groups of rats. MPO level was significantly higher in the diabetic group than the control group (P < 0.05; fig. 3). The administration of vildagliptin to nondiabetic and diabetic rats significantly reduced the MPO levels toward their corresponding control values (both P < 0.01; Fig. 3).
3.5 Cytokines levels
Statistical analysis with one-way ANOVA revealed that the administration of vildagliptin in control rats significantly reduced the levels of TNF-α (23.7 ± 2.2 vs. 32.1 ± 3.2; in pg/mg of total protein of sample) (P < 0.05; Fig. 4a) and of IL-1β (25.4 ± 4.1 vs. 34.8 ± 1.9) (P < 0.05; Fig. 4b), as compared with untreated control rats. In addition, although the levels of these inflammatory cytokines were not significantly different between diabetic and control hearts, pretreatment of diabetic rats with vildagliptin could significantly reduce the levels of TNF-α (26.8 ± 1.9 vs. 35.9 ± 1.8) (Fig. 4a) and of IL-1β (24.8 ± 1.7 vs. 37.7 ± 2.2 (Fig. 4b), in comparison with those of untreated diabetic rats (P < 0.01).
3.6 Expression levels of mitochondrial biogenesis-targeting genes
The expression levels of genes PGC-1α, SIRT-1, and NRF-2 in experimental groups are seen in Fig. 5. Chronic diabetes tended to significantly increase the expression of PGC-1α gene as compared with the control group (Fig. 5a). However, the effects of diabetes on the mRNA levels of SIRT-1 and NRF-2 were not statistically significant (Fig. 5b, c). While the administration of vildagliptin to control rats significantly increased only the mRNA levels of NRF-2 in comparison to control group (Fig. 5c), vildagliptin pretreatment in diabetic rats could significantly increase the expression of all three genes (P < 0.01 for PGC-1α and P < 0.05 for SIRT-1 and NRF-2) as compared with diabetic group (Fig. 5a–c).
4 Discussion
In the present study, the anti-arrhythmic effects of vildagliptin following myocardial injury of type II diabetic hearts were evaluated. Chronic diabetes significantly increased the number, duration, and incidence of ischemia-induced VF episodes and arrhythmias scoring. Pretreatment of diabetic rats with vildagliptin resulted in a significant decrease in number and duration of PVC, VT, and VF episodes and the severity of arrhythmias score during myocardial ischemia. Also, vildagliptin significantly reduced VT duration, VF count, and incidence of arrhythmias in nondiabetic hearts. Additionally, vildagliptin significantly increased the expression of mitochondrial biogenesis-targeting genes PGC-1α and SIRT-1 in diabetic rats and increased the expression of NRF-2 and declined MPO levels and CK release as well as myocardial content of inflammatory cytokines TNF-α and IL-1β in both nondiabetic and diabetic groups. Therefore, preconditioning of hearts with vildagliptin in type II diabetic rats can reduce the occurrence and severity of fatal ventricular arrhythmias-induced by myocardial ischemia. Increased activity of genes regulating mitochondrial biogenesis and reduction of inflammatory reactions may play significant impacts in this antiarrhythmic action.
Acute myocardial ischemia is associated with substantial alterations in intracellular and extracellular ionic, biochemical, and metabolic processes in cardiomyocytes. These alterations lead to cellular electrophysiological changes, inducing arrhythmias through several arrhythmogenic mechanisms [3, 18]. Following tachyarrhythmias, significant hemodynamic deteriorations can lead to left ventricular dysfunction. Ventricular arrhythmias such as PVCs, VT, and VF commonly occur early in ischemia and increase myocardial oxygen demand, resulting in exacerbation of ischemia and the development of infarction and acute MI. More importantly, these events are significantly worsening in diabetic conditions [3, 19]. Hyperglycemia, compensatory hyperinsulinemia, insulin resistance, and overproduction of fatty acids during type II diabetes create a certain systemic metabolic phenotype that ultimately exacerbates the production of advanced glycation products, increases oxidative stress and inflammatory responses, and reduces nitric oxide production and endothelial-dependent vascular responses [2, 3]. Ischemia-induced lack of ATP and hypoxia and resultant mitochondrial dysfunction cause abnormalities in impulse initiation and propagation along cardiomyocytes. All of these metabolic changes contribute to arrhythmogenesis. Thus, appropriate risk assessment and subsequent preventive treatments to impede these excessive responses are warranted in the cardiac injury of diabetic subjects.
To reduce the deleterious effects of ischemic injury in diabetic heart, the best candidates would be the use of medications that also have cardioprotective potentials beside their hypoglycemic effect. Vildagliptin has been shown that increase insulin secretion and decrease blood levels of glucagon-like peptides (such as GLP-1) [11] and also can preserve the normal function of the heart in diabetic rats by maintaining the mitochondrial biogenesis and enhancing the cell survival and intercellular mechanisms [14, 15]. Recently, it has been reported that vildagliptin can reduce the myocardial infarct size of diabetic rats and enhance the cardioprotective efficacy of ischemic post-conditioning on diabetic hearts following ischemia-reperfusion injury [13]. The drug has donated these protective effects in a variety of ways, such as the reduction of the myocardial levels of oxidative marker 8-isoprostane and the pro-inflammatory cytokine interleukin-6, and improved the mitochondrial function and autophagy process in the heart.
In our study, vildagliptin protected the diabetic heart against ischemic injury, as evidenced by a reduction in CK level, and demonstrated the antiarrhythmic effect by lessening the timings, incidences, and severity of PVC, VT, and VF. The protective and antiarrhythmogenic effects of vildagliptin pretreatment were accompanied by reduced levels of cardiac TNF-α and IL-1β. Even though the interaction between inflammatory mediators, arrhythmia, and diabetes is not well defined yet, inflammatory reactions have been known as a risk factor for myocardial arrhythmia [20, 21]. The overproduction of ROS during ischemia can trigger an inflammatory response and activate pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 following ischemic insult [22]. TNF-α, in turn, promotes IKKb/NF-kB activation, by which it negatively influences mitochondrial function and biogenesis, boosts ROS overproduction, and augments pro-inflammatory mediators expression and inflammatory responses [23]. Thus, the reduction of IL-1β level after administration of vildagliptin could be attributed to the parallel reduction in TNF-α activity.
In addition, according to our findings, diabetes led to increased systemic MPO levels and vildagliptin significantly prevented this effect. It has been reported that plasma levels of MPO are associated with ventricular arrhythmias and can promote pro-arrhythmic remodeling following myocardial ischemia [6]. MPO has potent pro-inflammatory properties and facilitates the generation of ROS [24]. Therefore, the reduction of MPO levels by vildagliptin can be another support for the anti-inflammatory and antiarrhythmic effect of this drug in diabetic heart.
The loss of mitochondrial function and biogenesis, which often occurs in diabetic conditions, has emerged as the main contributor to the arrhythmogenesis during ischemic injury [7]. This effect can be afforded by changes in the activity of energy-sensitive and redox signaling pathways that regulate ion homeostasis and electrophysiological activity of the heart. PGC-1α has a crucial role in regulating mitochondrial biogenesis [8, 9], and SIRT1 can directly regulate the activity of this coactivator [9, 25]. In the present study, the expression of both genes was upregulated following the administration of vildagliptin to diabetic rats, and this may indicate that vildagliptin-induced high expression of SIRT1 in cardiomyocytes can subsequently lead to the improvement of transcriptional activity of PGC-1α and thereby promotes the mitochondrial biogenesis and cardioprotection. Finally, the results obtained from real-time PCR revealed that vildagliptin also upregulated NRF-2 expression in the left ventricular ischemic area of both diabetic and nondiabetic rats. Activation of NRF-2 has been reported as cardioprotective. SIRT-1 and PGC-1α can promote the activity of the nuclear transcription factor NRF-2, which, in turn, upregulates the transcription of cytoprotective and antioxidative enzymes [26]. The expression of these target genes augments the heart resistance to oxidative stress and inflammatory reactions [26, 27]. Therefore, PGC-1α/SIRT-1/NRF-2 signaling pathway may act as a coordinated network to control mitochondrial dynamic and biogenesis and mediate the positive effect of vildagliptin on the activity of MPO and pro-inflammatory cytokines as well as myocardial arrhythmias.
In conclusion, the administration of vildagliptin as a preconditioning modality to type II diabetic rats could reduce the occurrence of ventricular arrhythmias and the severity of fatal arrhythmias following myocardial ischemia. The increased activity of genes regulating mitochondrial biogenesis and reduction of MPO levels and inflammatory mediators may play significant impacts in this antiarrhythmic action. Whether vildagliptin can modulate other mechanisms of arrhythmogenesis in diabetic hearts warrants further investigation.
Abbreviations
- VF :
-
Ventricular fibrillation
- VT :
-
Ventricular tachycardia
- VB :
-
Ventricular bigeminy
- VS :
-
Ventricular salvos
- VPB :
-
Single ventricular premature beat
References
Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diab. 2015;6:1246–58. https://doi.org/10.4239/wjd.v6.i13.1246.
Saeid F, Aniseh J, Reza B, Manouchehr VS. Signaling mediators modulated by cardioprotective interventions in healthy and diabetic myocardium with ischaemia–reperfusion injury. Eur J Prev Cardiol. 2018;25:1463–81. https://doi.org/10.1177/2047487318756420.
Severino P, D’Amato A, Netti L, Pucci M, Infusino F, Maestrini V, et al. Myocardial Ischemia and Diabetes Mellitus: Role of Oxidative Stress in the Connection between Cardiac Metabolism and Coronary Blood Flow. J Diabetes Res. 2019;(9489826). https://doi.org/10.1155/2019/9489826.
Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediat Inflamm. 2017;7018393. https://doi.org/10.1155/2017/7018393.
Badalzadeh R, Azimi A, Alihemmati A, Yousefi B. Chronic type-I diabetes could not impede the anti-inflammatory and anti-apoptotic effects of combined postconditioning with ischemia and cyclosporine a in myocardial reperfusion injury. J Physiol Biochem. 2017;73(1):111–20. https://doi.org/10.1007/s13105-016-0530-4.
Mollenhauer M, Friedrichs K, Lange M, Gesenberg J, Remane L, Kerkenpaß C, et al. Myeloperoxidase mediates Postischemic Arrhythmogenic ventricular remodeling. Circ Res. 2017;121(1):56–70. https://doi.org/10.1161/CIRCRESAHA.117.310870.
Song J, Yang R, Yang J, Zhou L. Mitochondrial dysfunction-associated Arrhythmogenic substrates in diabetes mellitus. Front Physiol. 2018;9:1670. https://doi.org/10.3389/fphys.2018.01670.
Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–90S. https://doi.org/10.3945/ajcn.110.001917.
Mohamed JS1, Hajira A, Pardo PS, Boriek AM. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1α network in skeletal muscle. Diabetes. 2014;63(5):1546–59. https://doi.org/10.2337/db13-1364.
Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–88. https://doi.org/10.1016/j.freeradbiomed.2015.04.036.
Bekiari E, Rizava C, Athanasiadou E, Papatheodorou K, Liakos A, Karagiannis T, et al. Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes. Endocrine. 2016;52(3):458–80. https://doi.org/10.1007/s12020-015-0841-1.
Williams R, de Vries F, Kothny W, Serban C, Lopez-Leon S, Chu C, et al. Cardiovascular safety of vildagliptin in patients with type 2 diabetes: a European multi-database, non-interventional post-authorization safety study. Diabetes Obes Metab. 2017;19(10):1473–8. https://doi.org/10.1111/dom.12951.
Bayrami G, Karimi P, Agha-Hosseini F, Feyzizadeh S, Badalzadeh R. Effect of ischemic Postconditioning on myocardial function and infarct size following reperfusion injury in diabetic rats pretreated with Vildagliptin. J Cardiovasc Pharmacol Ther. 2018;23(2):174–83. https://doi.org/10.1177/1074248417729881.
Apaijai N, Pintana H, Chattipakorn SC, Chattipakorn N. Effects of vildagliptin versus sitagliptin, on cardiac function, heart rate variability and mitochondrial function in obese insulin-resistant rats. Br J Pharmacol. 2013;169(5):1048–57. https://doi.org/10.1111/bph.12176.
Bayrami G, Alihemmati A, Karimi P. Combination of vildagliptin and ischemic postconditioning in diabetic hearts as a working strategy to reduce myocardial reperfusion injury by restoring mitochondrial function and autophagic activity. Adv Pharm Bull. 2018;8(2):319–29. https://doi.org/10.15171/apb.2018.037.
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20. https://doi.org/10.1016/j.phrs.2005.05.004.
Najafi M, Noroozi E, Javadi A, Badalzadeh R. Anti-arrhythmogenic and anti-inflammatory effects of troxerutin in ischemia/reperfusion injury of diabetic myocardium. Biomed Pharmacother. 2018;102:385–91. https://doi.org/10.1016/j.biopha.2018.03.047.
Bhar-Amato J, Davies W, Agarwal S. Ventricular arrhythmia after acute myocardial infarction: 'the perfect storm'. Arrhythm Electrophysiol Rev. 2017;6(3):134–9. https://doi.org/10.15420/aer.2017.24.1.
Koektuerk B, Aksoy M, Horlitz M, Bozdag-Turan I, Turan RG. Role of diabetes in heart rhythm disorders. World J Diabetes. 2016;7(3):45–9. https://doi.org/10.4239/wjd.v7.i3.45.
Korantzopoulos P, Letsas KP, Tse G, Fragakis N, Goudis CA, Liu T. Inflammation and atrial fibrillation: a comprehensive review. J Arrhythm. 2018;34(4):394–401. https://doi.org/10.1002/joa3.12077.
Lazzerini PE, Laghi-Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019 Jan;19(1):63–4. https://doi.org/10.1038/s41577-018-0098-z.
Saini HK, Xu Y-J, Zhang M, Liu PP, Kirshenbaum LA, Dhalla NS. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp Clin Cardiol. 2005;10(4):213–22.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. https://doi.org/10.1038/sigtrans.2017.23.
Anatoliotakis N, Deftereos S, Bouras G, Giannopoulos G, Tsounis D, Angelidis C, et al. Myeloperoxidase: expressing inflammation and oxidative stress in cardiovascular disease. Curr Top Med Chem. 2013;13(2):115–38.
Govender J, Loos B, Marais E, Engelbrecht AM. Melatonin improves cardiac and mitochondrial function during doxorubicin-induced cardiotoxicity: a possible role for peroxisome proliferator-activated receptor gamma coactivator 1-alpha and sirtuin activity? Toxicol Appl Pharmacol. 2018. https://doi.org/10.1016/j.taap.2018.06.031.
Holmström KM, Kostov RV, Dinkova-Kostova AT. The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol. 2016;1:80–91. https://doi.org/10.1016/j.cotox.2016.10.002.
Li X, Wang H, Gao Y. Protective effects of Quercetin on mitochondrial biogenesis in experimental traumatic brain injury via the Nrf2 signaling pathway. PLoS One. 2016;11(10):e0164237. https://doi.org/10.1371/journal.pone.0164237.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, data collection, and analysis. Su Wu and Qin Yang wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there was no conflict of interest in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Q., Ai, W., Nie, L. et al. Vildagliptin reduces myocardial ischemia-induced arrhythmogenesis via modulating inflammatory responses and promoting expression of genes regulating mitochondrial biogenesis in rats with type-II diabetes. J Interv Card Electrophysiol 59, 517–526 (2020). https://doi.org/10.1007/s10840-019-00679-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-019-00679-9