Abstract
Summary
A very high rate of osteoporosis, fractures, and low lean mass was observed in patients with chronic obstructive pulmonary disease (COPD). Disease severity was associated with bone and muscle adverse outcomes, while age ≥ 63.5 years old, low lean mass, higher iPTH, and a T-score below − 2.5 were all associated with higher risk of fracture.
Introduction
Osteoporosis is frequently neglected in patients with COPD. We aimed at evaluating the rate of osteoporosis, fractures, and low lean mass in patients with COPD.
Methods
Ninety-nine patients with COPD (53 women, 64.5 ± 9.6 years old, and 46 men, 65.9 ± 8.0 years old) underwent bone densitometry (DXA) with body composition analyses. Healthy individuals (N = 57) not exposed to tobacco matched by sex, age, and body mass index (BMI) were used as controls. Spirometry, routine laboratory workout, and conventional thoracolumbar radiography surveying for vertebral deformities were performed in all patients.
Results
Osteoporosis was found in 40.4% of the COPD patients against only 13.0% of the healthy controls (p = 0.001). Vertebral fractures were seen in 24.4% of the men and 22.0% of the women with COPD. Disease severity (GOLD 3 and 4) was significantly associated with higher risk of vitamin D deficiency (p = 0.032), lower BMD (both men and women at all sites), higher frequency of osteoporosis (in women at all sites), lower skeletal mass index, and higher rate of low lean mass (in both men and women) than healthy controls and COPD patients with milder disease (GOLD 1 and 2). Age was a main predictor of vertebral fractures (OR = 1.164 (1.078–9.297); p < 0.001), while high plasma iPTH (OR = 1.045 (1.005–1.088); p = 0.029) and low ALM (OR = 0.99965 (0.99933–0.99997); p = 0.031) were predictors of non-vertebral fractures.
Conclusion
Highly prevalent in COPD, osteoporosis and low lean mass were associated with FEV1% < 50%. Age, low lean mass, high iPTH, and low bone mass were all significantly associated with fractures in COPD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized as a persistent airflow limitation associated with inflammatory response caused by toxic particles and gases usually related to tobacco [1]. Highly prevalent, COPD affects quality of life and increases hospitalization and death [2, 3]. Patients with higher airflow limitation have higher risk for exacerbations, hospitalizations, and death [1]. Among other comorbidities, osteoporosis is highly common and frequently ignored in patients with COPD [4, 5]. The prevalence of osteoporosis in COPD patients has ranged from 17 to 35% [6, 7] and depends upon the definition criteria and methods used for the diagnosis [8].
Characterized by low bone mass and compromised bone quality and strength leading to an increased risk of fractures [8,9,10,11], osteoporosis is frequently neglected and only diagnosed after a fracture. Hip fractures increase mortality by 15 to 20% in 1 year [11]. Systemic inflammation in COPD patients contributes to loss of weight and lean mass in advanced stages [10, 12, 13]. Glucocorticoid use, sedentary lifestyle, and lower concentrations of vitamin D may all contribute to a higher incidence of osteoporosis in COPD patients [4, 5].
Only a few studies have analyzed predictors of osteoporosis and fractures in patients with COPD [8]. There is no specific recommendation to screen for bone fragility and loss of muscle mass or to initiate anti-osteoporosis drugs in these patients [14]. In the present study, we evaluate the rate of osteoporosis, fragility fractures, and low lean mass in a cohort of COPD patients seen at a tertiary hospital. Potential risk factors associated with skeletal fragility and body composition abnormalities were also investigated. The study was mainly interested in determining whether poor lung function in COPD is an independent risk factor for low lean mass and fragility fractures.
Patients and methods
Patients and healthy controls
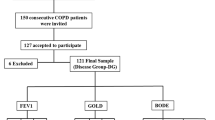
Patients with COPD were consecutively evaluated at the Pulmonology’s Outpatient Clinics at UNIFESP/EPM in São Paulo, Brazil, between November 2013 and December 2015. Patients were selected by convenience and underwent bone densitometry with body composition analyses, spirometry, laboratory tests, and thoracolumbar conventional X-ray. Healthy individuals with no exposure to tobacco matched by age, sex, and body mass index (BMI) were used as controls. Individuals with history of fragility fracture, chronic diseases, or use of medications that affect bone and mineral metabolism were excluded from the healthy controls. Patients answered a structured questionnaire on medical history, previous diagnosis, and treatment of osteoporosis [15]. Cumulative smoking was measured as pack-years. The local UNIFESP/EPM’s Ethics Committee approved the study protocol and all participants gave informed consent before entering the study.
Body composition analyses and bone mineral density measurements
Bone densitometry at the lumbar spine, proximal femur, and whole body using dual X-ray absorptiometry (DXA) technique was performed on a DPX MD+ (GE—Lunar DPX Medical Systems Radiation Corporation, Madison, WI, USA). Lumbar spine (L1L4), femoral neck, and total femur bone mineral density (BMD) were measured as recommended [16]. The following parameters of body composition were evaluated: appendicular lean mass (ALM) obtained by the sum of lean mass of arms and legs and body fat (BF) in kilograms and percentage. From the raw data obtained for ALM, skeletal mass index (SMI) was calculated, according to previously proposed model (SMI = ALM/height2) [17]. Accordingly, fat mass index (FMI) was calculated from BF (FMI = BF/height2). Bone densitometry precision expressed as the root mean square of the coefficients of variation (CV) [18] for lumbar spine and total femur BMD, percentage of BF, BF in grams, and ALM was 2, 3, 1.62, 1.53, and 1.64%, respectively, as previously published [19]. The same examiner performed acquisition and analysis of all DXA scans.
Diagnosis of osteoporosis was based on the International Society of Clinical Densitometry 2007’s criteria [16]. The frequency of low lean mass in the study population was investigated using the most recently proposed criteria: (1) the European Working Group for Sarcopenia in Older People—EWGSOP definition criteria SMI ≤ 7.26 kg/m2 in men and ≤ 5.5 kg/m2 in women [20] and (2) the criteria of the National Institutes of Health (FNIH) Sarcopenia Project ALM/BMI ratio < 0.789 in men and < 0.512 in women [21].
Spirometry
At the time of the DXA scan, pulmonary function tests were performed in the COPD patients on a Clinical Pulmonary Function-Spirometry (CPF-S, Medical Graphics Corporation, St. Paul, MN, USA). The same examiner performed all tests after daily calibration, according to manufacturer’s instructions. Analysis was performed according to GOLD recommendations [1, 22]. Briefly, airflow limitation as determined by spirometry is divided into four grades (GOLD 1 = FEV1% > 80, mild; GOLD 2 = FEV1% 50–79, moderate; GOLD 3 = FEV1% 30–49, severe; and GOLD 4 = FEV1% < 30, very severe) [1].
Fractures
Antero-posterior and lateral plain radiographs of the thoracic and lumbar spine were systematically performed to survey for vertebral fractures in the COPD patients. Genant’s criteria were used to classify the type and severity of prevalent spinal deformity [23]. Deformity grades II and III were considered as clinically relevant vertebral fractures. An experienced investigator (MMP) blinded to the clinical status of the subjects performed all the radiographic vertebral fracture assessment. Non-vertebral osteoporotic fractures were recorded from medical charts and patient’s interview. Osteoporotic non-vertebral fractures were defined as those occurring as a result of a low-energy trauma or less [24].
Physical function and activity
The Timed Up and Go test (TUG) was used to evaluate physical functioning and balance [25]. The Baecke questionnaire, measuring habitual physical activity levels performed over the past 12 months at work, during sports and at leisure time [26], was applied to all patients. Baecke questionnaire was applied as an interview by the same examiner.
Laboratory tests
All patients performed a complete laboratory workup including whole blood count, serum urea, creatinine, alanine transaminase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase, alkaline phosphatase, C-reactive protein, total calcium, ionized calcium, phosphate, magnesium, estradiol, follicle-stimulating hormone (FSH), luteinizing hormone (LH), total testosterone, arterial blood gas analysis, thyroid-stimulating hormone (TSH), and free T4, all performed under standard techniques. 25-hydroxyvitamin D (25(OH)D) and intact parathyroid hormone (iPTH) measurements were performed using chemiluminescence immunoassays (Siemens Advia Centaur XP and Siemens Immulite 2000 XPI, respectively, Tarrytown, NY). Coefficient of variation for 25(OH)D measurement using this method is 11.7%. The intra-assay CV for iPTH ranged from 5.5 to 6.6%, and the inter-assay CV ranged from 7.9 to 8.6% [27, 28].
Statistical analyses
Quantitative data are presented as mean ± standard deviation while categorical data are shown as n (%). Student’s t test was used to compare variables with normal distribution while chi-squared or Fisher’s exact test was used to compare COPD patients and their healthy controls. Variables with non-normal distribution were compared by nonparametric tests (Mann-Whitney). One-way ANOVA was used for the comparison of three or more quantitative variables with normal distribution and Tukey’s test as post hoc analysis. Correlation coefficient of Pearson or Spearman was used for variables with normal or non-normal distribution, respectively. Simple and multiple linear regression analyses were used to determine the independent variables associated with low bone and lean mass in the COPD patients. Multiple logistic regression analysis was performed to determine the main risk factors for fragility fractures in COPD patients. The association was measured as odds ratio (OR) with 95% confidence intervals. For both linear and logistic regression analyses, significance level to select independent variables was set as p < 0.1. Selected variables were then tested in multiple regression analyses by using the backward selection method. Parameters used to start linear regression analyses were age, ALM, SMI, BF, FMI, TUG, 25(OH)D, iPTH, FEV1%, and GOLD status 1 and 2 versus 3 and 4. Categorical variables used in the logistic regression analyses included having a T-score ≤ −2.5 (densitometric osteoporosis) and GOLD status (GOLD 1 and 2 versus 3 and 4). Continuous variables used in the logistic regression analyses were age, BMI, ALM, SMI, BF, FMI, TUG, iPTH, 25(OH)D, FEV1, and BMD measurements. Receiver operating characteristic (ROC) curve was used to define potential cut-offs in quantitative variables predicting fractures or low lean mass. The Statistical Package for Social Science SPSS 23.0 was used for all analyses. Significance level was set as p < 0.05.
Results
Population characteristics, osteoporosis, fractures, and low lean mass
From a total of 500 patients registered in our COPD outpatient’s clinics, 99 consecutive patients were contacted and agreed to participate in the present study. Fifty-seven healthy individuals matched by age, sex, and BMI were used as controls. Anthropometric characteristics and clinical and laboratorial parameters for the COPD patients and their healthy controls are shown in Table 1. Most of the COPD patients were smokers. Two COPD women were never smokers: one had passive exposure to cigarette smoke and the other had been exposed to fumes from wood oven. Cumulative smoking in COPD patients was 61 ± 35.8 pack-years. Spirometry showed mean percentage forced expiratory volume in the first second FEV1% of 51.3 ± 17.3%. Most of the COPD patients (94 individuals, 94.9%) were regularly using inhaled glucocorticoid while only six patients had a previous diagnosis of osteoporosis. As expected, COPD patients GOLD 3 and 4 had significantly higher PaCO2 and lower PaO2 than GOLD 1 and 2 patients. Patients with COPD GOLD 3 and 4 were significantly thinner and had lower prevalence of diabetes mellitus than patients GOLD 1 and 2 (Table 1).
Recent spirometry data were available for 89 patients included in the study. For our subsequent analyses, COPD patients were classified according to lung damage and airflow obstruction measured by FEV1% using the GOLD criteria [22] in two groups: COPD GOLD 1 and 2 (FEV1% ≥ 50%) and COPD GOLD 3 and 4 (FEV1% < 50%).
As seen in Table 1, most of the COPD patients were vitamin D deficient or insufficient (serum 25(OH)D concentration below 30 ng/mL). Patients with more severe disease (GOLD 3 and 4) also had significantly higher prevalence of vitamin D deficiency than patients with milder disease (GOLD 1 and 2). Secondary hyperparathyroidism (iPTH ≥ 65 pg/mL with normal serum calcium) was detected in 23.6% of the COPD patients. In those patients, serum 25(OH)D levels were on average 22.0 ng/mL (6.9–39.7 ng/mL). Testosterone, estradiol, FSH, and LH were within the normal range for age and sex.
Two patients did not have data on L1L4 BMD measurements due to structural artifacts in the spine while 9 other did not have total hip measurements due to technical problems. Using densitometric criteria [16], 40.4% of the COPD patients as a group had osteoporosis versus 13.0% of the healthy controls matched by sex, age, and BMI (p = 0.001), as shown in Table 1. Body composition parameters were available for 98 COPD patients. One patient did not have body composition parameters due to an acquisition error. Using the European definition criteria [20], low lean mass was significantly more prevalent in the COPD patients (men and women as a group) than in their controls. Patients with more severe disease (GOLD 3 and 4 men and women) had significantly higher rate of low lean mass than COPD men and women GOLD 1 and 2 (Table 1). About 42.2% of the men and 20.8% of the women with COPD had low lean mass versus 14.3 and 3.2% of healthy men and women, respectively (p = 0.027; not shown). No significant difference in the rate of low lean mass was observed when FNIH definition criteria [21] were used (46.7 and 34% versus 28.6 and 29%, respectively; p = 0.641).
Physical performance measured by the Baecke questionnaire showed that men with COPD had better performance (4.06 ± 1.52) when compared to women (3.44 ± 1.09; p = 0.047). Accordingly, men also had significantly better results in the TUG test (10.4 ± 2.8 s) when compared to women (10.9 ± 2.8 s; p = 0.018).
Table 2 shows the frequency of osteopenia and osteoporosis in our sample according to skeletal site, disease severity measured by the GOLD status, and sex. Bone abnormalities were significantly more prevalent in patients with more severe disease (GOLD 3 and 4) than in GOLD 1 and 2 patients at all skeletal sites taken together or separately and particularly among women. In fact, the frequency of bone abnormalities in men with COPD did not differ significantly between grades GOLD 1 and 2 versus GOLD 3 and 4.
BMD measurements and body composition
BMD measurements and body composition parameters according to disease severity and sex are shown in Table 3. BMD measurements differ significantly between healthy subjects and COPD patients. More severe disease (GOLD 3 and 4) was associated with significantly lower BMD particularly among women when compared to both healthy controls and GOLD 1 and 2 patients (milder disease). ALM was significantly lower in COPD women with more severe disease (GOLD 3 and 4) than their healthy controls and GOLD 1 and 2 women. COPD men with more severe disease had significantly lower SMI than their healthy controls (ANOVA). Women with COPD (both groups GOLD 1 and 2 and GOLD 3 and 4) had significantly higher FMI as compared to healthy controls matched for age and BMI (Table 3).
Correlations
Considering the findings of lower BMD and lean mass in COPD patients with more severe disease, potential associations between BMD, body composition parameters, and spirometry variables were then investigated. No significant correlation was detected between spirometry variables or DXA parameters and cumulative smoking measured as pack-years and physical activity level measured by the Baecke questionnaire. Univariate and multivariate linear regression analyses were performed to identify potential factors associated with BMD and body composition parameters in patients with COPD. Variables significantly associated in univariate linear regression (p < 0.1) were tested in multiple linear regression analyses and are shown in Table 4. Age, BMI, body composition parameters, and FEV1% were the main determinants of bone mass. A model including age, ALM, iPTH, and FEV1% was able to explain up to 62% of the variability of femoral neck BMD in COPD women. Age, weight, and FEV1 were factors associated with lean mass. A model including age and weight explained up to 58% of the variability of ALM in women with COPD.
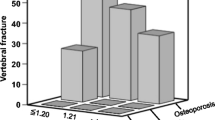
Radiographic assessment of vertebral fractures was available for 95 patients. Vertebral deformities were seen in 40 patients with COPD as follows: 14 patients had one vertebral deformity (14.7%), 10 patients had 2 vertebral deformities (10.5%), 11 patients had 3 (11.6%), and 5 patients had 4 to 6 vertebral deformities (5.3%). Vertebral deformities (Genant’s grades I to III) were detected in 42.2% of the men and in 42% of the women with COPD. Vertebral fractures (defined as Genant’s grades II and III) were detected in 24.4% of men and 22.0% of the women with COPD. The number of vertebral deformities (Genant I to III) did not significantly correlate with FEV1 or FEV1% for any the whole group, men or women with COPD. In women with COPD, vertebral fractures (Genant II and III) significantly associate with FEV1% on linear regression analysis (B = − 13.4; standard error = 6.5; p = 0.047; R2 = 0.071). On the other hand, vertebral deformities (Genant I to III) and the number of vertebral deformities did not significantly associate with FEV1%. Similar analysis in men with COPD did not find significant association between vertebral deformities (Genant I to III), vertebral fractures (Genant II and III), and the number of vertebral deformities with FEV1%. Nine women with COPD (17%) reported non-vertebral fragility fractures. Men with COPD did not report non-vertebral fractures.
As shown in Table 5, both men and women with COPD with vertebral deformities were significantly older than those without vertebral deformities. COPD women with vertebral deformities had also significantly higher serum iPTH concentrations, poorer performance on TUG test, lower BMD values at all sites, and higher rate of densitometric osteoporosis (T-score − 2.5 and below) than COPD women without vertebral deformities (Table 5). Logistic regression analyses were performed to identify potential factors associated with vertebral and non-vertebral fractures and low lean mass (as defined by the European criteria) in patients with COPD (Table 6). Increasing age was the only factor significantly associated with vertebral fractures in both men and women, while lower ALM and higher serum iPTH were significantly associated with non-vertebral fractures in the whole group of patients. Low femoral neck BMD on a DXA scan was the main factor associated with non-vertebral fractures in women with COPD. COPD men did not report non-vertebral fracture in the present study. FEV1% was the main predictor of low lean mass for the whole group and for men with COPD. In women with COPD, age was the only factor significantly associated with low lean mass.
Receiver operating characteristic (ROC) curve was used to evaluate the performance of factors significantly associated with fractures in logistic regression analyses. Age was significantly associated with vertebral fractures in our COPD patients. The areas under the curve for age as a predicting factor for vertebral deformities (Genant’s grades I to III) and fractures (Genant’s grades II and III) were 0.779 (p < 0.001) and 0.738 (p = 0.001), respectively (Supplemental Figure 1). Age above 63.5 years in both men and women with COPD was significantly associated with vertebral deformity Genant’s grades I to III with a sensitivity of 77.5% and a specificity of 65.5% (in those who have a vertebral fracture, 77.5% were 63.5 years and older while among those who do not have a fracture, 65.6% were younger than 63.5 years old). Similar analysis showed that age above 63.5 years old is significantly associated with vertebral fractures (Genant’s grades II and III) with a sensitivity of 86.4% and a specificity of 42.5% (Supplemental Figure 1).
Discussion
The association of musculoskeletal and bone disease in patients with COPD has been described and vertebral compression fractures are often missed or ignored in this clinical scenario [5]. Osteoporosis and its neglected fragility fractures are one of the most frequent comorbidities in COPD patients [8]. In the present work, we observed a very high rate of osteoporosis and fragility fractures in a series of COPD patients. Only a minority had previous diagnosis of osteoporosis. Most of the surveys have demonstrated a lack of care in COPD patients aiming to prevent, diagnose, and treat osteoporosis [5, 8, 10]. The rate of osteoporosis and fractures observed in our patients is clearly higher than that reported for the Brazilian general population [29,30,31]. In fact, COPD leads to a 1.5–2-fold increase in the relative risk of osteoporosis even after adjustments for confounding factors such as age and BMI [4, 32]. Our study also demonstrated higher rate of low lean mass in these patients, a factor associated with significant functional limitation in COPD regardless of lung function [33]. In women with COPD, we also observed a significant increase in body fat. Low bone mass, fractures, and body composition abnormalities (low lean mass and high body fat) were significantly associated with disease severity.
The real absolute risk of fractures in COPD patients is significantly higher than that estimated by FRAX®, similarly to what has been previously demonstrated for type 2 diabetes mellitus patients [34], suggesting the existence of some intrinsic element that is not predicted by other conventional risk factors [8]. In our sample, we observed a very high rate of vertebral deformities (42% for Genant’s grade I vertebral deformities and higher). It has been demonstrated that vertebral fractures have a strong negative impact on the course of COPD increasing the risk of hospitalization and death [5]. Accordingly, it has also been demonstrated that greater kyphosis can increase pulmonary impairment, further limiting function in COPD patients [35]. The very high rate of vertebral fractures (Genant’s grade II and III) in the present study (24.4% in men and 22% in women) contrasts with the low diagnosis rate and underscores the need for routine screening of bone fragility in COPD. The present study has also found that vertebral fractures are associated with increasing age and patients over 63 years old are at higher risk. Disease severity did not significantly associate with fractures when this outcome was analyzed by multiple logistic regression. Our limited sample size with relative small number of fractures may have precluded us from finding a significant association between FEV1 and fractures.
Lower serum 25(OH)D concentration is commonly seen in COPD patients and contributes to lower mineralization and osteomalacia, further reducing bone strength and increasing the risk of fractures. Vitamin D plays a significant role in calcium, phosphate, and bone homeostasis and may also be involved in the immune system, risk of infections, and many other extra-skeletal functions [36,37,38]. In our sample, 37.5% of the patients had 25(OH)D below 20 ng/mL. Disease severity with higher airflow limitation was significantly associated with lower serum vitamin D levels. Sedentary lifestyle with low sunlight exposure, insufficient diet, glucocorticoid use, renal impairment, and lower capacity for vitamin D storage in muscles and fatty tissue may all influence the vitamin D status in COPD [38, 39]. On the other hand, it is still not clear whether vitamin D supplementation may be able to significantly alter extra-skeletal outcomes in COPD or in other diseases [40].
A recent meta-analysis evaluated 25(OH)D status in COPD patients [39]. The authors demonstrated significantly lower serum 25(OH)D concentrations in COPD patients when compared to healthy individuals [39]. Similar to our results, serum 25(OH)D concentration was also significantly associated with pulmonary function: patients with lower FEV1 (GOLD 3 and 4) had significantly lower vitamin D concentrations than patients GOLD 1 and 2. Lower serum 25(OH)D concentration was still associated with a higher risk of COPD exacerbation [39]. Our data did not find any significant association between serum 25(OH)D levels and low bone mass or fractures. On the other hand, some of our analyses did show that increased plasma iPTH levels, a surrogate for vitamin D deficiency, were significantly associated with low bone mass and non-vertebral fractures. A study evaluating data from the Korean NHANES demonstrated that higher levels of iPTH were associated with lower FEV1 and CFV values and poor quality of life, associations not seen for serum vitamin D concentration. Taken together, these findings may suggest that plasma iPTH levels might be a better indirect marker of COPD and bone outcomes than serum 25(OH)D se concentration [41].
Many physical function tests may predict mortality- and disease-related outcomes in COPD patients. Simple and functional lower limb tests provide information about important clinical outcomes in patients with COPD. The TUG test is used to evaluate patient’s strength, mobility, and balance [17]. Some authors have shown that TUG may also predict mortality- and disease-related outcomes in COPD patients [42]. As expected, higher TUG results were associated with lower BMD at skeletal sites sensitive to mechanical load as the total femur and femoral neck. Cumulative smoking measured as pack-years and physical activity level measured by the Baecke questionnaire did not correlate significantly with spirometry parameters or DXA variables. The known poor sensitivity and specificity of both pack-years calculation and Baecke questionnaire would explain the lack of association with spirometry and DXA data. Those variables are much dependent on patient’s recall and so may not correlate significantly with disease outcome measures (pulmonary function and bone density).
Our results showed that increased severity of COPD (GOLD 3 and 4) was associated with lower BMD values in both men and women and higher rates of osteoporosis at all skeletal sites in women. Previous studies have also demonstrated higher risk of osteoporosis in GOLD 4 patients as compared to patients classified as GOLD 1 [10, 43]. Other analyses have also pointed that disease phenotype and severity might affect the risk of comorbidities in COPD patients. Severe emphysematous disease detected by tomography increases the risk of osteoporosis in COPD patients [44]. In our current study, COPD severity was significantly associated with low bone mass, low lean mass, and fragility fractures.
Treatment with inhaled or oral glucocorticoid was associated with lower BMD at the lumbar spine, femoral neck, and total femur and with a threefold increase in the risk of vertebral fracture in 714 men with COPD or asthma followed in the MrOs cohort [4]. No pulmonary function test was performed in that cohort so the data was mainly based on subjects report on the presence of pulmonary disease. In our study, the use of inhaled glucocorticoid was not significantly associated with bone outcomes. This is probably due to the fact that around 95% of the patients had been on long-term treatment with inhaled glucocorticoid.
Our data showed that lower FEV1 were significantly associated with lower BMD and lower lean mass. Lower FEV1 was also associated with increased fat mass in women. The risk of osteoporosis and low lean mass was thus increased in patients with more severe disease. Age, lean mass, high plasma levels of iPTH, and low femoral neck BMD were independently associated with increased risk of fracture. Age above 63.5 years old was associated with significantly increased risk of vertebral fractures (Supplemental Figure 1). On its turn, FEV1% and age were significantly associated with low lean mass as defined by the recently proposed European criteria [20]. These definition criteria are meant to be used in the elderly and have not been validated in COPD patients. On the other hand, it is important to emphasize the significant decline in skeletal muscle function and its negative effects in COPD patients. The fact that patients and controls were matched by BMI in our study might help explaining the lack of difference in low lean mass prevalence when FNIH criteria were used since the cutoffs are defined based on ALM corrected to BMI.
Some important limitations of the current study need to be pointed out. Patients in this cohort knowingly used systemic oral glucocorticoid during the exacerbating periods. Thus, we could not precisely control for cumulative use of glucocorticoids once this modality of treatment was used frequently without prescription. The very high rate of use of inhaled glucocorticoid (95% of the patients) makes it difficult to establish any differential effect of such medication on BMD and fracture risk. The cross-sectional nature of the study design precludes us from establishing a cause-effect relationship between disease-related parameters and bone and body composition variables and vice versa.
Conclusion
Our findings demonstrate a very high frequency of osteoporosis, fragility fractures, and low lean mass in patients with COPD. Disease severity was significantly correlated with low bone mass and body composition abnormalities. These results underscore the need for routine screening of osteoporosis and fragility fractures in patients with COPD. While it is well established that glucocorticoid increases the risk of fragility fractures, it remains to be proven whether reversal or improvement of pulmonary function through medication or rehabilitation might have a positive impact on bone and muscle outcomes in this clinical scenario.
References
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agusti A (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J 49:1700214
Schwab P, Dhamane AD, Hopson SD, Moretz C, Annavarapu S, Burslem K, Renda A, Kaila S (2017) Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis 12:735–744
Silva DR, Coelho AC, Dumke A, Valentini JD, de Nunes JN, Stefani CL, da Silva Mendes LF, Knorst MM (2011) Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care 56:961–968
Dam TT, Harrison S, Fink HA, Ramsdell J, Barrett-Connor E, Osteoporotic fractures in men research G (2010) Bone mineral density and fractures in older men with chronic obstructive pulmonary disease or asthma. Osteoporos Int 21:1341–1349
Pascual-Guardia S, Badenes-Bonet D, Martin-Ontiyuelo C, Zuccarino F, Marin-Corral J, Rodriguez A, Barreiro E, Gea J (2017) Hospital admissions and mortality in patients with COPD exacerbations and vertebral body compression fractures. Int J Chron Obstruct Pulmon Dis 12:1837–1845
Harrison RA, Siminoski K, Vethanayagam D, Majumdar SR (2007) Osteoporosis-related kyphosis and impairments in pulmonary function: a systematic review. J Bone and Mineral Res : Off J Am Soc Bone and Mineral Res 22:447–457
Graat-Verboom L, Spruit MA, van den Borne BE, Smeenk FW, Martens EJ, Lunde R, Wouters EF, Network C (2009) Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med 103:1143–1151
Okazaki R, Watanabe R, Inoue D (2016) Osteoporosis associated with chronic obstructive pulmonary disease. J Bone metab 23:111–120
NIH Consensus Development Panel on Osteoporosis Prevention D, Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA : J Am Med Assoc 285:785–795
Graat-Verboom L, van den Borne BE, Smeenk FW, Spruit MA, Wouters EF (2011) Osteoporosis in COPD outpatients based on bone mineral density and vertebral fractures. J Bone Miner Res 26:561–568
Sozen T, Ozisik L, Basaran NC (2017) An overview and management of osteoporosis. Eur J Rheumatol 4:46–56
Buist AS, Vollmer WM, McBurnie MA (2008) Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis 12:703–708
Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits LR, Yates JC, Vestbo J, Celli B (2009) Prevalence and progression of osteoporosis in patients with COPD: results from the TOwards a revolution in COPD health study. Chest 136:1456–1465
Briot K, Geusens P, Em Bultink I, Lems WF, Roux C (2017) Inflammatory diseases and bone fragility. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA
Pinheiro MM, Ciconelli RM, Martini LA, Ferraz MB (2009) Clinical risk factors for osteoporotic fractures in Brazilian women and men: the Brazilian osteoporosis study (BRAZOS). Osteoporos Int : J Established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 20:399–408
Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, Zemel BS, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Silverman S (2008) International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 43:1115–1121
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK (1995) Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporosis International : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 5:262–270
Sousa MD, Pinheiro MM, Szejnfeld VL, Castro CH (2013) Body composition parameters in healthy Brazilian women differ from White, Black, and Hispanic American women reference range. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TTL, Vassileva MT (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69:547–558
Vestbo J, Hurd SS, Agusti AG et al (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187:347–365
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone and Mineral Res : Off J Am Soc Bone and Mineral Res 8:1137–1148
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study group. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 4:368–381
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148
Baecke JA, Burema J, Frijters JE (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942
Eloi M, Horvath DV, Szejnfeld VL, Ortega JC, Rocha DA, Szejnfeld J, Castro CH (2016) Vitamin D deficiency and seasonal variation over the years in Sao Paulo, Brazil. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27:3449–3456
Eloi M, Horvath DV, Ortega JC, Prado MS, Andrade LE, Szejnfeld VL, de Moura Castro CH (2017) 25-Hydroxivitamin D serum concentration, not free and bioavailable vitamin D, is associated with disease activity in systemic lupus erythematosus patients. PLoS One 12:e0170323
Arantes HP, Gimeno SG, Chiang AY, Bilezikian JP, Lazaretti-Castro M (2016) Incidence of vertebral fractures in calcium and vitamin D-supplemented postmenopausal Brazilian women with osteopenia or osteoporosis: data from Arzoxifene generations trial. Arch Endocrinol Metab 60:54–59
Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G (2017) Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 28:1531–1542
Lopes JB, Danilevicius CF, Takayama L, Caparbo VF, Menezes PR, Scazufca M, Kuroishi ME, Pereira RM (2011) Prevalence and risk factors of radiographic vertebral fracture in Brazilian community-dwelling elderly. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 22:711–719
Fouda MA, Alhamad EH, Al-Hajjaj MS, Shaik SA, Alboukai AA, Al-Kassimi FA (2017) A study of chronic obstructive pulmonary disease-specific causes of osteoporosis with emphasis on the emphysema phenotype. Ann Thorac Med 12:101–106
Eisner MD, Blanc PD, Sidney S, Yelin EH, Lathon PV, Katz PP, Tolstykh I, Ackerson L, Iribarren C (2007) Body composition and functional limitation in COPD. Respir Res 8:7
Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, Bone IOF, Diabetes Working G (2016) Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol
Lorbergs AL, O'Connor GT, Zhou Y, Travison TG, Kiel DP, Cupples LA, Rosen H, Samelson EJ (2017) Severity of kyphosis and decline in lung function: the Framingham study. J Gerontol series Biol Sci Med Sci 72:689–694
Holick MF (2017) The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocrine Metab Disorders 18:153–165
Trochoutsou AI, Kloukina V, Samitas K, Xanthou G (2015) Vitamin-D in the immune system: genomic and non-genomic actions. Mini Rev Med Chem 15:953–963
Kokturk N, Baha A, Oh YM, Young Ju J, Jones PW (2016) Vitamin D deficiency: what does it mean for chronic obstructive pulmonary disease (COPD)? A comprehensive review for pulmonologists. Clin Respir J
Zhu M, Wang T, Wang C, Ji Y (2016) The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 11:2597–2607
Autier P, Boniol M, Pizot C, Mullie P (2014) Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2:76–89
Park JH, Park HK, Jung H, Lee SS, Koo HK (2015) Parathyroid hormone as a novel biomarker for chronic obstructive pulmonary disease: Korean National Health and nutrition examination survey. PLoS One 10:e0138482
Mesquita R, Wilke S, Smid DE, Janssen DJ, Franssen FM, Probst VS, Wouters EF, Muris JW, Pitta F, Spruit MA (2016) Measurement properties of the Timed Up & Go test in patients with COPD. Chron Respir Dis
Franco CB, Paz-Filho G, Gomes PE, Nascimento VB, Kulak CA, Boguszewski CL, Borba VZ (2009) Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int 20:1881–1887
Camiciottoli G, Bigazzi F, Magni C, Bonti V, Diciotti S, Bartolucci M, Mascalchi M, Pistolesi M (2016) Prevalence of comorbidities according to predominant phenotype and severity of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 11:2229–2236
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
Supplemental Fig. 1
(DOCX 244 kb)
Rights and permissions
About this article
Cite this article
Graumam, R.Q., Pinheiro, M.M., Nery, L.E. et al. Increased rate of osteoporosis, low lean mass, and fragility fractures in COPD patients: association with disease severity. Osteoporos Int 29, 1457–1468 (2018). https://doi.org/10.1007/s00198-018-4483-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4483-z