Abstract
Summary
Fractures are common in individuals with COPD and occur at higher bone mass values than expected. COPD appears to be an important risk factor for bone fragility.
Introduction
Patients with chronic obstructive pulmonary disease (COPD) have an increased risk of osteoporosis and fractures, but screening and prophylactic measures to prevent both disorders are often neglected in this population. This case-control study assessed the prevalence of osteopenia, osteoporosis, and fractures in patients with COPD, and identified potential risk factors for fractures in this population.
Methods
Overall, 91 patients with COPD (COPD group; COPDG) and 81 age- and sex-matched controls (control group; CG) were assessed with bone mineral density (BMD), thoracic/lumbar spine radiographs, and serum PTH and 25-hydroxyvitamin D (25[OH]D) levels. The occurrence of prior fractures was retrieved from clinical history.
Results
The prevalence of total fractures in the COPDG was 57.1% (odds of fracture 4.7 times greater compared with the CG), and the femoral neck T-score emerged as the best predictor of fractures. Compared with the CG, the COPDG had lower spine and femoral BMD (p ≤ 0.01) and 25(OH)D levels (p = 0.01) and 2.6 times greater odds of osteoporosis. Among men, vertebral fractures were more prevalent in the COPDG versus CG (25.9% vs. 6.5%, respectively, p = 0.01). The odds of fracture increased with femoral neck T-scores ≤ − 2.7 in the CG and ≤ − 0.6 in the COPDG.
Conclusion
These results add robust evidence to an increased odds of osteoporosis and fractures in COPD. Fractures in the COPDG occurred at higher BMD values than expected, suggesting that COPD may be an independent marker of fracture risk, reinforcing a need for regular osteoporosis screening with BMD measurement and prophylaxis of fractures in patients with this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is a universal condition with worldwide repercussion and the fourth current leading cause of death worldwide [1]. The chronic inflammatory process associated with the disease extends beyond the lungs and leads to cardiovascular diseases, psychiatric conditions, anaemia, and sarcopenia [2].
Osteoporosis has been gaining relevance as a COPD-associated comorbidity [3] since it increases the risk of fractures [4] and the morbidity and mortality associated with the disease. Osteoporosis is defined as a change in bone microarchitecture and/or decrease in bone mass, resulting in an increased risk of fractures. Bone densitometry (dual-energy x-ray absorptiometry, DXA) is currently used to classify the risk of fracture. According to the T-score value (the number of standard deviations (SDs) of the comparison of the subject’s bone mineral density [BMD] value with the mean BMD value of a young adult population of the same sex), the subject is classified as normal (T-score ≥ − 1.0) or as having osteopenia (T-score < − 1.0 and > − 2.5) or osteoporosis (T-score ≤ − 2.5).
COPD is associated with a higher occurrence of fractures. Data show that vertebral fractures compromise lung function and are a common complication of the disease, with prevalence rates between 24 and 79% [5]. Also, mortality after hip fracture is increased in subjects with COPD when compared with individuals without the disease [6].
Despite having a recognized role in morbidity and mortality associated with COPD, osteoporosis is still neglected during the follow-up of patients affected with this pulmonary condition [7, 8]. Based on these considerations, we conducted this study to assess the prevalence of osteoporosis, osteopenia, and fractures in a population of patients with COPD, and to identify potential risk factors for fractures in this cohort.
Material and methods
Study subjects
This study was approved by the Research Ethics Committee of the Federal University of São Paulo (CEP [REC] 1738/10) and was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
The COPD group (COPDG) comprised subjects undergoing regular follow-up at the Pulmonary Rehabilitation Centre of the EPM/UNIFESP (Escola Paulista de Medicina/Universidade Federal de São Paulo), who were invited to participate in the study. The group included patients of both sexes and older than 40 years of age who were diagnosed with stable COPD and had not used oral glucocorticoids in the previous 3 months. Based on spirometry results, the COPDG was further divided into two subgroups (GOLD I/II and GOLD III/IV) according to disease severity [1].
The control group (CG) comprised volunteers recruited in the university (staff or patients’ companions) and subjects from the community who were non-smokers or were former smokers for more than 1 year, without previous pulmonary disease and with a normal spirometry test.
We excluded from both groups those subjects using drugs that could affect bone mass (with the exception of calcium and vitamin D) or with a history of chronic diseases, active cancer, renal insufficiency, primary hyperparathyroidism, and hyperthyroidism, as well as individuals unable to complete the questionnaire or to undergo the pulmonary function test.
Of 199 recruited subjects, 27 were excluded for not meeting the inclusion criteria or for meeting the exclusion criteria (10 participants in the COPDG and 17 in the CG). The final cohort comprised 91 individuals in the COPDG (49 GOLD I/II and 42 GOLD III/IV) and 81 age- and sex-matched individuals in the CG (Fig. 1).
Study design
This was a cross-sectional study conducted to assess the prevalence of osteoporosis, osteopenia, and fractures in a population of patients with COPD, and identify potential risk factors for fractures in this cohort. Data collection and assessments were performed between August 2012 and December 2014.
All subjects answered a questionnaire about personal medical history (current and previous illnesses), alcoholism and smoking habits (quantified in pack-years), regular medications in use, number of times using oral glucocorticoids in the previous year, fractures in the previous 5 years resulting or not from falls (except fractures of the skull, hands, and feet), and physical activity (at least twice a week). Blood samples were collected in the morning after at least 8 h of fasting for measurement of levels of parathyroid hormone (PTH), 25-hydroxyvitamin D (25[OH]D), C-terminal telopeptide of type I collagen (CTX), procollagen type 1 N-terminal propeptide (P1NP), osteocalcin, total calcium, creatinine, and thyroid-stimulating hormone (TSH). The samples were processed and frozen at − 70 °C for further laboratory testing.
Following anthropometric assessments (weight, height, and body mass index [BMI]), all subjects underwent lumbar spine, femur, and whole-body DXA.
Methods
Laboratory analyses
PTH was measured by immunoelectrochemical assay (Roche Elecsys 2010, Roche, Roche Diagnostic, Indianapolis, IN, USA). Serum 25(OH)D was measured by chemiluminescence assay (Roche Elecsys 2010, intra-assay variation 4.1%, interassay variations 17.5% and 17%). Serum levels of bone formation (P1NP) and resorption (CTX) markers were assessed by chemiluminescence (Roche Elecsys 2010). Serum total calcium, creatinine, and TSH were measured with standard automatic assays and by colorimetric, colorimetric kinetic, and electrochemiluminometric methods (Cobas 6000; Roche Diagnostics, Indianapolis, IN, USA), respectively.
Bone densitometry
All bone densitometry (DXA) measurements were performed by the same operator at the Endocrinology Centre of UNIFESP/EPM on a Lunar-GE device (model NT GE Medical Systems, Madison, WI, USA). Positioning and image acquisition were carried out according to the International Society for Clinical Densitometry (ISCD) protocol [9]. BMD values (g/cm2) and their respective T-scores were obtained from the lumbar spine (L1–L4), total hip, and femoral neck for a diagnostic classification according to the criteria by the World Health Organisation (WHO) [10] and the ISCD.
In terms of body composition, we assessed the patients’ appendicular lean mass (ALM, lean mass of arms and legs), appendicular lean mass index or Baumgartner index (ALM/height2), and fat mass index (FMI; total fat mass/height2) [11].
Spirometry
The subjects were instructed to avoid caffeinated products and bronchodilators 12 h before the test. All participants underwent spirometry testing with qualified professionals using the Easy One spirometer (ndd Medical Technologies, Zurich, Switzerland) according to the 1994 guidelines of the American Thoracic Society [12] for measurement of the forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1), and calculation of the FEV1/FVC ratio. Three consecutive measurements were performed, followed by a fourth measurement after bronchodilation with salbutamol 400 μg.
Fracture assessment
-
A)
Clinical evaluation: all patients completed a questionnaire assessing the occurrence of fractures resulting or not from falls in the previous 5 years. All reported fractures were considered in the analysis, except for those in the skull, hands, and feet, and fractures due to large traumas (high-impact fractures).
-
B)
Spinal radiographs: 162 subjects (85 COPDG and 77 CG) underwent posteroanterior and lateral thoracic and lumbar spine radiographs for detection of thoracic and/or lumbar spinal fractures. Only fractures grade II and III according to Genant’s semiquantitative assessment were considered [13]. All radiographs were read by the same radiologist.
The term “total fractures” was used to refer to the number of fractures reported by the patient plus the number of radiographic spine fractures. Each individual was considered only once in the analysis of fractures.
Statistical analysis
Statistical analyses were performed with the R Core Team program (2016) [14]. Variables are represented as mean and SD values. The Kolmogorov-Smirnov test was used to evaluate normality. Student’s t test was applied to compare bone markers, age, BMI, pack-years, BMD, fat mass, and lean mass indexes between the COPDG and CG. These same variables in the GOLD I/II and GOLD III/IV COPDG subgroups were compared with ANOVA, paired Student’s t test, and Bonferroni correction to detect differences when ANOVA was significant. These tests were also used to compare body composition in the three groups.
Fisher’s exact test was used to evaluate qualitative variables in the COPDG and CG and identify differences between the subgroups of men and women in relation to the prevalence of fracture. This test was also used to analyze comorbidities between the groups. Univariate models evaluated the correlation between all variables and the occurrence of osteoporosis, osteopenia, and fractures, and the correlations with p values < 0.05 were explored further on a multivariate analysis. Subsequently, multiple regression models, based on the backward and forward stepwise methods, with tests for the input and output of each variable, were performed as follows: (1) multiple normal models to assess the association between BMD at the three sites analysed and other variables of the database and for BMD and COPD grades (groups GOLDI/II and GOLD II/IV); (2) multinomial regression model to assess in the COPDG the odds ratio (OR) of osteopenia and osteoporosis in relation to normal subjects; (3) logistic regression model to assess the odds of fracture in the study population, resulting in the calculation of the OR for fractures in COPDG compared with the CG. The same risk was also calculated for patients with GOLD I/II and III/IV using the multiple logistic model.
Univariate logistic regression models were adjusted for the association between the presence of radiographic fractures in men in the COPDG and in all men. Breslow-Day test assessed the homogeneity between the OR of fractures in the COPDG and CG for the categories “normal”, “osteopenia”, and “osteoporosis”. Additionally, a ROC curve analysis was performed to estimate the OR of fracture in GOLD I/II and III/IV patients and controls and calculate the T-score cut-off point, increasing the chance of a correct classification of fractured subjects. With that, we established T-score values from which the OR of fracture would be higher for the group. P ≤ 0.05 was considered significant.
Results
Table 1 presents a comparison of general and demographic characteristics, medical history, lifestyle habits, physical activity and body composition data, and laboratory results of the subjects in the COPDG and CG. Men predominated in both groups (60.4% and 58.0% in the COPDG and CG, respectively), but the distribution of participants by sex and age was comparable in both groups (Table 1). Practice of regular physical activity (at least twice a week) and alcohol consumption was comparable between groups, but smoking and prior smoking were significantly more frequent in the COPDG. Both groups had comparable distributions of the comorbidities hypertension, diabetes mellitus (DM), dyslipidemia, and coronary disease, as well as continuous use of medications (diuretics, insulin, and oral antidiabetic agents) (Table 1). When compared with subjects in the CG, those in the COPDG presented lower mean weight and BMI values, as well as differences in body composition, indicated by lower ALM, FMI, and Baumgartner index values (Table 1).
Serum levels of total calcium and TSH were comparable in both groups (Table 1). The mean creatinine value was significantly higher in the COPDG compared with the CG, but all participants had a normal renal function (Table 1). Evaluation of serum bone metabolic parameters revealed higher PTH levels and lower 25(OH)D and CTX levels in the COPDG compared with the CG. Table 2 shows the variables that correlated significantly with osteopenia, osteoporosis, and fractures in the univariate model.
Mean BMD values were lower in the COPDG compared with the CG at all sites evaluated (Table 3). Within the COPDG, the mean BMD values were significantly higher in GOLD I/II versus GOLD III/IV subjects at all sites: L1–L4 (1.098 ± 0.200 g/cm2 vs. 0.988 ± 0.180 g/cm2, respectively, p < 0.01), femoral neck (0.889 ± 0.130 g/cm2 vs. 0.830 ± 0.130 g/cm2, respectively, p = 0.03), and total hip (0.942 ± 0.140 g/cm2 vs. 0.883 ± 0.130 g/cm2, respectively, p = 0.04).
Rates of osteopenia and osteoporosis were significantly higher in the COPDG than the CG (Table 3). Individuals in the COPDG were 2.4 times and 2.6 times more likely to present osteopenia and osteoporosis, respectively, than those in the CG according to the following adjusted multinomial regression model equations:
*CG = 0 and COPDG = 1
(p < 0.05 for the variables group, age, and weight)
Fractures due to falls within the previous 5 years were more frequent in the COPDG compared with the CG (Table 3). Subjects with more severe pulmonary disease also had a higher percentage of falls with fractures (GOLD I/II group 26.5%, GOLD III/IV group 47.6%, and CG 7.4%, p < 0.01).
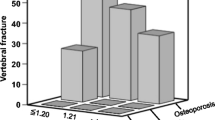
The percentage of subjects with vertebral radiographic fractures was not significantly different between the groups (Table 3). However, when analyzed by sex, men with COPD had about 4 times more radiographic fractures than men in the CG (p = 0.01). A similar analysis including only women showed no significant differences.
Individuals in the COPDG had a higher prevalence of total fractures (reported and observed) than those in the CG.
In subjects in the COPDG, total fractures were more prevalent among those with more severe lung disease (CG: 17.2%, GOLD I/II: 51%, and GOLD III/IV: 64.2%, p < 0.01). This difference remained significant even when the groups were analyzed by sex (Table 3).
Bone densitometry data and body composition parameters were also different in men in the COPDG versus men in the CG (Table 4). These differences were not significant among women (data not shown). When we compared men with and without radiographic fractures in both groups (COPDG and CG), those with fractures were older (72.2 ± 7.4 vs. 65.3 ± 9.3 years, p = 0.008) and had a greater smoking burden (96.6 ± 31.4 pack-years vs. 41.5 ± 26.8 pack-years, p < 0.01) in relation to those without radiographic fractures. Men with fractures compared with those without fractures had lower mean BMD values at the femoral neck (0.828 ± 0.126 vs. 0.942 ± 0.166 g/cm2, respectively, p = 0.008) and total hip (0.900 ± 0.137 g/cm2 vs. 1.013 ± 0.172 g/cm2, respectively, p = 0.01). When only men with COPD were analyzed, those with radiographic fractures, compared with those without fractures, were also older (72.5 ± 6.03 vs. 66.2 ± 9.72 years, respectively, p = 0.03) and had a greater smoking burden (96.6 ± 31.4 vs. 42.1 ± 27.6 pack-years, p < 0.01), but the BMD values did not differ between individuals with or without fractures: L1–L4 (1.048 ± 0.190 vs. 1.093 ± 0.190, respectively, p = 0.649), femoral neck (0.823 ± 0.140 vs. 0.884 ± 0.13, respectively, p = 0.146), and total hip (0.894 ± 0.150 vs. 0.941 ± 0.130, respectively, p = 0.343).
Considering total fractures (reported and radiographic), the odds of fracture when both groups were considered according to the following adjusted multinomial regression model equation:
*CG = 0 and COPDG = 1
(p < 0.05 for the variables group and femoral neck T-score)
Based on this equation, each 1 SD reduction in femoral neck T-score increased the odds of fractures by 2.07 times. However, the OR of fracture was 4.7 times greater in the COPDG compared with the CG. For example, in subjects with a T-score value of − 3.0, the ORs of fracture were 4.17 in the COPDG and 0.87 in the CG. In addition, the odds of fracture increased exponentially with a T-score < − 0.6 in the COPDG, while in the CG, this occurred at the − 2.7 SD threshold (Fig. 2). In the assessment according to the severity of the disease, the odds of fracture were 4.3 and 5.4 times higher in GOLD I/II and GOLD III/IV patients, respectively, when compared with CG subjects. The thresholds for an exponential increase in the OR of fractures according to femoral neck T-score were − 0.6 and − 0.3 for GOLD I/II and III/IV, respectively.
Subjects in the COPDG had significantly higher odds of fracture than those in the CG across all DXA categories (Table 5). In the categories “normal”, “osteopenia”, and “osteoporosis”, individuals in the COPDG presented 5.3, 5.7, and 4.09 times greater ORs, respectively, than CG subjects in the same categories. There were no differences among the ORs of the three categories (OR 0.93; Breslow-Day test) (Table5).
Discussion
In this study, subjects with COPD, compared with controls, had a lower BMD and 2.6 times increased odds of osteoporosis. They also had nearly 5 times increased odds of bone fractures than matched CG individuals.
Men with COPD had more radiographically documented vertebral fractures than control men, and more falls resulting in fractures. This finding is clinically relevant, considering that vertebral fractures may further reduce lung capacity, resulting in a greater limitation and worsening of the respiratory condition [15]. Hip fractures in patients with COPD are associated with increased mortality in men when compared with women [16]. Of note, screening of osteoporosis in men is usually not performed routinely. Morden et al. found that a male population with COPD performed fewer densitometry tests and received less treatment for osteoporosis, despite a high rate of fractures [7]. Considering that COPD is more prevalent in men [17], assessment of fracture risk in this subgroup becomes even more relevant. Adding to this evidence, our results suggest that men with COPD should be better evaluated for bone fragility.
BMD is a DXA parameter that estimates the risk of fracture with high specificity according to the T-score threshold adopted as a cut-off value. The WHO has defined T-scores ≤ − 2.5 as diagnostic of osteoporosis. This threshold was valid for our control population, in which the cut-off value for increased odds of fracture was − 2.7, but in the COPDG, increased odds of fractures were defined at a femoral neck T-score below − 0.6. This occurred in 62.9% of all the subjects classified as osteoporotic by DXA and in a similar proportion (63%) of those with osteopenia, suggesting that the interpretation of DXA in this population should be done carefully. Our results demonstrate that BMD is unable to fully capture the determinants of bone strength in COPD, and that the T-score should not be solely interpreted according to the WHO classification for the purpose of evaluating the risk of fracture in this population. Our study showed that for the same T-score, individuals in the COPDG had much higher odds of fracture (4.7 times) compared to those in the CG (Fig. 1). Parameters other than BMD should also be considered. For example, assessment with thoracic and lumbar spine radiographs could improve the detection of vertebral fractures, even in asymptomatic individuals.
In evaluating a patient’s absolute risk of fracture, consideration of BMD-unrelated factors, some that in fact impairs the structural bone strength such as age, smoking, and associated diseases [18,19,20], can yield greater accuracy. Among tools developed to assess the risk of fracture, the most popular is FRAX [21]. Although FRAX has a good accuracy [22] and has been validated in several studies, in cases of secondary osteoporosis, FRAX may underestimate the importance of specific conditions, like diabetes [23]. Additionally, the value of risk assessment tools for the male population, in particular, is lacking [24].
The GLOW study showed that the addition of several comorbidities to FRAX, including COPD, increases the prediction of fractures [25]. Based on that, we conclude that COPD is an important risk factor and may be considered an independent risk factor for the occurrence of fractures. However, longitudinal studies are needed and should be incorporated into existing fracture prediction tools.
The pathophysiology of bone disease in COPD is still unclear. Studies with high-resolution computed microtomography and bone biopsy in women with COPD reveal not only decreased bone mass, but also inferior bone quality and dynamic parameters suggestive of low remodelling, reduced osteoblastic activity, and a higher prevalence of vertebral fractures [26]. Our findings support this evidence, as patients with COPD had a lower concentration of serum CTX, a marker of bone turnover. Chronic hypoxemia may be one of the causes of reduced bone mass in individuals with chronic respiratory failure [27].
Hypoxia-inducible factor 1α (HIF-1α), an important transcription factor in cellular response to hypoxia [28], is increased in COPD [29]. Chen et al. demonstrated that HIF-1α inhibits bone formation by suppressing the Wnt pathway [30]. Additionally, runt-related transcription factor 2 (RUNX2), an important modulator of bone formation, appears to have lower expression in this situation [31], while the production of mediators of inflammation that suppress bone formation, like IL-1beta [32], TNF-alpha [33], and IL-6 [34], seems to be increased. Some authors have observed an increased secretion of receptor activator of nuclear factor kappa-B ligand (RANKL) and a reduction of osteoprotegerin, suggesting that bone resorption may also be increased in COPD [35].
In the present study, the COPDG had higher PTH levels, possibly due to lower 25(OH)D concentrations. Several studies have shown individuals with pulmonary disease to be vitamin D deficient [36, 37]. This may be attributed to decreased sun exposure due to restricted mobility, as many studies have shown the vitamin deficiency to be related to greater pulmonary disease severity, lower ability to exercise, and reduced muscle strength [38, 39], in addition to increased muscle catabolism related to inflammation [36].
Aligned with the literature [39], our results of body composition assessment showed that individuals with COPD had lower BMI and fat mass compared with controls. Compared with participants in the CG, individuals in the COPDG presented lower lean mass values assessed by the Baumgartner index, a finding associated with worse outcomes, according to some authors [40]. However, none of the body composition parameters assessed in our study correlated with BMD or fractures.
One of the limitations of our study was the cross-sectional design, which allowed us to observe the prevalence but not the incidence of fractures, since our subjects were not treated for bone fragility. Other limitations included the consideration of reported fractures, since only vertebral fractures were documented by radiography, and also the absence of functional tests to document the sarcopenia (only body composition by DXA was performed). The fact that we were unable to match the groups according to anthropometric parameters other than age and sex, or to determine the ethnicity of our population during data collection, was also a limitation of the study.
Our study has several strengths, including the comparison of the results against controls matched by sex and age, which increases the power of associations and risks of events. The evaluation of bone remodelling markers allowed a potential understanding of the pathophysiology of bone changes in subjects with COPD. Other strengths included the analysis of thoracic and lumbar spine radiographs, which allowed documentation of the most frequent fractures in our cohort, and quantification of falls resulting in fractures.
Conclusions
This cross-sectional study with participants matched by sex and age revealed an increased prevalence of bone fragility and fractures in individuals with COPD, regardless of bone mass, as fractures occurred in individuals with either osteopenia or osteoporosis. We emphasize the importance of the assessment of fracture risk in individuals with COPD, since this pulmonary dysfunction could be considered a relevant risk factor for the occurrence of such events. Fractures have an important impact on the morbidity and mortality in this already frail population. Our findings also indicate the importance of COPD as an independent risk factor for the occurrence of these events in the general population.
References
Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/. Accessed April 11, 2019
Agusti A (2007) Systemic effects of chronic obstructive pulmonary disease: what we know and what we don’t know (but should). Proc Am Thorac Soc 4(7):522–525. https://doi.org/10.1513/pats.200701-004FM
Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, Zhang Y, Leader JK, Gur D, Greenspan SL, Sciurba FC (2011) Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med 183(7):885–890. https://doi.org/10.1164/rccm.201004-0666OC
Gazzotti MR, Roco CM, Pradella CO, Nascimento OA, Porto EF, Adas M, Lazaretti-Castro M, Jardim JR (2019) Frequency of osteoporosis and vertebral fractures in chronic obstructive pulmonary disease (COPD) patients. Arch Bronconeumol 55(5):252–257. https://doi.org/10.1016/j.arbres.2018.10.010
Okazaki R, Watanabe R, Inoue D (2016) Osteoporosis associated with chronic obstructive pulmonary disease. J Bone Metab 23(3):111–120. https://doi.org/10.11005/jbm.2016.23.3.111
de Luise C, Brimacombe M, Pedersen L, Sorensen HT (2008) Chronic obstructive pulmonary disease and mortality following hip fracture: a population-based cohort study. Eur J Epidemiol 23(2):115–122. https://doi.org/10.1007/s10654-007-9211-5
Morden NE, Sullivan SD, Bartle B, Lee TA (2011) Skeletal health in men with chronic lung disease: rates of testing, treatment, and fractures. Osteoporos Int 22(6):1855–1862. https://doi.org/10.1007/s00198-010-1423-y
Ferguson GT, Calverley PMA, Anderson JA, Jenkins CR, Jones PW, Willits LR, Yates JC, Vestbo J, Celli B (2009) Prevalence and progression of osteoporosis in patients with COPD: results from the towards a revolution in COPD health study. Chest 136(6):1456–1465. https://doi.org/10.1378/chest.08-3016
Densitometry TISfC Offi cial Positions (2015) ISCD Combined - Official Positions of the International Society for Clinical Densitometry. https://iscd.app.box.com/v/OP-ISCD-2015-Adult. Accessed May 16, 2019
NIH Consensus Development Panel on Osteoporosis Prevention D, Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285(6):785–795
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One 4(9):e7038. https://doi.org/10.1371/journal.pone.0007038
Standardization of Spirometry (1994) Update. American Thoracic Society (1995). Am J Respir Crit Care Med 152(3):1107–1136. https://doi.org/10.1164/ajrccm.152.3.7663792
Genant HK, Engelke K, Fuerst T, Gluer CC, Grampp S, Harris ST, Jergas M, Lang T, Lu Y, Majumdar S, Mathur A, Takada M (1996) Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 11(6):707–730. https://doi.org/10.1002/jbmr.5650110602
R: The R Project for Statistical Computing. https://www.R-project.org/. Accessed September 4, 2017
Biskobing DM (2002) COPD and osteoporosis. Chest 121(2):609–620. https://doi.org/10.1378/chest.121.2.609
Penrod JD, Litke A, Hawkes WG, Magaziner J, Doucette JT, Koval KJ, Silberzweig SB, Egol KA, Siu AL (2008) The association of race, gender, and comorbidity with mortality and function after hip fracture. J Gerontol A Biol Sci Med Sci 63(8):867–872. https://doi.org/10.1093/gerona/63.8.867
Rycroft CE, Heyes A, Lanza L, Becker K (2012) Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis 7:457–494. https://doi.org/10.2147/COPD.S32330
Civitelli R, Armamento-Villareal R, Napoli N (2009) Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int 20(6):843–851. https://doi.org/10.1007/s00198-009-0838-9
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML (2004) Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164(10):1108–1112. https://doi.org/10.1001/archinte.164.10.1108
Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B (2001) Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 12(12):989–995. https://doi.org/10.1007/s001980170006
McCloskey EV, Harvey NC, Johansson H, Kanis JA (2016) FRAX updates 2016. Curr Opin Rheumatol 28(4):433–441. https://doi.org/10.1097/BOR.0000000000000304
Marques A, Ferreira RJ, Santos E, Loza E, Carmona L, da Silva JA (2015) The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis 74(11):1958–1967. https://doi.org/10.1136/annrheumdis-2015-207907
Kurra S, Fink DA, Siris ES (2014) Osteoporosis-associated fracture and diabetes. Endocrinol Metab Clin N Am 43(1):233–243. https://doi.org/10.1016/j.ecl.2013.09.004
Nayak S, Edwards DL, Saleh AA, Greenspan SL (2014) Performance of risk assessment instruments for predicting osteoporotic fracture risk: a systematic review. Osteoporos Int 25(1):23–49. https://doi.org/10.1007/s00198-013-2504-5
Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD, Boonen S, Chapurlat R, Diez-Perez A, Anderson FA Jr, Hooven FH, LaCroix AZ, Lindsay R, Netelenbos JC, Pfeilschifter J, Rossini M, Roux C, Saag KG, Sambrook P, Silverman S, Watts NB, Greenspan SL, Premaor M, Cooper C, Investigators G (2012) Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 50(6):1288–1293. https://doi.org/10.1016/j.bone.2012.02.639
Kulak CA, Borba VC, Jorgetti V, Dos Reis LM, Liu XS, Kimmel DB, Kulak J Jr, Rabelo LM, Zhou H, Guo XE, Bilezikian JP, Boguszewski CL, Dempster DW (2010) Skeletal microstructural abnormalities in postmenopausal women with chronic obstructive pulmonary disease. J Bone Miner Res 25(9):1931–1940. https://doi.org/10.1002/jbmr.88
Fujimoto H, Fujimoto K, Ueda A, Ohata M (1999) Hypoxemia is a risk factor for bone mass loss. J Bone Miner Metab 17(3):211–216
Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, Schipani E, Clemens TL (2010) Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci 1192:322–326. https://doi.org/10.1111/j.1749-6632.2009.05238.x
Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, Parlow JL, Archer SL (2017) Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest 151(1):181–192. https://doi.org/10.1016/j.chest.2016.09.001
Chen D, Li Y, Zhou Z, Wu C, Xing Y, Zou X, Tian W, Zhang C (2013) HIF-1alpha inhibits Wnt signaling pathway by activating Sost expression in osteoblasts. PLoS One 8(6):e65940. https://doi.org/10.1371/journal.pone.0065940
Park JH, Park BH, Kim HK, Park TS, Baek HS (2002) Hypoxia decreases Runx2/Cbfa1 expression in human osteoblast-like cells. Mol Cell Endocrinol 192(1–2):197–203
Sapey E, Ahmad A, Bayley D, Newbold P, Snell N, Rugman P, Stockley RA (2009) Imbalances between interleukin-1 and tumor necrosis factor agonists and antagonists in stable COPD. J Clin Immunol 29(4):508–516. https://doi.org/10.1007/s10875-009-9286-8
Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H (2000) The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161(4 Pt 1):1179–1184. https://doi.org/10.1164/ajrccm.161.4.9903022
Garcia-Rio F, Miravitlles M, Soriano JB, Munoz L, Duran-Tauleria E, Sanchez G, Sobradillo V, Ancochea J, Committee E-SS (2010) Systemic inflammation in chronic obstructive pulmonary disease: a population-based study. Respir Res 11:63. https://doi.org/10.1186/1465-9921-11-63
Bai P, Sun Y, Jin J, Hou J, Li R, Zhang Q, Wang Y (2011) Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir Res 12:157. https://doi.org/10.1186/1465-9921-12-157
Mathyssen C, Gayan-Ramirez G, Bouillon R, Janssens W (2017) Vitamin D supplementation in respiratory diseases: evidence from randomized controlled trials. Pol Arch Intern Med 127(11):775–784. https://doi.org/10.20452/pamw.4134
Kokturk N, Baha A, Oh YM, Young Ju J, Jones PW (2018) Vitamin D deficiency: what does it mean for chronic obstructive pulmonary disease (COPD)? A compherensive review for pulmonologists. Clin Respir J 12(2):382–397. https://doi.org/10.1111/crj.12588
Nolasco R, Moreira LDF, Bocalin DS, Fronza FCAO, Marin RV, Lazaretti-Castro M (2017) Effects of vitamin D supplementation on pulmonary function in postmenopausal women following an aquatic exercise program. Arch Endocrinol Metab 2017:61/1. https://doi.org/10.1590/2359-3997000000211
Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Zhou Y, Liu S, Wang X, Wang D, Lu J, Zheng J, Ran P (2007) Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 176(8):753–760. https://doi.org/10.1164/rccm.200612-1749OC
Costa TM, Costa FM, Moreira CA, Rabelo LM, Boguszewski CL, Borba VZ (2015) Sarcopenia in COPD: relationship with COPD severity and prognosis. J Bras Pneumol 41(5):415–421. https://doi.org/10.1590/S1806-37132015000000040
Acknowledgements
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support of the authors M.G.A.O. and M.R.G. and the laboratory tests, and to Roche Diagnostica Brasil for providing the kits for the measurement of bone metabolic markers. We also thank Milena Braga-Basaria (Voxmed Medical Communications) for critically reviewing and suggesting improvements to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adas-Okuma, M., Maeda, S., Gazzotti, M. et al. COPD as an independent risk factor for osteoporosis and fractures. Osteoporos Int 31, 687–697 (2020). https://doi.org/10.1007/s00198-019-05235-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05235-9