Abstract
Summary
We did a cross-sectional analysis of chronic pulmonary obstructive disease (COPD) patients without chronic use of systemic glucocorticoids (CUG). Osteoporosis was found in 51% and bone mineral density (BMD) was correlated with severity of disease. Low levels of vitamin D were found in 94%. All COPD patients may benefit from vitamin D supplementation and screening for low BMD.
Introduction
Patients with chronic pulmonary obstructive disease have low bone mineral density, caused by chronic use of systemic glucocorticoids and hypovitaminosis D. However, patients without CUG may also have low BMD.
Methods
We performed a cross-sectional analysis in 49 patients (21 men, 28 postmenopausal women), with COPD without CUG, from Brazil (25° 25' S). Several markers of bone metabolism were measured, plus BMD. Osteoporosis risk factors and history of fractures were investigated. Respiratory function was assessed by venous gasometry, spirometry, and oximetry. BMD results were compared to those of 40 healthy non-smokers controls.
Results
COPD patients had lower BMD at all sites (p < 0.01). Osteoporosis was observed in 51%. BMD independently correlated with stage of disease (lumbar spine, R = 0.38, p = 0.01; total femur, R = 0.36, p = 0.01; femoral neck, R = 0.40, p < 0.01). Ninety-four percent had low levels of vitamin D (<30 ng/mL) and 67% had secondary hyperparathyroidism. Vitamin D was correlated with oxygen saturation (R = 0.36, p = 0.01), with lower levels in those with saturation <88% (p = 0.01).
Conclusion
Patients with COPD without CUG have increased risk for osteoporosis. Such patients have hypovitaminosis D, which is correlated with the severity of disease. Screening for low BMD and vitamin D supplementation may be warranted to all COPD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic pulmonary obstructive disease (COPD) is a complex disease that affects not only respiratory function, but is also accompanied by other comorbidities, such as decreased physical activity, right ventricular heart failure, and decreased quality of life.
Osteoporosis is a common finding in patients with COPD, especially in those with advanced disease [1] with high risk of fractures [2]. The pathophysiology of osteoporosis in patients with COPD is not well established, and it has been suggested that excess tobacco consumption, malnutrition, vitamin D deficiency, hypogonadism, and inactivity may play a role [3]. In addition, chronic use of systemic and inhaled glucocorticoids also contributes to increase the risk of bone loss [4, 5] and their use in the treatment of COPD is controversial. Currently, systemic glucocorticoids are limited to the most severely affected patients with tendency to exacerbations.
In patients with COPD, prevalence of osteoporosis can be as high as 60%, which increases with the progression of the pulmonary disease [5, 6]. At least one vertebral fracture can be found in 4% to 63% of the patients with COPD, which leads to impairment of respiratory function. It is estimated that each vertebrae that is fractured determines a decrease by 9% of the predicted forced vital capacity (FVC). Even though the prevalence of vertebral fractures in patients with COPD is high, there is discordance in the literature about the need for BMD evaluation in these patients. Many studies suggest that only high-risk patients for osteoporosis should be evaluated, such as the ones in use of glucocorticoids, postmenopausal or amenorrheic women, hypogonadic and patients with past history of fractures or body mass index (BMI) below 22 kg/m2 [1, 7, 9]. Others, however, suggest that all patients with advanced forms of COPD should be screened for osteoporosis [3].
Data in the literature on BMD and fracture risk in patients with COPD is scarce [1, 9]. The aim of this study was to evaluate the prevalence of low bone mass in a group of patients with COPD without chronic use of systemic glucocorticoids. In addition, we investigated other factors that could affect the bone mass, such as low levels of vitamin D.
Materials and methods
Patients
The study protocol was approved by the HC-UFPR ethics committee. Informed written consents were obtained from all subjects.
We consecutively recruited 49 patients (21 males, 28 females, mean age 65.4 ± 9.2 years) from the Pulmonary Outpatient Clinic of the Hospital de Clínicas da Universidade Federal do Paraná (HC-UFPR), in Curitiba, Brazil (25° 25' S). Recruitment was undertaken between March and September 2004. We included only men and postmenopausal women who fulfilled the COPD diagnostic criteria according to the Global Strategy for the Diagnosis, Management, and Prevention of COPD (GOLD) [10]. Exclusion criteria were: (1) prolonged immobilization within the past 6 months, (2) presence of comorbidities or use of drugs that cause interference on bone metabolism, and (3) use of oral or intravenous glucocorticoids for three or more consecutive months, in an equivalent dose of ≥ 5 mg per day of prednisone, or in a cumulative dose of prednisolone >1,000 mg [11].
Methods
This is a cross-sectional study. Participants were evaluated during a single visit that included a medical interview, physical examination, laboratory testing, BMD measurement, and spirometry. Medical history was obtained through a questionnaire applied by a single investigator, or from the patients' charts. Data obtained included age, race, BMI, menstrual status, frequency of physical activity, drinking, smoking and dietary habits, age at diagnosis of COPD, duration of disease (years), history of fractures, and use of medications (past and present). Patients who engaged three or more hours of physical activity per week were considered physically active. Patterns of alcohol consumption were evaluated according to the National Council on Alcohol Abuse and Alcoholism, and categorized into past or present drinking, and mild, moderate, or heavy drinking [12]. Present or past smoking history was determined, as well as smoking intensity, expressed in number of cigarette packs per day per year of smoking. Patients that had stopped smoking less than 6 months prior to the evaluation were considered to have a present smoking history. In case of pipe smoking or use of hand-rolled cigarettes, the following equivalency was considered: one puff on a pipe was equal to one hand-rolled cigarette or to two cigarettes [13]. Calcium intake was estimated by the amount of dairy products ingested daily [14].
In order to quantify the use of oral or intravenous glucocorticoids, we recorded the duration and number of cycles, and converted the cumulative doses into their respective equivalent doses of prednisolone. Use of inhalatory glucocorticoids was categorized by potency as high (>800 mcg/day of beclometasone, >400 mcg/day of budesonide, >500 mcg/day of fluticasone, and >1,200 mcg/day of triamcinolone or flunisolide), moderate (400–800 mcg/day of beclometasone, 200–400 mcg/day of budesonide, 200–500 mcg/day of fluticasone, 800–1,200 mcg/day of triancinolone or flunisolide) or low dose (100–400 mcg/day of beclometasone, 100–200 mcg/day of budesonide or fluticasone, and 400–800 mcg/day of triamcinolone or flunisolide) [15].
Fasting blood was drawn in spring of 2005. Urine was collected during 24 h, starting the day before the blood draw. We measured total serum calcium, phosphorus, alkaline phosphatase, and albumin by standard kits, which were analyzed in automated spectrophotometric equipment (ADVIA1650, Bayer, Leverkusen, Germany). Twenty-four-hour urine calcium was measured by the same methods. Venous gasometric parameters were assessed by standard gasometry (Blood gas analyzer pH348, Bayer, Leverkusen, Germany). In addition, levels of intact parathyroid hormone (iPTH) and total testosterone (only in men) were determined by chemiluminescence (DPC, Immulite 2000, Los Angeles, CA, USA). Levels of 25-hydroxi vitamin D (25OHD) were determined by radioimmunoassay (I125 DiaSorin Stillwater, Minnesota, USA). A level of 25OHD equal or greater than 30 ng/mL (75 nmol/L) was considered to indicate sufficient vitamin D. Patients with levels of 25OHD between 21 to 29 ng/mL (52 to 72 nmol/L) were considered vitamin D-insufficient. Those with 25OHD levels <20 ng/mL (50 nmol/L) were considered vitamin D-deficient [16].
Bone mineral density was measured at the lumbar spine, femoral neck, and total femur by dual energy X-ray absorptiometry (DXA) using a Hologic 1000 densitometer (Hologic, Bedford, MA, USA). BMD was classified as normal, low BMD, or osteoporosis according to the International Society for Clinical Densitometry [17]. Results were compared to a non-smoker group of 40 healthy individuals matched by age, gender, ethnicity, and BMI, without previous history of pulmonary diseases. All COPD patients were submitted to a pulmonary function test. Spirometry was undertaken with a Survey Plus spirometer (Collins Medical, Louisville, KY, USA), using the Knudson 76 protocol [18]. For this study, we considered the results of the forced expiratory volume in 1 s (FEV1), the forced vital capacity (FVC), and the FEV1/FVC ratio. Patients with FEV1/FVC < 0.7 are diagnosed with COPD [10]. The results were compared to the parameters published by the American Thoracic Society [19]. Arterial oxygen saturation (O2Sat) was determined by a non-invasive device (Oxypleth Oximeter, Novametrix Medical System, Wallingford, USA).
Statistics
Data was analyzed using the software SPSS 13.0® for Windows (SPSS, Chicago, IL, USA). Results were expressed as mean ± SD or median (range). Groups were compared by the Student t-test or by non-parametric tests. One-way analysis of variance (ANOVA) or the chi-square test was used when appropriate. Pearson and Spearman coefficients were used for correlation analysis. Two-sided tests were used with p < 0.05 being considered significant. Multiple linear regression analysis was undertaken in order to assess the relationship between BMD and other variables.
Results
Medical history and spirometry
Medical history and spirometric parameters are illustrated in Table 1. Patients were divided into four groups, according to the results of the spirometry [10]: stage I—mild COPD (n = 14, 28.6%; six males and eight females); stage II—moderate COPD (n = 21, 43.0%; eight males and 13 females); and stage III—severe COPD (n = 11, 22.4%; six males and five females); and stage IV—very severe COPD (n = 3, 6%; one male and two females).
Twenty-five patients (51%) had past or present history of use of inhalatory glucocorticoids (stage I—six patients, stage II—eight patients, stage III—eight patients, stage IV—three patients). Nine patients had used high doses of inhalatory glucocorticoids for a mean of 1.4 ± 1.2 years, 13 had used a moderate dose for a median of 0.4 year (0.08 to 5 years), and three had used a low dose for a median of 0.3 year (0.08 to 1.5 years). None of the patients had chronic use of systemic glucocorticoids and 13 patients (26.5%) had used systemic glucocorticoids only during an acute exacerbation, with a lifetime cumulative dose of 398.3 ± 265.4 mg of prednisolone.
Laboratory assays and BMD
The laboratory results are shown in Table 1. Secondary hyperparathyroidism was observed in 33 patients (67%). There was an inverse correlation between serum calcium and iPTH (R = −0.35, p = 0.01). Mean levels of 25OHD were 20.8 ± 0.9 ng/mL. Only three patients (6.1%) had normal levels of 25OHD (≥30 ng/mL), 59.2% (n = 29) were vitamin D-insufficient, and 34.7% (n = 17) were vitamin D-deficient. The mean O2Sat was 93%. We found a positive correlation between O2Sat and levels of 25OHD (R = 0.36, p = 0.01). Patients with O2Sat ≥ 88% had higher mean levels of 25OHD, when compared with patients with O2Sat < 88% (22.1 ± 5.1 ng/mL vs. 14.5 ± 7.1 ng/mL, p = 0.01), as seen in Fig. 1. All patients had normal levels of phosphorus, alkaline phosphatase, pH, and bicarbonate. All men had normal levels of total testosterone.
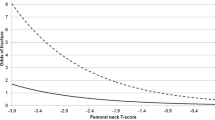
Normal BMD was observed in six patients (12.2%; five males and one female). Low BMD was seen in 43 participants (87.7%; 16 males and 27 females). Osteoporosis was diagnosed in 25 patients (51.0%; five males and 20 females). To evaluate the effects of systemic and inhalatory glucocorticoids on the bone, patients were analyzed separately in three groups: patients who had never used systemic glucocorticoids (all participants), patients who had never used inhalatory glucocorticoids, and patients who had past or present history of use of glucocorticoids. We observed no differences regarding BMD at the lumbar spine (p = 0.987), femoral neck (p = 0.865), and total femur (p = 0.848). Moreover, no differences were observed regarding T-scores at the lumbar spine (p = 0.974), femoral neck (p = 0.927), and total femur (p = 0.872). The use of inhalatory glucocorticoids in high doses and/or for more than 2.5 years also determined no differences in BMD and in T-scores, as shown by the comparison of the results of that subgroup, with those of patients who had never used inhalatory glucocorticoids (p = 0.471, 0.339, and 0.625, for lumbar, femoral neck, and total femur BMDs, respectively; p = 0.381, 0.313, and 0.179, for lumbar, femoral neck, and total femur T-scores, respectively). The absolute bone mineral densities are seen in Table 2. The groups of patients with more severe forms of COPD presented higher prevalence of osteoporosis, as shown in Fig. 2. We found inverse correlations between FEV1 and BMD at all sites (lumbar spine R = 0.38, p < 0.01; femoral neck R = 0.40, p < 0.01; total femur R = 0.36, p = 0.01), which was maintained after adjustments for weight and age (Fig. 3).
The following variables were considered in the multiple regression analysis for their relationship to the BMD: weight, levels of 25OHD, use of inhalatory glucocorticoids, present smoking history, and FEV1. Statistically significant correlations were seen only for body weight (lumbar spine, B = 0.05, R 2 = 0.402, p < 0.01; femoral neck, B = 0.04, R 2 = 0.395, p < 0.01; total femur, B = 0.05, R 2 = 0.407, R = 0.36, p < 0.01) and VEF1 (lumbar spine, B = 0.06, R 2 = 0.02, p < 0.01; femoral neck, B = 0.07, R 2 = 0.39, p < 0.01; total femur, B = 0.07, R 2 = 0.407, R = 0.36 p < 0.01). Body weight and VEF1 explained together 40.2% of the lumbar spine, 39.5% of the femoral neck, and 40.7% of total femur BMD variability. This analysis showed that a decrease of FEV1 by 1 l led to a decrease of lumbar spine BMD by 0.065 g/cm2, of femoral neck BMD by 0.066 g/cm2, and of total femur BMD by 0.067 g/cm2.
Linear regression analysis did not show any correlation between BMD or levels of 25OHD, and use of inhalatory glucocorticoids or smoking habits.
Discussion
In our study, we showed that, in a population of patients with COPD without chronic use of systemic glucocorticoids, prevalence of osteoporosis was 51%. This is concordant with the results obtained by similar studies [7, 20, 21]. In addition, our results confirmed that the prevalence of osteoporosis is correlated with the severity of COPD, as suggested by other studies [6, 22, 23]. Moreover, low levels of 25OH vitamin D were observed in 94% of the patients with COPD, which was correlated with the oxygen saturation.
Karadag et al. observed a 35% prevalence of osteoporosis in the lumbar spine and 10% in the femoral neck, in patients with COPD. This prevalence was not significantly different from the prevalence observed in a control group, suggesting that patients with mild to moderate COPD do not need to be routinely assessed for osteoporosis [9]. According to our findings, screening for osteoporosis is justifiable, due to the observance of a high prevalence of osteoporosis in all patients with COPD, even though most of them had mild to moderate disease. In general population studies, several data have shown a correlation between FEV1 and BMD, which suggests that impaired pulmonary function may affect bone health [24–26]. Similarly, we found a positive correlation between FEV1 and BMD in all sites. However, other studies had opposite findings [7, 20, 21, 23, 27], possibly due to the use of different samples and methodologies.
Most of the studies that evaluated the effect of COPD on BMD did not exclude patients who had used systemic glucocorticoids for long periods. This is an important confounding factor that hinders the role of COPD progression on BMD [7, 23–25, 29]. This confounding factor was avoided in our study by the exclusion of patients who had been exposed to a cumulative dose of systemic glucocorticoids equal or higher than 1,000 mg of prednisolone [11]. Nevertheless, 13 patients (26.5%) had received systemic glucocorticoids for the treatment of acute exacerbations. In these patients, the cumulative dose was still below 1,000 mg of prednisolone (398 mg) and their BMD was not significantly different from the BMD of the patients who had never used systemic glucocorticoids. The use of inhalatory glucocorticoids may also have negative effects on BMD, which could have been a confounding factor to our study [28]. However, in our study, previous use of inhalatory glucocorticoids was not associated with changes in BMD or in T-scores, in concordance with other studies [29–32]. In discordance with another study [33], we showed no association between use of inhalatory glucocorticoids and reductions in BMD, when 11 patients who had used inhalatory glucocorticoids for more than 2.5 years or in high doses were analyzed separately.
Our patients lived in Curitiba, Brazil (25° 25' S), and were evaluated during spring. We observed high prevalence of vitamin D insufficiency (59.2%) and deficiency (34.7%). The prevalence of insufficiency was higher than the one observed in a study conducted in postmenopausal women living in Recife, Brazil (12º S), where 43% of them had vitamin D insufficiency [34]. However, our findings were similar to the prevalence observed in a group of elderly in São Paulo, Brazil (23º 34’ S) [35]. In our study, the mean levels of vitamin D were comparable to the levels found in similar studies that evaluated patients with COPD, without chronic use of systemic glucocorticoids [21, 27, 36]. The low levels of vitamin D led to secondary hyperparathyroidism, which was seen in 67% of our patients and could justify the low bone mass.
We found a correlation between O2Sat and levels of vitamin D, in discordance with other studies [20, 36, 37]. Frequently, oxygen therapy is recommended to patients with O2Sat < 88%, which is a marker of severity of COPD [38]. In our patients with O2Sat < 88%, we observed lower levels of vitamin D. This finding could be explained by the fact that patients with severe forms of COPD are less active and, therefore, less exposed to sunlight. However, we did not observe differences regarding physical activity between patients with O2Sat < 88% or ≥88%. In a general population, levels of vitamin D and FEV1 are independently associated [39]. We believe that the levels of vitamin D can be considered as a marker of the severity of COPD, similarly to the correlation between hypovitaminosis D and increased risk of several comorbidities and mortality [40, 41].
In concordance with other studies, we could not find any correlations between smoking habits and BMD [9, 20–25, 27]. However, in general populations, this correlation may exist [42–45]. This discordance may be attributed to the small sample size, to the design of the study, and to the method of evaluation of smoking habits.
Past history of traumatic fractures was present in 22.5% of our patients. In this subgroup, BMD was lower. Studies that evaluated fractures in patients with COPD have controversial findings. For some of them, the risk of fracture may not be increased in patients with COPD [9, 27]. Other study showed more severe fractures in patients with COPD [8]. Our study had a small number of patients with severe disease, which may explain the lack of correlation between fractures and severity of disease.
Our study has some limitations. First, we did not perform thoracic X-ray for morphometric vertebral evaluations, which may have contributed to the underestimation of the prevalence of fractures. Second, we did not include a control group for the evaluation of fractures and levels of vitamin D. The possible presence of vertebral fractures may have contributed to the underestimation of FEV1, which may have influenced the correlation between BMD and FEV1.
In conclusion, our findings clearly show that patients with COPD, without chronic use of systemic glucocorticoids, have increased risk for osteoporosis and low levels of vitamin D, which is correlated with the severity of the disease. Use of inhalatory glucocorticoids by part of our sample was not associated with changes in BMD. We suggest that patients with COPD should routinely have their BMD evaluated, even in the absence of severe disease or use of glucocorticoids. This approach allows the reduction of risk of fractures, and allows adequate treatment of osteoporosis. In addition, supplementation of vitamin D may be needed in all patients with COPD, especially to those with O2Sat less than 88%. Future studies need to evaluate long-term risk of fractures and the outcomes after routine BMD screening and vitamin D supplementation in patients with COPD.
References
Biskobing DM (2002) COPD and osteoporosis. Chest 121(2):609–620
Iqbal F, Michaelson J, Thaler L et al (1999) Declining bone mass in men with chronic pulmonary disease: contribution of glucocorticoid treatment, body mass index, and gonadal function. Chest 116(6):1616–1624
Jorgensen NR, Schwarz P (2008) Osteoporosis in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 14(2):122–127
Kanis JA, Johansson H, Oden A et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19(6):893–899
McEvoy CE, Ensrud KE, Bender E et al (1998) Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157(3 Pt 1):704–709
Vrieze A, de Greef MH, Wijkstra PJ, Wempe JB (2007) Low bone mineral density in COPD patients related to worse lung function, low weight and decreased fat-free mass. Osteoporos Int 18(9):1197–1202
Incalzi RA, Caradonna P, Ranieri P et al (2000) Correlates of osteoporosis in chronic obstructive pulmonary disease. Respir Med 94(11):1079–1084
Papaioannou A, Parkinson W, Ferko N et al (2003) Prevalence of vertebral fractures among patients with chronic obstructive pulmonary disease in Canada. Osteoporos Int 14(11):913–917
Karadag F, Cildag O, Yurekli Y, Gurgey O (2003) Should COPD patients be routinely evaluated for bone mineral density? J Bone Miner Metab 21(4):242–246
Fabbri LM, Hurd SS (2003) Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J 22(1):1–2
Dubois EF, Roder E, Dekhuijzen PN et al (2002) Dual energy X-ray absorptiometry outcomes in male COPD patients after treatment with different glucocorticoid regimens. Chest 121(5):1456–1463
NCAAA. Task Force on Recommended Alcohol Questions—National Council on Alcohol Abuse and Alcoholism Recommended Sets of Alcohol Consumption Questions 2003 [cited 2008 September 15]; Available from:
Lolio C, Souza J, Santo A, Buchalla C (1993) Prevalence of smoking in a city of southeastern Brazil. Rev Saúde Pública 27:4
Lloyd T, Rollings N, Eggli DF et al (1997) Dietary caffeine intake and bone status of postmenopausal women. Am J Clin Nutr 65(6):1826–1830
Expert Panel Report 3 (EPR-3) (2007) Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol 120(5 Suppl):S94–S138
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Hans D, Downs RW Jr, Duboeuf F et al (2006) Skeletal sites for osteoporosis diagnosis: the 2005 ISCD Official Positions. J Clin Densitom 9(1):15–21
Knudson RJ, Slatin RC, Lebowitz MD, Burrows B (1976) The maximal expiratory flow-volume curves normal standards variability and effect of age. Am Rev Respir Dis 113:587–600
Standardization of Spirometry (1995) 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 152(3):1107–1136
Jorgensen NR, Schwarz P, Holme I et al (2007) The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med 101(1):177–185
Katsura H, Kida K (2002) A comparison of bone mineral density in elderly female patients with COPD and bronchial asthma. Chest 122(6):1949–1955
Forli L, Halse J, Haug E et al (2004) Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J Intern Med 256(1):56–62
Kjensli A, Mowinckel P, Ryg MS, Falch JA (2007) Low bone mineral density is related to severity of chronic obstructive pulmonary disease. Bone 40(2):493–497
Lekamwasam S, Trivedi DP, Khaw KT (2002) An association between respiratory function and bone mineral density in women from the general community: a cross sectional study. Osteoporos Int 13(9):710–715
Lekamwasam S, Trivedi DP, Khaw KT (2005) An association between respiratory function and hip bone mineral density in older men: a cross-sectional study. Osteoporos Int 16(2):204–207
Sin DD, Man JP, Man SF (2003) The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med 114(1):10–14
Riancho JA, Gonzalez Macias J, Del Arco C et al (1987) Vertebral compression fractures and mineral metabolism in chronic obstructive lung disease. Thorax 42(12):962–966
Goldstein MF, Fallon JJ Jr, Harning R (1999) Chronic glucocorticoid therapy-induced osteoporosis in patients with obstructive lung disease. Chest 116(6):1733–1749
de Vries F, van Staa TP, Bracke MS et al (2005) Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J 25(5):879–884
Elmstahl S, Ekstrom H, Johnell O et al (2006) No association between inhaled corticosteroids and whole body DXA in postmenopausal women. Pharmacoepidemiol Drug Saf 15(7):527–535
Halpern MT, Schmier JK, Van Kerkhove MD et al (2004) Impact of long-term inhaled corticosteroid therapy on bone mineral density: results of a meta-analysis. Ann Allergy Asthma Immunol 92(2):201–207 quiz 7-8, 67
Jones AM, Munavvar M, Vail A et al (2002) Prospective, placebo-controlled trial of 5 vs 10 days of oral prednisolone in acute adult asthma. Respir Med 96(11):950–954
Richy F, Bousquet J, Ehrlich GE et al (2003) Inhaled corticosteroids effects on bone in asthmatic and COPD patients: a quantitative systematic review. Osteoporos Int 14(3):179–190
Bandeira F, Griz L, Dreyer P et al (2006) Vitamin D deficiency: a global perspective. Arq Bras Endocrinol Metabol 50(4):640–646
Saraiva GL, Cendoroglo MS, Ramos LR et al (2005) Influence of ultraviolet radiation on the production of 25 hydroxyvitamin D in the elderly population in the city of Sao Paulo (23 degrees 34'S), Brazil. Osteoporos Int 16(12):1649–1654
Dimai HP, Domej W, Leb G, Lau KH (2001) Bone loss in patients with untreated chronic obstructive pulmonary disease is mediated by an increase in bone resorption associated with hypercapnia. J Bone Miner Res 16(11):2132–2141
Donovan DS Jr, Papadopoulos A, Staron RB et al (1998) Bone mass and vitamin D deficiency in adults with advanced cystic fibrosis lung disease. Am J Respir Crit Care Med 157(6 Pt 1):1892–1899
Sutherland ER, Cherniack RM (2004) Management of chronic obstructive pulmonary disease. N Engl J Med 350(26):2689–2697
Black PN, Scragg R (2005) Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest 128(6):3792–3798
Melamed ML, Michos ED, Post W, Astor B (2008) 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168(15):1629–1637
Thomas MK, Lloyd-Jones DM, Thadhani RI et al (1998) Hypovitaminosis D in medical inpatients. N Engl J Med 338(12):777–783
Gerdhem P, Obrant KJ (2002) Effects of cigarette-smoking on bone mass as assessed by dual-energy X-ray absorptiometry and ultrasound. Osteoporos Int 13(12):932–936
Law MR, Hackshaw AK (1997) A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 315(7112):841–846
Szulc P, Garnero P, Claustrat B et al (2002) Increased bone resorption in moderate smokers with low body weight: the Minos study. J Clin Endocrinol Metab 87(2):666–674
Ward KD, Klesges RC (2001) A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 68(5):259–270
Acknowledgments
We thank Dr. Lêda Maria Rabelo for her assistance during the preparation of this manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franco, C.B., Paz-Filho, G., Gomes, P.E. et al. Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int 20, 1881–1887 (2009). https://doi.org/10.1007/s00198-009-0890-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0890-5