Abstract
Introduction and hypothesis
Pelvic organ mobility is defined as the displacement of pelvic organs between rest and maximal straining. We hypothesized that pelvic organ mobility after vaginal sacrospinous hysteropexy (SSHP) might be increased compared with other surgeries for uterine descent, which may contribute to the high occurrence of postoperative cystocele after this surgery. Pelvic organ mobility and the vaginal axes after SSHP are compared with other surgical procedures for uterine descent: vaginal hysterectomy with uterosacral suspension (VH) and laparoscopic sacrohysteropexy (LSH).
Methods
In this prospective pilot study, 15 women were included (5 for each procedure). Six months postoperatively, POP-Q examination and dynamic MRI were performed and questionnaires were filled out regarding prolapse complaints. Pelvic organ mobility on MRI was defined as vertical displacement of pelvic organs at rest and maximal straining. The displacements and angles were measured using an image registration method. Furthermore, the angle of displacement of cervix/vaginal vault and vaginal axes were assessed.
Results
No anatomical recurrences of pelvic organ prolapse were found. No difference in pelvic organ mobility was demonstrated. After VH, a more posterior position of the upper vagina was found compared with SSHP and LSH.
Conclusions
Based on these data, the higher recurrence risk in the anterior compartment after SSHP cannot be explained. Larger sample sizes, studying women with recurrence or de novo cystocele after SSHP or using an upright MRI scanner would be of interest to further assess the relationship between pelvic organ mobility and the occurrence of anterior vaginal wall prolapse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, vaginal hysterectomy (VH) with uterosacral suspension of the vaginal vault is the most important treatment for symptomatic uterovaginal prolapse [1]. However, a high proportion of women with prolapse symptoms prefer uterine preservation instead of hysterectomy [2, 3]. As a consequence, these preserving procedures are becoming more popular [4, 5]. Recent studies have demonstrated that suspension of the cervix to the sacrospinous ligament, the so-called vaginal sacrospinous hysteropexy (SSHP), is as effective as the VH [6, 7].
The risk for recurrent prolapse of the anterior vaginal wall after SSHP is often discussed, with incidences ranging from 5.8 to 21.3% [8, 9]. A recent randomized controlled trial comparing SSHP with VH demonstrated more anterior vaginal wall recurrences after SSHP (40% vs 36%), but more posterior vaginal wall recurrences after VH (18% vs 5%) [6]. As for anatomical recurrence after SSHP, the cervix generally remains well fixed to the sacrospinous ligament, but the weak point is supposed to be the anterior compartment [10]. It is hypothesized that the high rates of recurrence in the anterior vaginal wall after SSHP might be related to the previously incurred damage of muscular supports (e.g. levator ani muscle injury, reduced muscle strength), the change in vaginal axis to a more posterior and horizontal position or a combination of the two [9, 11].
Imaging has become an important complementary tool in the assessment of pelvic floor disorders. Magnetic resonance imaging (MRI) can simultaneously evaluate all the compartments of the pelvic floor, and provide information about muscles and ligaments. Furthermore, dynamic MRI of the pelvic floor allows detailed functional evaluation of the pelvic floor [12].
Pelvic organ mobility is defined as the displacement of pelvic organs between rest and maximal straining. Studying pelvic organ mobility after surgery for uterine prolapse is of interest, because it can provide more insight into the cause of recurrence of anterior vaginal wall prolapse after SSHP. It is unknown how mechanical forces after pelvic organ prolapse (POP) surgery change and whether or not they contribute to this recurrence. Numerical simulation methods have been developed to analyze pelvic organ mobility and to evaluate the role of support structure on mobility in general and in the case of POP, or it can be used to simulate surgery [13,14,15]. These methods may also help to reveal the distribution of mechanical forces on pelvic structures after surgery and compare surgical procedures with regard to these forces. With patient-specific analysis and simulations, the process of decision-making in the case of surgical intervention could be improved. However, all these numerical methods have not been validated by quantitative measurements, especially after POP surgery.
We hypothesized that, after SSHP, pelvic organ mobility of the cervix might be decreased owing to fixation of the cervix. Therefore, more pressure on the anterior vaginal wall occurs, resulting in an increase in pelvic organ mobility of the anterior vaginal wall. This may contribute to the occurrence of postoperative cystocele. In addition, the vaginal axis and its change between rest and maximal straining is assessed, because this might contribute to the recurrence of anterior vaginal wall prolapse as well. In this study, the mobility of the pelvic organs and the vaginal axis after vaginal SSHP are compared with VH plus uterosacral suspension of the vaginal vault and laparoscopic sacrohysteropexy (LSH).
Materials and methods
This prospective observational pilot study was performed in the Isala hospital (Zwolle, the Netherlands) from November 2014 to May 2017. After approval from the medical ethics committee, eligible women were asked to participate in this study. Three different surgical procedures for uterine descent were analyzed: vaginal sacrospinous hysteropexy (SSHP), laparoscopic sacrohysteropexy (LSH) and vaginal hysterectomy (VH). All women who recently underwent one of those procedures as a primary treatment for uterine descent were eligible to participate in this study. Exclusion criteria were factors that precludes MRI interpretation (e.g. prosthetic hip), contraindications for MRI (e.g. claustrophobia), patients with neurological disease affecting the pelvic floor or with previous pelvic floor surgery and patients who were not able to maintain the Valsalva maneuver for at least 10 s (e.g. because of pulmonary problems). A total of 15 women were enrolled in this study (5 for each procedure). All women gave written informed consent. Six months after surgery, women underwent gynecological examination and dynamic MRI, and filled out validated questionnaires.
Surgical techniques
Sacrospinous hysteropexy was carried out by fixing the posterior side of the cervix to the sacrospinous ligament using two permanent sutures (Prolene 1/0; Ethicon, Sommerville, NJ, USA). The sutures were placed unilaterally to the right sacrospinous ligament at least 2 cm from the ischial spine using a needle driver under direct visualization. Additional anterior or posterior vaginal wall repair was performed if indicated. LSH was performed using four laparoscopic ports (umbilical, suprapubic, two lateral ports). The peritoneum over the sacral promontory was incised and the broad ligament at the level of the cervico-uterine junction was opened bilaterally. The arms of a bifurcated polypropylene mesh were introduced into these windows of the broad ligament, and the mesh was attached to the anterior and posterior sides of the cervix using permanent sutures. Afterwards, the mesh was attached to the sacral promontory using titanium staples. Finally, additional anterior and/or posterior colporrhaphy was performed if indicated.
Regarding VH, the vaginal wall around the cervix was circumcised and, after bowel and bladder dissection, the anterior and posterior peritoneum were opened. The uterosacral ligaments were ligated and transected. Next, the uterus was released in several steps using clamps and sutures. After removing the uterus, the peritoneum was closed with absorbable sutures. Vault suspension was performed by suspension of the uterosacral ligaments. Such suspension involves the attachment of the uterosacral ligaments to the vaginal vault, thereby restoring normal support to the apical compartment. Concomitant anterior or posterior vaginal wall repair was performed afterwards if indicated.
Gynecological examination
All women underwent gynecological examination, which includes vaginal inspection in a 45° semi-upright position for staging prolapse of the anterior, apical, and posterior compartments using the Pelvic Organ Prolapse Quantification system (POP-Q) [16]. Maximum prolapse was demonstrated and identified by performing a Valsalva maneuver while each vaginal wall was individually exposed.
Questionnaires
To assess the presence and discomfort of prolapse symptoms, women were asked to fill out a validated questionnaire. This questionnaire covers quality of life (Short Form-36, EQ-5D), urogenital and defecation symptoms (UDI, DDI, IIQ), and sexual dysfunction (PISQ-12) [17,18,19,20,21]. Furthermore, the questionnaire contained several questions with respect to parity, mode of delivery, and BMI.
Dynamic MRI
All women underwent MRI in the supine position using a 1.5-T MR scanner (Ingenia; Philips Healthcare, Best, the Netherlands) with an anterior coil centered low on the pelvis for better signal reception. Women were asked not to void their bladder 1 h before examination, to ensure a moderately filled bladder. No intravenously administered contrast medium was used. Prior to the MRI, the rectum was filled with an aqueous gel (200 ml) with the patient in a left lateral decubitus position, using a rectal catheter. Furthermore, the vagina was filled with 30 ml aqueous gel.
Static MRI for anatomical reference was based on the acquisition of a multishot turbo spin echo (TSE) T2-weighted sequence in axial and sagittal planes (field-of-view [FOV] 350 × 350 mm2, slice thickness 4 mm, slices 35, slice gap 0.4 mm, TR/TE 1,846/100 ms, in-plane resolution of 1.0 × 1.0 mm2).
Dynamic MRI of the pelvis was performed using a single-shot balanced fast field echo sequence in the midsagittal and axial plane with a temporal resolution of 1.5 s (FOV 230 × 230 mm2 and 325 x 252 mm2), slice thickness 4 and 6 mm, TR/TE 4.8/2.3 ms, 66 dynamics, in-plane resolution of 1.6 × 1.5 mm2 and 1.5 × 1.2 mm2).
Images were obtained at rest and during maximal straining. Instructions were given by the technologist prior to each separate series. Average scanning time was 20 min, and the total duration of the MRI examination was 35 min.
Analysis of MR images
All MR images of the dynamic sequence were analyzed by one experienced researcher; measurements were done in a midsagittal plane. The researcher was not blinded to the surgical procedure. However, the researcher is a physician/engineer who was not involved in the subject matter of this study and, as a result, could not influence the outcomes.
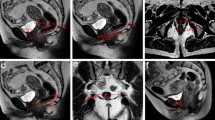
Pelvic organ mobility was defined as the displacement of pelvic organs between rest and maximal straining. The displacement field was calculated by comparison of the initial image at rest and images of the dynamic sequence during straining by an image registration method using Elastix Software. In medical applications, the Image Registration technique is commonly used to modify one image to correspond to another taken at a different time or under different conditions [22]. This technique consists in finding the spatial relation between the position of pixels of the initial image (at rest) and the position of pixels in images taken during straining. A detailed version of the protocol used for analysis has been published previously [23]. This protocol was used to determine the displacement field on the contour of the bladder, vagina, and rectum, as shown in Fig. 1.
Three areas of the vagina were more particularly analyzed to compare the mobility of each surgical procedures: the anterior vagina (AV), the posterior vagina (PV), and the cervix/vaginal vault (Fig. 2). On each area, the mean vertical displacement was quantified in pixels (and converted into millimeters) by calculating the difference in position of these points at rest and during straining. Furthermore, the angle of displacement of the cervix/vaginal vault was also assessed.
Moreover, each patient is applying loading more or less progressively. To compare mobility at same time or level of straining, a percentage of maximum straining has been defined as follow:
where the percentage of strain is the percentage of progress of the loading time t before the maximum strain, t0 is the time of the beginning of the straining, and tmax the time at maximum straining of the dynamic sequence. Figure 3 provides an example of the determination of pelvic organ mobility. In this sagittal plane, the organs are contoured at rest (in white) and during straining, the color bar corresponding to the displacement intensity on the contour of organs at four levels of straining.
Vaginal axes
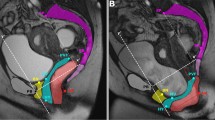
To compare the vaginal axes at rest, the vagina was separated into three distinct regions: upper, middle, and lower vagina [24, 25]. The upper region was defined as a straight line drawn from the anterior to the posterior fornix or from the anterior to the posterior aspect of the vaginal cuff in the case of hysterectomy. The anterior vaginal wall (from the introitus to the anterior vaginal fornix or vaginal cuff) was divided in half; the proximal portion was defined as the middle vaginal region, and the distal portion was defined as the lower vaginal region. Straight lines were drawn to fit both of these regions.
To correct for variations in pelvic inclination and to allow standardized measurements within the pelvis, the vaginal axis was measured as the angle between the vaginal axis and the pelvic inclination correction system (PICS) line [26]. This line is obtained by rotating a line from the inferior point of the pubic symphysis to the junction between the fifth lumbar vertebra and first sacral coccygeal bone (sacro-coccygeal inferior pubic point [SCIPP] line) by 34° in a clockwise direction. For each vaginal region, the vaginal axis was measured using this PICS line.
Statistics
All statistical analyses were performed using SPSS for Windows (version 24.0.0.1). Because of the small number of participants in each group, data were assumed to not be normally distributed. Consequently, medians and interquartile ranges were used as measures of central tendency and dispersion respectively. Comparisons of continuous variables between the three surgeries were calculated using Kruskal–Wallis tests, or, in the case of two groups, Mann—Whitney U tests. Categorical variables were compared using Chi-squared tests. P values below 0.05 were considered to be significant.
Results
The baseline characteristics of the three groups were similar (Table 1) with a median time between surgery and MRI of 31 weeks (LSH and VH group) and 33 weeks (SSHP group). Furthermore, concomitant surgeries were comparable in the three groups. The number of sexually active women was higher in the LSH and VH groups (n = 4, 80%) than in the SSHP group (n = 1, 33.3%). However, only 3 out of 5 women of the SSHP group filled out the questionnaire regarding sexual functioning. In all groups, no anatomical recurrences of pelvic organ prolapse were found. Postoperative POP-Q assessment was comparable in the three groups (Table 1).
In Table 2, the postoperative prolapse complaints (assessed using UDI, DDI, and IIQ) and health-related quality of life (SF-36) are shown. After LSH, more women reported bothersome pain during defecation than after SSHP and VH. Women of the SSHP group scored significantly higher on the item “embarrassment due to urinary incontinence and/or prolapse and/or defecatory problems” of the IIQ. No other significant differences among the groups were found regarding prolapse complaints 6 months after prolapse surgery. Furthermore, health-related quality of life was comparable in all three groups.
Table 3 demonstrates the mean displacement of the anterior vaginal wall, the posterior vaginal wall, and the cervix/vaginal vault during straining for each surgical procedure. Furthermore, the angle of displacement of the cervix/vaginal vault during straining is displayed in this table. There were no significant differences in pelvic organ mobility among the three groups. In addition, no difference was found in the angle of displacement of the cervix/vaginal vault during straining among the three groups. Figure 4 illustrates the range of pelvic organ mobility among the groups. The largest dispersion was found in the VH group.
After VH, the vaginal axis of the upper vaginal region was significantly inferior compared with SSHP and LSH (median 13.7°, compared with 36.3 and 43.3 respectively, p = 0.042, Table 3). After VH, the upper part of the vagina seems to be more posterior than after LSH and SSHP (Fig. 5). Regarding the middle and lower vaginal regions, no difference in the vaginal axes was found among procedures.
Discussion
In the present study, dynamic MRI 6 months after SSHP demonstrated no difference in pelvic organ mobility of the anterior vaginal wall, nor in the posterior vaginal wall and cervix/vaginal vault as compared to the LSH and VH. Furthermore, the angle of displacement of the cervix/vaginal vault was comparable among the three groups and no significant difference was found in the vaginal axes after SSHP compared with LSH and VH. Based on these data, no explanation for the higher recurrence rate of cystocele after SSHP was found.
Little is known about pelvic organ mobility after prolapse surgery. To the best of our knowledge, this is the first study to compare pelvic organ mobility after hysteropexy and hysterectomy. One prospective clinical trial compared preoperative pelvic organ mobility with postoperative pelvic organ mobility in women who underwent anterior and/or posterior mesh-repair surgery [27]. In this study, pelvic organ mobility improved significantly after surgery.
Vaginal configuration after POP surgery has been described in several studies. After attaching the post-hysterectomy vaginal vault to the sacrospinous ligaments, static MRI demonstrates a distortion of the vaginal anatomy by pulling the upper vaginal plane superiorly and at times anteriorly [24, 28]. This finding is contrary to the common belief that the vagina is pulled posteriorly by sacrospinous fixation. A possible explanation may be that the vaginal vault is normally situated below the level of the ischial spines and sacrospinous ligaments, but suspension to these ligaments would pull the vagina superiorly, resulting in a “straining” effect in the sagittal plane [28]. These findings are mainly in line with our study results. After SSHP, we found the vagina to be pulled more superiorly compared with VH, but the vaginal axis after SSHP was comparable with that after LSH. A retrospective cohort study assessed vaginal axes using dynamic MRI in women after hysterectomy compared with women with a uterus in place [25]. After hysterectomy, the middle vaginal axis was positioned more anteriorly compared with the middle vaginal axis with a uterus in place. Furthermore, the angles between the upper and middle vagina and between the middle and lower vagina were more obtuse in women after hysterectomy, suggesting straightening of the vaginal axis. These findings are not in line with our study. After VH, we found a more posterior position of the upper vagina compared with SSHP and LSH, with no differences in the middle and lower vaginal regions. A possible explanation for this difference might be the fact that in the latter study hysterectomy was performed for various conditions, whereas in our study only women after POP treatment were included.
This study has several strengths and limitations. The strength of this report is that this is the first study to compare anatomical and functional results of SSHP, LSH and VH by MRI measurement, including dynamic evaluation. Furthermore, MRI was analyzed by one experienced researcher, with no risk of inter-observer variability. In addition, images were obtained by a standardized protocol.
A limitation of this study is the small sample size. In this pilot study only 15 women were included in total (5 of each group). Furthermore, we did not investigate recurrences of POP in this study. It is unclear whether or not the angle of displacement and vaginal axes are different in these women compared with our study group. All measurements were performed in the midsagittal plane. The vaginal axis, especially in the case of SSHP, may point to the right side, which is not reflected by midsagittal measurements. The lack of the 3D aspect of the vaginal axis could be considered a weakness of this study. To evaluate pelvic organ mobility, patients were instructed to put strain on their pelvic floor by performing a Valsalva maneuver. However, the effect of Valsalva on the extent of POP (and thus pelvic organ mobility) is dependent on the instructions by the physician and the knowledge and the ability of the patients to relax (strain) their pelvic floor muscles [29]. In addition, repeating the Valsalva maneuver increases the induced maximal strain. Interpreting pelvic organ mobility using this method could be subjective. Literature suggests that upright MRI scanning, both at rest and maximal straining, shows a larger extent of the prolapse than that observed during supine straining [30]. At the time of this study, an upright MRI scanner was not available in our clinic. However, it would be interesting to assess pelvic organ mobility using an upright MRI scanner.
In conclusion, we found no difference in pelvic organ mobility after SSHP compared with other POP operations. Based on these data, we cannot explain the higher recurrence risk in the anterior compartment after SSHP. This might be due to the small sample size of this pilot study. It will be interesting to evaluate pelvic organ mobility after SSHP in a larger sample size and in women who have recurrence or de novo cystocele to further assess the relation between pelvic organ mobility and the occurrence of anterior vaginal wall prolapse. Furthermore, assessing pelvic organ mobility using an upright MRI scanner could provide additional insights.
References
Jha S, Cutner A, Moran P. The UK national prolapse survey: 10 years on. Int Urogynecol J. 2018;29:795–801.
Van IJsselmuiden MN, Detollenaere RJ, Gerritse MBE, Kluivers KB, Bongers MY, van Eijndhoven HWF. Dutch women’s attitudes towards hysterectomy and uterus preservation in surgical treatment of pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2018;220:79–83.
Korbly NB, Kassis NC, Good MM, Richardson ML, Book NM, Yip S, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209(5):470.
Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23(6):365–71.
Detollenaere RJ, den Boon J, Kluivers KB, Vierhout ME, van Eijndhoven HW. Surgical management of pelvic organ prolapse and uterine descent in the Netherlands. Int Urogynecol J. 2013;24(5):781–8.
Schulten SFM, Detollenaere RJ, Stekelenburg J, IntHout J, Kluivers KB, van Eijndhoven HWF. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational follow-up of a multicentre randomized controlled trial. BMJ. 2019;366:I5149.
Dietz V, van der Vaart CH, van der Graaf Y, Heintz P, Schraffordt Koops SE. One-year follow-up after sacrospinous hysteropexy and vaginal hysterectomy for uterine descent: a randomized study. Int Urogynecol J. 2010;21(2):209–16.
Petri E, Ashok K. Sacrospinous vaginal fixation—current status. Acta Obstet Gynecol Scand. 2011;90:429–36.
Morgan DM, Rogers MA, Huebner M, Wei JT, Delancey JO. Heterogeneity in anatomic outcome of sacrospinous ligament fixation for prolapse: a systematic review. Obstet Gynecol. 2007;109:1424–33.
Aigmueller T, Riss P, Dungl A, Bauer H. Long-term follow-up after vaginal sacrospinous fixation: patient satisfaction, anatomical results and quality of life. Urogynecol J Pelvic Floor Dysfunct. 2008;19:965–9.
DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302.
Kobi M, Flusberg M, Paroder V, Chernyak V. Practical guide to dynamic pelvic floor MRI. J Magn Reson Imaging. 2018;47:1155–70.
Rubod C, Lecomte-Grosbas P, Brieu M, Giraudet G, Betrouni N, Cosson M. 3D simulation of pelvic system numerical simulation for a better understanding of the contribution of the uterine ligaments. Int Urogynecol J. 2013;24:2093.
Chen L, Ashton-Miller JA, DeLancey JO. A 3D finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech. 2009;42(10):1371–7.
Jeanditgautier E, Mayeur O, Brieu M, Lamblin G, Rubod C, Cosson M. Mobility and stress analysis of different surgical simulations during a sacral colpopexy, using a finite element model of the pelvic system. Int Urogynecol J. 2016;27(6):951–7.
Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7.
Ware JE, Kosinski M, Keller SD. SF-36 physical and mental component summary measures—a user’s manual. 1994. Boston, MA: New England Medical Center, The Health Institute.
Van der Vaart CH, de Leeuw JR, Roovers JP, Heintz AP. Measuring health-related quality of life in women with urogenital dysfunction: the urogenital distress inventory and incontinence impact questionnaire revisited. Neurourol Urodyn. 2003;22:97–104.
Roovers JP, van der Bom JG, van der Vaart CH, Heintz AP. Prediction of findings at defecography om patients with genital prolapse. Br J Obstet Gynaecol. 2005;112:1547–53.
Lamers LM, Stalmeier PF, McDonnell K, Krabbe PF, van Busschbach JJ. Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff. Ned Tijdschr Geneeskd. 2005;149:1574–8.
Schweitzer KJ, de Jong M, Milani AL. Prolaps en seks: hoe meten we de relatie. Ned Tijdschr Obstet Gynaecol. 2008;121:79–82.
Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29(1):196–205.
Lecomte-Grosbras P, Witz JF, Faye N, Cosson M, Rubod C. Quantification of pelvic mobility on dynamic magnetic resonance images: using mechanical insight to help diagnose pelvic pathologies. Strain. 2015;51:301–10.
Sze EH, Meranus J, Kohli N, Miklos JR, Karram MM. Vaginal configuration on MRI after abdominal sacrocolpopexy and sacrospinous ligament suspension. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:375–9.
Lee DD, Siegelman ES, Chua WY, Arya LA, Harvie HS. Comparison of vaginal axis in women who have undergone hysterectomy versus women with an intact uterus. Female Pelvic Med Reconstr Surg. 2019;25(4):313–7.
Betschart C, Chen L, Ashton-Miller JA, Delancey JO. On pelvic reference lines and the MR evaluation of genital prolapse: a proposal for standardization using the pelvic inclination correction system. Int Urogynecol J. 2013;24(9):1421–8.
Alt CD, Brocker KA, Lenz F, Sohn C, Kauczor HU, Hallscheidt P. MRI findings before and after prolapse surgery. Acta Radiol. 2014;55(4):495–504.
Rane A, Lim YN, Withey G, Muller R. Magnetic resonance imaging findings following three different vaginal vault prolapse repair procedures: a randomized study. Aust N Z J Obstet Gynaecol. 2004;44:135–9.
Tumbarello JA, Hsu Y, Lewicky-Gaupp C, Rohrer S, DeLancey JO. Do repetitive Valsalva maneuvers change maximum prolapse on dynamic MRI? Int Urogynecol J. 2010;21(10):1247–51.
Grob ATM, Olde Heuvel J, Futterer JJ, Massop D, Veenstra van Nieuwenhoven AL, Simonis FFJ, van der Vaart CH. Underestimation of pelvic organ prolapse in the supine straining position based on magnetic resonance imaging findings. Int Urogynecol J. 2019;30(11):1939–44.
Author information
Authors and Affiliations
Contributions
M.B.: manuscript writing; M.C.: manuscript writing; H.W.F.v.E.: protocol/project development, data collection, manuscript writing; M.N.v.I.: protocol/project development, data collection and analysis, manuscript writing; P.L.-G.: protocol/project development, data collection and analysis, manuscript writing; J.F.W.: manuscript writing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van IJsselmuiden, M.N., Lecomte-Grosbras, P., Witz, JF. et al. Dynamic magnetic resonance imaging to quantify pelvic organ mobility after treatment for uterine descent: differences between surgical procedures. Int Urogynecol J 31, 2119–2127 (2020). https://doi.org/10.1007/s00192-020-04278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04278-5