Abstract
Introduction and hypothesis
We aim to analyze the combined influence of the size of the mesh, the number of sutures, the combined use of an anterior and posterior mesh, and the tension applied to the promontory, on the mobility of the pelvic organs and on the sutures, using a Finite Element (FE) model of the female pelvic system during abdominal sacral colpopexy.
Methods
We used a FE model of the female pelvic system, which allowed us to simulate the mobility of the pelvic system and to evaluate problems related to female prolapse. The meshes were added to the geometrical model and then transferred to computing software. This analysis allowed us to compare the stress and mobility during a thrust effort in different situations.
Results
The bigger the mesh, the less mobility of both anterior and posterior organs there would be. This is accompanied by an increase in stress at the suture level. The combination of a posterior mesh with an anterior one decreases mobility and stress at the suture level. There is a particularly relevant stressing zone on the suture at the cervix. The increase in the number of sutures induces a decrease in the tension applied at each suture zone and has no impact on organ mobility.
Conclusion
Our model enables us to simulate and analyze an infinite number of surgical hypotheses. Even if these results are not validated at a clinical level, we can observe the importance of the association of both an anterior and a posterior mesh or the number of sutures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past few years, laparoscopic sacral colpopexy has become a commonly performed procedure for the surgical treatment of female prolapse [1]. Although the surgical technique is well designed, different surgeons have different practices, e.g., in the size of the mesh used, the systematic use of a posterior mesh combined with the anterior, and the number or type of sutures used to fix the mesh.
The erosion rate (3.4 %) and the re-intervention rate (4.4 %) for recurrence of prolapse is low, but the change in certain practices could reduce these failures further [2]. Nevertheless, the clinical study of the potential impact of these different elements is difficult because it would require carrying out studies involving a large population to highlight significant differences.
Thanks to the making of a biomechanical female pelvic system we have already been able to analyze the different elements implicated in the prolapse [3, 4]. We used this model again to evaluate the potential impact of these techniques on mobility and stresses on different organs during a sacral colpopexy.
The purpose of our study is to analyze the influence of the size of the mesh, the number of sutures, and the combined use of both an anterior and a posterior mesh on the mobility of pelvic organs and the constraints on sutures using a Finite Element model of the female pelvic system during an abdominal sacral colpopexy.
Materials and methods
We used a 3D Finite Element model of the female pelvic system, already made by our team [5]. It allowed us to simulate the mobility of the pelvic system and to evaluate the troubles related to female prolapse [3, 6]. The Institutional Review Board (Comité Ethique de la Recherche en Obstétrique et Gynécologie) approved this research (CEROG 2012-GYN-06-01-R1).
To make this model, the following steps were necessary, according to the protocol used by our team [5]. First, we created a geometrical model based on an MRI of a woman, to specify the position of the different anatomical structures. The segmentation of the anatomical structures on the MRI, were made using the software AVIZO.
The areas obtained are then smoothened via the software CATIA to obtain more realistic forms (Fig. 1).
Finally, the model represents a patient-specific woman with normal support (without any pathological conditions). We know that prolapse results from a defect of the anatomical suspension system [7, 8]. As we aimed to analyze the mobility of the organs with surgical mesh, we chose to consider a dysfunction of anatomical support system by disabling the different ligaments such as the broad and round ligaments. We conserved the interface between the organs, such as that between the rectum and the vagina or between the bladder and vagina. Nevertheless, no model could exactly reproduce the situation of a real female pelvis, where an active blood supply system is also present.
Then, using the CAD software CATIA V5 (Dassault Systèmes), we modeled a subtotal hysterectomy, leaving only the cervix, and fixed the meshes onto the promontory. They recovered the anterior or posterior vaginal wall by passing through the cervix. The mesh interacted with the fasciae and vagina.
Thanks to this protocol, we were able to test different sizes of meshes (Fig. 2).
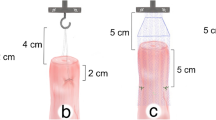
To our knowledge, there is no recommendation with regard to mesh size. Thus, we based it on the experience of our surgical team. A large mesh is 3 cm wide and 11 cm long covering 4 cm of the vaginal wall (distance between the cervix and the lower end of the mesh). For the small mesh, we used one 1.5 cm wide and 9.5 cm long, covering 2.5 cm of the vaginal wall. Initially, these meshes were installed on our FE model without mechanical stress, corresponding only to the normal setting up of organs. It is to be noted that the distance between the cervix and the promontory has been fixed at 7 cm. We used a polypropylene mesh and nonresorbing sutures.
Second, we transferred this geometrical model to a computing software program using the Finite Elements method (Abaqus/CAE 6.12-2; Dassault Systèmes Simulia) based on a standard protocol described in the literature [5]. At this stage, it was necessary to implement the mechanical properties of tissues. To do so, we used the recently published studies using either cadaveric or fresh tissue from pregnant and nonpregnant women [9]. The FE mesh generation has also been realized through Abaqus. The organs have been modeled using Finite Element, such as shell elements with constant thickness or hexahedral elements for the uterus and fasciae. We conducted an h-convergence study to determine sufficient mesh quality. This protocol follows the same method employed in a previous study [5], where more details are provided concerning the description of elements, material properties, loading, and boundary conditions.
In our review of the literature we found some information on the mechanical properties of meshes [10]. According to these works, we have calculated an average of the uniaxial properties values of the meshes and chose a value of 0.5 MPa (N/mm2). Some studies worked on the importance of the stiffness of the mesh or pore dimensions [11, 12]. In our work, the modeling of the mesh does not take into account the pores and is considered a homogeneous surface on our FE model. Thus, it has a constant thickness of 500 μm, corresponding to the achieved value of the mesh after healing.
After these steps, we analyzed the mobility of all organs, especially those of the vagina and stresses at sutures. To do so, we subjected the FE model to an effort comparable to a coughing effort [13]. This corresponds to a pressure on the pelvic organs of 10−3 MPa at an inclination of 45° from the sagittal axis in an antero-posterior direction (Fig. 1).
The first phase has been conducted by comparing the use of a large and a small mesh, either in an anterior or a posterior position, or both together. Meshes were fixed by two sutures on the anterior vagina wall and by one on the cervix. The number of sutures used is not clearly discussed in the literature. The mesh interacts with the fasciae and vagina with a contact between the surfaces. Therefore, we decided to follow the surgical customs of our team to evaluate our practices and to highlight significant potential differences and the need to modify them.
During the second phase we compared the stress applied to each suture zone (3 × 3 mm) on the vaginal wall based on the number of suture points and their spacing. We compared two or four points evenly spread on the vaginal wall and one on the cervix. Then we measured the distances between suture zones on each of our simulations, with both the large and the small meshes in the anterior and posterior positions.
During the third phase, we also analyzed stress induced on the sutures when we changed the tension applied to the mesh fixed to the promontory. To do so, we shortened the initially 7 cm long mesh by 1 cm. The variation of a 7 cm mesh to 6 cm, keeping the same position of promontory fixation, concretely translates into an upward movement of organs or a displacement of the mesh. Several simulations were performed with a step-by-step method (shortening of 2.5 mm) to analyze the impact on stress.
Results
During the first phase, the increase in the size of the mesh led to a decrease in organ mobility, both anteriorly and posteriorly (Fig. 3). In our case study, we observed a 9-mm shift at the level of the cervix following the use of a small anterior mesh (Fig. 3c). This shifting measured 4 mm using the large mesh (Fig. 3b). The combination of a large anterior mesh and a large posterior one showed a slightly shorter shift of 2.5 mm (Fig. 3e). In each case, this analysis of mobility based on the number of sutures on the vaginal wall has not revealed any significant differences in the displacement (Fig. 3a, b).
During the second phase, we focused on the Von Mises’ stress at a suture level. The first assessment shows a wide zone of stress at the cervix (220 Pa), compared with that applied to the vaginal wall. Whatever the number of sutures on the latter (Fig. 4), this stress zone is 10 times higher than the one applied to the vaginal sutures.
Presentation of the Von Mises’ stress for different configurations. A dark circle represents each suture. a Anterior large mesh fixed with four sutures on the vaginal wall. b Large anterior mesh fixed with two sutures. c Small anterior mesh fixed by two sutures. d Large posterior mesh fixed by two sutures
Using a larger mesh with two sutures induces an average stress of 31.5 Pa on each suture, whereas it reaches only 5.9 Pa with a smaller mesh. According to our precedent results, this seem consistent, as the use of a wider mesh induced better support, which led to greater efforts of the sutures along with greater stress. A sharp decrease in mobility induces far stronger stress on the same number of sutures; therefore, it seems necessary to increase the number of sutures to correct this phenomenon (Fig. 4a, b). Stress on the vaginal wall is then more evenly distributed. This conclusion remains the same with the other configuration: small and large mesh, both anterior and posterior (Fig. 5).
The combination of a posterior mesh with an anterior one decreases the stress applied to each suture on the anterior vaginal wall. There is no impact on structural mobility following the change in the number of sutures (Fig. 3a, b).
The increase in spaces between sutures increases the stress on the vaginal wall. For example, a spacing smaller than 1.5 cm induces a small stress (less than 0.008 MPa). This rise is not linear. Indeed, there is a plateau between 1 and 2.5 cm spacing (stress close to 0.005 MPa). But after 2.5 cm, a 1-cm increase in spacing induces a doubling of the average stress (Fig. 6). Moreover, the comparison between the different layouts shows great stress in the anterior layout than in the posterior one for the same spacing. For example, a spacing between sutures of 3.5 cm induces a stress of 0.01 MPa in the posterior layout and 0.025 in the anterior one (x2.5).
A moderate increase in tension by shortening the mesh by 1 cm on the promontory induces a strong rise in stress on the suture area at an organ level. Thus, a shortening of 1 cm (14.3 %) for a large anterior mesh induces a doubling of the stress at the suture level on the cervix (from 220 to 450 Pa). We witnessed a linear correlation between different shortenings and the related stress. Indeed, shortening the mesh by 0.25, 0.5, and 0.75 cm induced a rise of 301, 358, and 401 Pa respectively.
Discussion
Surgical techniques
Several authors have published lengthy descriptions of the technique of sacral colpopexy [1, 2, 14, 15]. Several variations exist among the different surgical teams, and no clinical studies were ever able to find differences between these technical points.
Thanks to our model, we were able to simulate any technical surgical variation. In our study, we primarily focused on variations of the surgical technique we perform in our department. For example, we performed a subtotal hysterectomy instead of a total one, as this seemed to decrease the risk of mesh exposure [16].
Mesh size
It is now widely admitted that sacral colpopexy has to be realized using a mesh. Several studies dealt with the type of mesh [17–19], but only few dealt with its size. For example, it was shown that a mesh measuring 50 × 50 mm led to more complications than a 35 × 35 mm one. In our study, the bigger mesh measured 30 × mm [20]. Nowadays, the anterior dissection has to reach the balloon of the bladder catheter corresponding to the vesical trigone [21], without any precision related to the width of the dissection.
According to our results, the use of a 3-cm wide mesh would lead to better results in terms of mobility compared with a smaller mesh. This would need an anterior dissection at least 3 cm wide down to the vesical trigone. We are aware that it is sometimes difficult to obtain a satisfactory dissection especially on a scarred area; nevertheless, the mesh should cover the whole vaginal wall dissection and be as wide as possible. However, we did not take into consideration the early or late shrinkage phenomenon that is sometimes observed [22].
Combination of an anterior and posterior mesh
Nowadays, most surgeons systematically combine a posterior mesh with an anterior one to decrease the risk of a secondary rectocele [23]. This seems to be facilitated by laparoscopy, because of the better visibility and the help brought by the pneumodissection. Nevertheless, some teams question this systematic combination because of a higher complication rate, especially concerning anal wouns, and only use this combination in relation to a Burch colposuspension [24].
The use of a posterior mesh in our analysis goes beyond the prevention of a prolapse relapse, as it decreases the mobility in a better way than the sole use of an isolated anterior mesh. Therefore, it seems that the posterior mesh would reinforce the efficiency of the anterior one in addition to the risk limitation of a secondary rectocele, especially while performing a Burch technique. Besides, the use of a posterior mesh decreases the stress applied on the anterior one, leading to a potential decrease in the failure rate.
This study will bring about a new development, which is the systematic use of a posterior mesh. To this day, it has never been suggested that a posterior mesh could reinforce the efficiency of an anterior one. Its role seemed to be limited to the prevention of a potential secondary posterior prolapse.
Number and type of sutures
As mentioned before, the suture at the promontory is well described in the literature. Nevertheless, as the sutures of the vaginal wall are less well studied, they can vary greatly, depending on the surgeons. It seems that the vaginal fixation could be done using either resorbing or nonresorbing surgical threads or Taker® [15]. To date, no studies have shown the potential benefits of one method compared with the other. However, some studies have focused on the impact on the host response of mesh fixation and the amount of tension a mesh could experience [25].
According to our results, the suture of the mesh to the cervix is a high-stress area, however many sutures there are on the vaginal wall. Thus, it seems necessary to pay particular attention to this specific area.
Our study suggests that the use of four sutures evenly spread across the anterior part of the vagina wall (roughly a 2.5-cm spacing) might help to decrease the stress on each suture area and then potentially decrease the breaking point of these sutures. The number of sutures is especially relevant in case of the use of a large mesh. We came to the same conclusion as Barone et al., who worked on the importance of the fixation points to reduce the surface curvature using photogrammetry [25]. Indeed, the wider the mesh, the stronger the stress on each suture. Therefore, in this situation it seems to be necessary to increase the number of sutures. Although these conclusions bring about a first approach to changing our practices, it is unfortunately difficult to evaluate precisely the spacing of our sutures during surgery.
Tension on the mesh at the promontory
The technique of the ligamentoplasty is well described. We use a nonresorbing suture to fix the mesh to the presacral ligament. The same thread is then used to fix the anterior and posterior meshes [23]. Some studies proved that it was better to perform this suture using a thread instead of Taker® [26, 27]. Although it is not officially admitted, we usually leave a space between the mesh and the promontory to limit the infection risk and to allow easier resection in the event of complications [23].
Our analysis related to stress on meshes at the promontory seems to corroborate this practice. Indeed, tension that is too strong on the mesh at the promontory would place severe stress on the vaginal sutures.
Limitations of our analysis
We are aware that the results of this study are based on empirical results through mechanical analysis. Thus, these results alone cannot modify our practices.
We tried to answer some persistent questions that remain, despite the widespread development of this type of surgery. On the one hand, we aimed to justify or challenge our own practices. On the other hand, we aimed to open a discussion on the recommendations for this particular surgical technique. It would help to standardize the practices, leading to better evaluation, and to a decrease in the complication and failure rates. Nevertheless, complementary analyses are required, perhaps at a mechanical level with an FE model even closer to reality or with studies on cadavers.
Some studies worked on the importance of the stiffness of the mesh or pore dimensions and highlighted that less mobility induces complications [11, 12]. We did not deal with these factors because our study focuses on the size of the mesh (length and width), the number of sutures, and the position. In subsequent work, it would be interesting to vary the stiffness of the mesh and corroborate the results of these different studies with our model. Moreover, this FE model is subject-specific. Also, FE models are generally modeling acute responses and are largely dependent on the material properties chosen.
It seems especially relevant to validate these results with clinical studies to better incorporate these elements into our practice. Therefore, it could be interesting to conduct postoperative MRI or perineal ultrasound to evaluate even more accurately the benefits of these different techniques.
Conclusion
Our model enables us to simulate and analyze an infinite number of surgical choices and hypotheses. We can try to bring answers to uncertainties, which persist in the surgical treatment of prolapse by sacral colpopexy. Although these results are not validated at a clinical level, we can observe the importance of the association of both an anterior and posterior mesh and the number of sutures. These elements could be used to give a more accurate description of the surgery and homogenize our practices.
References
Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ (2004) Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol 190(1):20–26
Nygaard IE, McCreery R, Brubaker L, Connolly A, Cundiff G, Weber AM et al (2004) Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol 104(4):805–823
Cosson M, Rubod C, Vallet A, Witz JF, Dubois P, Brieu M (2013) Simulation of normal pelvic mobilities in building an MRI-validated biomechanical model. Int Urogynecol J 24(1):105–112
Cosson M, Rubod C, Vallet A, Witz J-F, Brieu M (2011) Biomechanical modeling of pelvic organ mobility: towards personalized medicine. Bull Acad Natl Med 195(8):1869–1883; discussion 1883
Mayeur O, Witz J-F, Lecomte P, Brieu M, Cosson M, Miller K (2015) Influence of geometry and mechanical properties on the accuracy of patient-specific simulation of women pelvic floor. Ann Biomed Eng doi:10.1007/s10439-015-1401-9
Rubod C, Lecomte-Grosbras P, Brieu M, Giraudet G, Betrouni N, Cosson M (2013) 3D simulation of pelvic system numerical simulation for a better understanding of the contribution of the uterine ligaments. Int Urogynecol J 24(12):2093–2098
Petros PEP, Woodman PJ (2008) The integral theory of continence. Int Urogynecol J Pelvic Floor Dysfunct 19(1):35–40
DeLancey JO (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166(6 Pt 1):1717–1724; discussion 1724–1728
Rubod C, Boukerrou M, Brieu M, Jean-Charles C, Dubois P, Cosson M (2008) Biomechanical properties of vaginal tissue: preliminary results. Int Urogynecol J Pelvic Floor Dysfunct 19(6):811–816
Shepherd JP, Feola AJ, Abramowitch SD, Moalli PA (2012) Uniaxial biomechanical properties of seven different vaginally implanted meshes for pelvic organ prolapse. Int Urogynecol J 23(5):613–620
Liang R, Abramowitch S, Knight K, Palcsey S, Nolfi A, Feola A et al (2013) Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG Int J Obstet Gynaecol 120(2):233–243
Feola A, Abramowitch S, Jallah Z, Stein S, Barone W, Palcsey S et al (2013) Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG Int J Obstet Gynaecol 120(2):224–232
Kamina P (2008) Anatomie clinique, vol 4. Organes urinaires et génitaux, pelvis, coupes du tronc. Maloine, Paris
Nezhat CH, Nezhat F, Nezhat C (1994) Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol 84(5):885–888
Maher C, Feiner B, Baessler K, Schmid C (2013) Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 4:CD004014
Bensinger G, Lind L, Lesser M, Guess M, Winkler HA (2005) Abdominal sacral suspensions: analysis of complications using permanent mesh. Am J Obstet Gynecol 193(6):2094–2098
Cundiff GW, Varner E, Visco AG, Zyczynski HM, Nager CW, Norton PA et al (2008) Risk factors for mesh/suture erosion following sacral colpopexy. Am J Obstet Gynecol 199(6):688.e1–688.e5
Govier FE, Kobashi KC, Kozlowski PM, Kuznetsov DD, Begley SJ, McGonigle KF et al (2005) High complication rate identified in sacrocolpopexy patients attributed to silicone mesh. Urology 65(6):1099–1103
Quiroz LH, Gutman RE, Shippey S, Cundiff GW, Sanses T, Blomquist JL et al (2008) Abdominal sacrocolpopexy: anatomic outcomes and complications with Pelvicol, autologous and synthetic graft materials. Am J Obstet Gynecol 198(5):557.e1–557.e5
Manodoro S, Endo M, Uvin P, Albersen M, Vláčil J, Engels A et al (2013) Graft-related complications and biaxial tensiometry following experimental vaginal implantation of flat mesh of variable dimensions. BJOG Int J Obstet Gynaecol 120(2):244–250
Gadonneix P, Ercoli A, Salet-Lizée D, Cotelle O, Bolner B, Van Den Akker M et al (2004) Laparoscopic sacrocolpopexy with two separate meshes along the anterior and posterior vaginal walls for multicompartment pelvic organ prolapse. J Am Assoc Gynecol Laparosc 11(1):29–35
Feiner B, Maher C (2010) Vaginal mesh contraction: definition, clinical presentation, and management. Obstet Gynecol 115(2, Part 1):325–330
Wattiez A, Canis M, Mage G, Pouly JL, Bruhat MA (2001) Promontofixation for the treatment of prolapse. Urol Clin N Am 28(1):151–157
Antiphon P, Elard S, Benyoussef A, Fofana M, Yiou R, Gettman M et al (2004) Laparoscopic promontory sacral colpopexy: is the posterior, recto-vaginal, mesh mandatory? Eur Urol 45(5):655–661
Barone WR, Amini R, Maiti S, Moalli PA, Abramowitch SD (2015) The impact of boundary conditions on surface curvature of polypropylene mesh in response to uniaxial loading. J Biomech 48(9):1566–1574
Boukerrou M, Orazi G, Nayama M, Boodhun R, Crépin G, Cosson M (2003) Promontofixation procedure: use of non-absorbable sutures or tackers? J Gynecol Obstet Biol Reprod 32(6):524–528
Cosson M, Narducci F, Querleu D, Crépin G (2001) Experimental use of laparoscopic material: report of a case of spondylodiscitis after laparoscopic sacropexy with Taker. Ann Chir 126(6):554–556
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
E. Jeanditgautier, O. Mayeur, G. Lamblin, and M. Brieu report no conflicts of interest. C. Rubod is a consultant for Olympus. M. Cosson led training courses with Boston, AMS, and Olympus, and is also a consultant, accepting honoraria and payment for research from Allergan, Boston, and AMS.
Rights and permissions
About this article
Cite this article
Jeanditgautier, E., Mayeur, O., Brieu, M. et al. Mobility and stress analysis of different surgical simulations during a sacral colpopexy, using a finite element model of the pelvic system. Int Urogynecol J 27, 951–957 (2016). https://doi.org/10.1007/s00192-015-2917-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-015-2917-0