Abstract
Introduction and hypothesis
Genital prolapse remains a complex pathological condition. Physiopathology remains poorly understood, aetiology is multi-factorial, surgery is not always satisfying, as the rate of relapse cannot be overlooked. More over a good anatomical result will not always guarantee functional satisfaction. The aim of our study is to have a better understanding of the involvement of uterine ligaments in pelvic statics via 3D simulation.
Methods
Simulation of pelvic mobility is performed with a validated numerical model in a normal situation (standing up to lying down) or induced pathological ones where parts of the constitutive elements of the model are virtually “cut” independently. Displacements are then discussed.
Results
Numerical results have been compared with dynamic MRI for two volunteers. Dynamic sequences had 90 images, and 180 simulations have been validated. Results are coherent with clinical data and the literature, thus validating our mechanical approach. Uterine ligaments are involved in pelvic statics, but their lesions are not sufficient to generate a genital prolapse. Round ligaments play a part in uterine orientation; the utero-sacral ligaments support the uterus when standing up.
Conclusions
Pelvic normal and pathological mobility study via modelling and 3D simulation is a new strategy in understanding the complex multifactorial physiopathology of genital prolapse. This approach must be validated in a larger series of patients. Nevertheless, pelvic ligaments seem to play an important role in statics, especially, in agreement with a literature survey, utero-sacral ligaments in a standing position.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genital prolapse is a significant problem for gynaecologists. This static pelvic disorder affects one woman in three in all age groups [1] and more than 60 % of the patients are aged over 60 [2]. Faced with the ageing female population, there is an increasing demand for surgical treatment aimed at improving quality of life, reaching a level of intervention of 11.8 % at the age of 80. [3]. Many surgical techniques are developed with different approach (traditional or mesh kits), but all have their limits underlined in the literature. Long-term results are disappointing, in a systematic review of 2009 [4], showing that total reoperation rates for prolapse recurrence were 4 % in the traditional vaginal surgery group versus 1.3 % in the vaginal mesh group. However, the total reoperation rate was 8.5 % for vaginal mesh kits because of a higher rate of reoperations for complications such as mesh erosion. Toh et al. [5] underlined the necessity of requiring more well-designed studies for evaluation of recurrent vault prolapse.
Many epidemiological risks of pelvic prolapse have been described in the literature [6] and other “local traumas” (pregnancy, physical exercise) or iatrogenic factors. Several physiopathological hypotheses have been proposed. For Goh, prolapse of the pelvic organs is due to mechanical and neurological factors and also to the properties of connective tissue [7]. The genesis of prolapse could thus be linked to ligament and neuromuscular dysfunction under abnormally high loads.
The aetiology of genital prolapse now seems multifactorial. It is important to determine the involvement of each factor, especially for pelvic ligaments. The structures fixing pelvic organs are many and not well defined. Most are tissue layers, fascias and linking organs with no true anatomical organisation. Discordances in the literature are enhanced by the variations of nomenclature. Nevertheless, pelvic ligaments seem to be in the core of the problem of statics, owing to their contribution to suspension [8, 9]. Understanding the involvement of each of them in this balance could help to progress the understanding of the physiopathology of genital prolapse.
To solve these problems, we analysed the respective importance of uterine ligaments in pelvic statics by the bias of a validated model of pelvic mobility (developed by our team and explained in the section Materials) [10–13]. Our aim is to obtain a better understanding of the physiological role of uterine ligaments by a 3D simulation of the pelvic system. Via this approach, we studied the loading of ligaments when the patient is standing up or lying down and the consequences when suppressing one of the ligaments.
Materials and methods
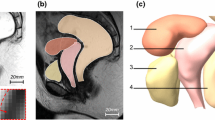
In our approach to the analysis of the involvement of uterine ligaments in pelvic statics, we developed a complete numerical 3D model of the female pelvic system. Our numerical model of pelvic system mobility (Fig. 1) was built up and validated using data from medical imaging, anatomy and mechanics [9–16]. The Institutional Review Board (Comité d’Ethique de la Recherche en Obstétrique et Gynécologie) approved our research (CEROG 2012-GYN-06-01-R1).
To build up a valid numerical biomechanical model several steps were required [17]. The 3D model must represent all anatomical elements, the mechanical behaviour of all tissues involved, subject to the boundary conditions of displacement and loads that organs and fixations may support. Finally, simulation of physiology and/or physiopathology will be performed by a well-improved numerical method such as the finite elements method.
Geometry of organs and pelvis was obtained by rebuilding volumes with static MR images [10–13]. The geometries of the relevant organs and bones were determined by the segmentation of static MR images in the three different planes (sagittal, axial and coronal). The surfaces were then meshed by triangulation and regularised using the software program BLENDER. A finite elements model was developed in ABAQUS EXPLICIT software (version 6.101) whose use has been validated several times for numerical simulation of structures for industrial use or biomechanical studies [18]. Each organ, muscle and ligament is meshed with shell elements. Pelvic floor and muscles were added following clinical and anatomical data. The ligaments are also difficult to individualise via MRI. They were progressively incorporated in order to make numerical simulation of displacements converge towards MR image quantification [11, 12]. Ligaments were introduced in agreement with the anatomical localisation and static data presented in the literature. This approach was validated in two patients without prolapse, but the methodology may be applied to all MR images of patients.
To complete the finite element problem, the behaviour of the different materials involved was introduced. The properties of the broad, round and utero-sacral ligaments were defined with regard to literature data [9]. Broad and cardinal ligaments were assumed to have mechanical properties equivalent to round ligaments. Such an assumption has been revealed to provide accurate numerical results [12]. We considered tissues to be elastic and isotropic under great deformation, as shown in our recent work [9, 14–16]. The behaviour of tissues was modelled as elastic and linear under large transformations to simplify numerical simulation and reduce the computation time without impairing the quality of the simulations. Global behaviour of the structures is thus elastic, but non-linear. Tissues that are well hydrated are not very compressible and quasi-incompressibility is assumed.
Limits of displacements and their conditions were fixed by qualitative and quantitative evaluation of displacements on dynamic MRI of a patient during pushing [10–12]; limits of pressure were given by literature data [13, 19]. Simulations of this pelvic mobility were compared with dynamic MRI. The numerical model has been defined by comparison and topological optimisation procedures [20] in two nulliparous volunteers (20 and 21 years old) without any pathological features of prolapse. The optimisation process was performed during coughing or pushing, comparing 90 MRI sequences taken during a dynamic procedure.
To allow the finite element simulation to estimate the mobility measured on dynamic MRI, pelvic ligaments, difficult to identify on MRI images (round, umbilical, utero-sacral, broad and cardinal ligaments), were successively added. They were localised following literature and anatomy results [8, 19]. Their respective contributions were highlighted when comparing simulation with medical imaging. For each patient, 90 images and numerical simulations were compared. Our model was validated with a maximal difference of 8 mm between simulated displacements and MR images [12]. Pelvic floor and muscles were added following clinical and anatomical data. However, we have not yet evaluated their possible lesions and aptitude to contract.
Under physiological conditions, the pelvic system is subjected to gravity and pushing. To respect physiological conditions and our biped condition, the numerical model needs to simulate the upright position of the patient. Because MR images in a sitting or upright position were not available, the numerical model was defined in the lying down position. Even though MRI acquired when standing or sitting would have been interesting, the numerical approach used allows a standing position, or any position, to be simulated, by modifying the direction of gravity.
In this context, to obtain a better understanding of the physiopathology of genital prolapse, simulation of pelvic mobility was performed in a normal situation or the pathological condition was induced after the virtual “sectioning”, successive and independent, of the different ligaments of the model. This allowed testing of the different physiopathological involvement of pelvic ligaments by comparison with data obtained by numerical simulation.
Results
The results presented in this section do not deal with a statistical analysis, but are aimed at showing, thanks to numerical simulation, the consequences of the evolution of a specific anatomy for women’s pelvic mobility.
Displacement measures
Different uterine ligaments involved in pelvic statics were studied. Each pair of ligaments was studied individually by deletion or not (proximal section [section at the uterine insertion]). For each simulation and each topology (with or without ligaments), displacement was measured. Our tool of numerical simulation, based on the finite elements method, gives approximate values of strain and displacement for each node point of the mesh. This approximation directly correlates with the size of the finite element mesh and the polynomial approximation. In the present case, accuracy of the displacement is about 0.01 mm.
Simulation without lesions and standing up
These simulations allow study of how body position may influence tension in the sustaining structures. The results are reported in Fig. 2. When rotation allows a standing up position of the model the utero-sacral ligaments have more tension than in decubitus (14 N versus 2 N). Utero-sacral ligaments have a higher tension when the patient is standing up (14 N versus 0 to 4 N in the other ligaments). In decubitus configuration it will be higher for round ligaments (16 N versus 2 to 6 N in the other ligaments).
Simulations in a standing position with sections of uterine ligaments
We wanted to study the consequences induced by partial degradation of ligaments; we had seen their importance when building up the numerical model, and tried to study extreme degradation of the system by successive and independent suppression of the uterine ligaments theoretically involved.
Figures 3 to 4 illustrate the magnitude of the displacement (plotted in centimetres) at each point of the organs (blue shows low displacement, red large displacement with regard to the colour map in the centre of the figure).
Only deleting round ligaments induces excessive lateral mobility of the uterus with nearly complete rotation on itself or even retroversion (Fig. 3). A rotation of 83° has been noticed.
Isolated bilateral deletion of utero-sacral ligaments does not induce patent genital prolapse. Excessive relative mobility of the uterus can only be seen without total exteriorisation (Fig. 4). In such a case, it was noticed that the upper part of the vagina was falling by 1.3 cm.
Deletion of the broad ligament induced a maximal displacement of 6 mm, while cardinal ligament deletion induced 8 mm. As long as the differences between the numerical model and the physiological situation are of the order of 8 mm, the numerical simulation of isolated bilateral deletion of broad or cardinal ligaments do not induce significant displacement, which may lead the proposed numerical model to understand their contribution to pelvic statics.
Discussion
The aim of the proposed study is to use a numerical approach in order to provide more tools for understanding the physiology of the pelvic mobility and the physiopathology of genital prolapse. This approach offers information that is not proposed by conventional methods of investigation (MRI analysis, clinical studies, anatomical investigations). Indeed, 3D numerical study provides out-of-plane information with regard to dynamic MRI [12] and provides an opportunity to test and/or validate physiopathological hypotheses available in the literature.
The present reference method for studying pelvic system mobility and detecting possible pathological conditions remains iconography, especially dynamic MRI or perineal echography (we did not use this in our study). Unfortunately, most MRI or echography equipment requires patients to lie down. Forces of gravity and strain, an important factor for mobility, do not exert in the same planes as physiologically. The numerical tool partially allowed this problem to be solved. It is easy, numerically, “to tip up the patient and her pelvis” and to simulate the incidence of gravity in a normal upright position. The numerical tool can also evaluate the intensity of strain when pushing in different positions of the pelvis. The most stretched ligaments are not the same in the standing or lying positions. When standing up, the utero-sacral ligaments support the most significant stress, while when lying down the round ligaments are the most heavily involved. To our knowledge, this is the first numerical approach showing these results. However, a review of the literature corroborates the involvement of the utero-sacral ligaments in pelvic statics. Iconography and anatomy allowed DeLancey et al. [8, 21, 22] to suppose that utero-sacral ligaments were the major factors in pelvic statics. A study of the comparative anatomy [23] showed that quadrupeds have no ligaments equivalent to utero-sacral ligaments, perhaps because their contribution in this situation may be neglected. They would only play a major role in the upright position, such as for bipeds.
The results of our simulations in pathological situations are consistent with clinical and anatomical data already known. For example, round ligaments are known to maintain the uterus laterally. Their involvement in uterine retroversions and Allen–Masters syndrome is known. Figure 3 shows logically a uterine retroversion after sectioning. Rivaux et al. showed in a recent study that the mechanical stiffness of the ligaments is not symmetrical, i.e. not the same for one pair of ligaments on either side of the utero-vaginal axis [9]. The relative rotation of the uterus on one side or other according to the different rigidity of the round ligament is thus not surprising.
The numerical deletion of ligaments we realised also tended to show that impairment of pelvic statics is not induced by simple degradation of one pelvic sustaining element. Destruction of one ligament, being an extreme situation, could not create a patent prolapse. Our results are corroborated by other authors with totally different approaches, thus proving that the physiopathology of prolapse is extremely complex and aetiologies multifactorial. For instance, an isolated section of a utero-sacral ligament does not create a genital prolapse, underlining the multifactorial origin of genital prolapse, although this ligament probably plays a major role in statics [8, 9]. On the contrary, ligaments involved in pelvic statics change properties in pathological conditions, especially altered pelvic statics [24]. Mengert reported in an experimental study on cadavers that only when the paravaginal tissues have been cut has prolapse developed [25]. This hypothesis was not tested in our study.
The numerical approach to solving health problems is a new and promising tool. The first results provided by simulation are encouraging and comforting in our initiative to develop a model of the pelvic system. Numerical ascertainment agrees with the clinical and anatomic data known. Several studies in our team follow this approach to the understanding of genital prolapse physiopathology and stretch the field to use these simulations. We could in the same way simulate a delivery and localise mechanical tensions or even lesions. But our model must be improved by an anatomically validated pelvic floor, and by several muscles identified by their real and intrinsic mechanical properties. Modelling of the different muscles of the pelvic floor could be more accurate, modifying some muscular tracts and not the complete pelvic floor. We could then master the stresses on our model and their distribution. The software we used did not allow testing of several hypotheses at the same time; ligaments were cut successively and independently. It would be interesting to simulate lesions and combine several situations to obtain real pathophysiological conditions. It is more realistic to study lesions because of ageing or trauma than after complete sectioning in our model. Our model cannot yet show physiologic modifications of scarring, or evolution of tissues under loadings or ageing. It will be difficult to reach that stage. In this last case, it might not be sufficient to damage the system without taking into account prolapsed tissues which are more rigid than non-prolapsed tissue as shown in the literature [15].
Studying normal and pathological pelvic mobility via modelling and 3D simulation is an innovative strategy. With the proposed model, we were able to analyse the involvement of uterine ligaments in pelvic statics. Our results are coherent with clinical, anatomical data, bibliography and anthropology, but must be validated on a larger series of patients. This approach underlines the complex physiopathology of genital prolapse and how multifactorial it is. The role of pelvic ligaments seems to be important, especially for the utero-sacral ligaments and especially in a standing position.
References
Samuelsson E, Victor F, Tibblin G, Svardsudd K (1999) Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol 180:299–305
Swift S (2000) The distribution of pelvis organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol 183:277–285
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark A (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501–506
Diwakar GB, Barber MD, Feiner B, Maher C, Jelovsek JE (2009) Complication and reoperation rates after apical vaginal prolapse surgical repair : a systematic review. Obstet Gynecol 113:367–373
Toh VV, Bogne V, Bako A (2012) Management of recurrent vault prolapse. Int Urogynecol J 23:29–34
Baden WF, Walker TA, Lindsey JH (1968) The vaginal profile. Tex Med 64:56–58
Goh JT (2003) Biomechanical and biochemical assessments for pelvic organ prolapse. Curr Opin Obstet Gynecol 15:391–394
Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JO (2004) Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol 103:447–451
Rivaux G, Rubod C, Dedet B, Brieu M, Gabriel B, Cosson M (2013) Comparative analysis of pelvic ligaments : a biomechanics study. Int Urogynecol J 24:135–139
Vallet A, Brieu M, Rubod C, Cosson M (2011) Simulation of pelvic mobility: topology optimization of ligamentous system. Comp Meth Biomech Eng 14:159–163
Cosson M, Rubod C, Vallet A, Witz JF, Brieu M (2012) Biomechanical modelling of pelvic organ mobility : towards personalized medicine. Bull Acad Natl Med 195:1869–1883
Cosson M, Rubod C, Vallet A, Witz JF, Dubois P, Brieu M (2013) Simulation of normal pelvic mobilities in building an MRI-validated biomechanical model. Int Urogynecol J 24:105–112
Rao GV, Rubod C, Brieu M, Bhatnagar N, Cosson M (2010) Experiments and finite element modeling for the study of prolapse in the pelvic floor system. Comput Methods Biomech Biomed Eng 13:349–357
Rubod C, Brieu M, Cosson M, Rivaux G, Clay JC, De Landsheere L, Gabriel B (2012) Biomechanical properties of human pelvic organs. Urology 79:968.e17–22
Jean-Charles C, Rubod C, Brieu M, Boukerrou M, Fasel J, Cosson M (2010) Biomechanical properties of prolapsed or non prolapsed vaginal tissue: impact on prolapse surgery. Int Urogynecol J Pelvic Floor Dysfunct 21:1535–1538
Gabriel B, Rubod C, Brieu M, Dedet B, De Landsheere L, Delmas V, Cosson M (2011) Vagina, abdominal skin and aponeurosis: do they have similar biomechanical properties? Int Urogynecol J Pelvic Floor Dysfunct 22:23–27
Fung Y (1993) Biomechanics: mechanical properties of living tissues, 2nd edn. Springer, Heidelberg
Avril S, Badel P, Duprey A (2010) Anisotropic and hyperelastic identification of in vitro human arteries from full-field optical measurements. J Biomech 43:2978–2985
Kamina P (1995) Anatomie clinique du petit bassin et périnée. Editions Maloine, Paris
Grédiac M, Toussaint E, Pierron F (2002) Special virtual fields for the direct determination of material parameters with the virtual fields method. Int J Solids Struct 39:2707–2730
Ramanah R, Berger MB, Chen L, Riethmuller D, Delancey JO (2012) See it in 3D : researchers examined structural links between the cardinal and uterosacral ligaments. Am J Obstet Gynecol 207:437.e1–7
Summers A, Winkel LA, Hussain HK, DeLancey JO (2006) The relationship between anterior and apical compartment support. Am J Obstet Gynecol 194:1438–1443
Barone R (2009) Anatomie comparée des mammifères domestiques. Editions Vigot, Paris
Querleu D (1998) Techniques chirurgicales en gynécologie, 2nd edn. Masson, Paris
Mengert WF (1936) Mechanics of uterine support and position. Am J Obstet Gynecol 31:775
Conflict of interest
C. Rubod, M. Brieu, P. Lecomte-Grosbras have declared that they have no conflict of interest. G. Giraudet accepts travel expenses or honoraria from Ethicon. N. Betrouni is a paid consultant of STEBA Biotech. M. Cosson is a consultant, accepts travel expenses or honoraria and accepts payment for research from Ethicon, AMS and Baston.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rubod, C., Lecomte-Grosbras, P., Brieu, M. et al. 3D simulation of pelvic system numerical simulation for a better understanding of the contribution of the uterine ligaments. Int Urogynecol J 24, 2093–2098 (2013). https://doi.org/10.1007/s00192-013-2135-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2135-6