Abstract
Purpose
In the present study, the early results of sensor-assisted versus manually balanced posterior-stabilized total knee arthroplasty (TKA) for osteoarthritis with varus deformities were prospectively compared.
Methods
Fifty patients undergoing sensor-assisted TKA (group S) and 50 patients receiving manually balanced TKA (group M) were prospectively compared. The groups did not differ in terms of demographics, preoperative clinical status, or severity of deformity. The knee and function scores (KS and FS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and range of motion (ROM) were evaluated clinically. The mechanical axes and positions of components were assessed radiographically. In sensor-assisted TKA, the medial and lateral compartment loads were compared based on the patellar positions of inversion and eversion.
Results
There was no between-group difference in the postoperative KS or FS (n.s., respectively). The average postoperative WOMAC score was 17.0 in group S and 18.0 in group M (n.s.). The ROM was 131.2° in group S and 130.8° in group M (n.s.). Neither the postoperative alignment of the mechanical axis nor the component positioning differed between the groups (n.s.). In sensor-assisted TKA, the difference between the medial and lateral compartment loads was less than 15 lbs (6.8 kg) in each knee. The lateral compartment load increased after patellar eversion (p < 0.001).
Conclusion
There are concerns about the cost–benefit ratio of the intraoperative load sensor, despite its advantage of more precisely assessing ligament balance without patellar eversion, which resulted in a smaller lateral gap. A long-term follow-up study with a large cohort is required.
Level of evidence
II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) is performed to create a stable knee exhibiting functional improvement and correction of deformity [15, 17, 25, 31]. Imbalances in the soft tissue create patient dissatisfaction and functional limitations [6, 18, 19, 33]. In addition, unequal loading of the medial and lateral compartments increases the risk for accelerated wear and premature failure of the polyethylene (PE) insert; in fact, unbalanced ligaments are responsible for 35% of early TKA revisions [13]. Although balancing is important, it is often based on subjective surgical assessment, so success requires training, operative experience, and skill [8, 23].
A wireless intraoperative load sensor was created to measure the intercompartmental loads of the trial and final implants placed during TKA [23]. This instrument provides real-time feedback on the tibiofemoral position and the loads at the contact points of both compartments. The sensor data allow the surgeon to correct imbalances in the soft tissue. In the previous studies, the use of a sensor reduced gap imbalances, improved patient-reported clinical outcomes, and reduced the rate of arthrofibrosis [7, 10, 13, 22, 23].
However, these studies have certainly limitations. The study reporting reductions in gap imbalances was performed on cadavers, so the results cannot be generalized to the living [22]. Several studies reporting improved clinical outcomes did not compare sensor-assisted and manually balanced TKA, but rather balanced and unbalanced sensor-assisted TKA [13, 23]. In addition, no study matched patients based on the condition of the knee, which of course affects clinical outcomes; patients were only matched for general demographics such as age, sex, and body mass index (BMI) [7, 10]. For the purpose of cost–benefit analysis, it will be important for orthopedic surgeons to perform the appropriate comparison of postoperative results between sensor-assisted and manually balanced TKA.
The patella is lateralized (with or without eversion) during assessments of transepicondylar axis or mediolateral gap balancing. Such lateralization can affect the intraoperative compartment loads, because the extensor mechanism may act as a lateral tether; thus, balancing when the patella is everted may be inappropriate [29, 32, 35]. In addition, accurate component tracking is impossible when the patella is dislocated [12]. Thus, many surgeons measure the mediolateral gap using a specific offset tension device when the patella is relocated [16, 34]. However, such devices do not read the direct peak load on the tibiofemoral contact point. Only a real or trial implant can reproduce the rotatory movement and conforming joint between the femur and tibia.
The main purpose of the present study was to compare the early clinical and radiographic outcomes of patients with similar preoperative knee conditions undergoing sensor-assisted or manually balanced TKA. The outcomes of sensor-assisted TKA were hypothesized to be similar to or better than those of manually balanced TKA. The other purpose was to use the sensor to evaluate changes in the loads of the medial and lateral compartments when the patella was inverted and everted. It was hypothesized that the position of the patella during TKA would affect the load distributions of the medial and lateral compartments.
Materials and methods
This study was approved by the Institutional Review Board of our hospital (Kyung Hee University hospital; KHUH 06-039). A detailed informed consent form was signed by each patient, and all information was kept confidential.
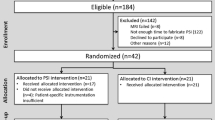
A prospective randomized study was conducted between August and October 2017. In total, 174 patients were recruited during the study period. Patients were included if they received a primary TKA due to degenerative osteoarthritis of Kellgren–Lawrence grade 4 with varus deformities, or a posterior-stabilized (PS) TKA performed with a NexGen-Legacy prosthesis (Zimmer, Warsaw, IN, USA). The exclusion criteria were inflammatory, infectious, or posttraumatic arthritis; a history of knee infection, fracture, dislocation, or ligament injury; knee instability or a history of reconstructive ligament surgery or high-tibial osteotomy; severe coronal deformities (> 20°); and/or severe flexion contracture (> 20°). According to the criteria, 74 patients were excluded and 100 patients were included in the study (Fig. 1). The patients in whom an intraoperative load sensor was to be applied were determined by a randomized number previously obtained from an online number generator [30]. The 50 sensor-assisted TKAs (group S) and 50 manually balanced TKAs (group M) were performed by a senior surgeon. The preoperative demographics and knee conditions were well matched between the two groups (Tables 1, 2).
All primary TKAs were performed with a PS prosthesis by a modified measured resection technique with patellar resurfacing [3]. A medial parapatellar approach was used with a midline skin incision. The transepicondylar axis was used for femoral component rotation. The size of the femoral component was selected by the anterior-referencing method, and efforts were made to reduce the change in posterior condylar offset. The reference line for tibial rotation was accurately aimed at a line passing through the medial third of the tibial tubercle and the second metatarsal or the middle of the talus. All osteophytes were removed and patellae resurfaced. Patellofemoral articulation was carefully evaluated by the no-thumb technique. Meticulous soft-tissue balancing was performed.
Trial implants were placed and stability and knee kinematics were evaluated after the initial balancing. A keeled plastic tibial trial device was used to avoid mismatching of the tibiofemoral contact point between the trial and real implants. Then, the intraoperative load sensor (VERASENSE™; Orthosensor, Dania Beach, FL, USA) was inserted in group S. The VERASENSE™ device is a wireless and disposable tibial trial insert within which a micro-force sensor is embedded. This device can objectively quantify contact pressures across the medial and lateral compartments (Fig. 2a). The geometry of the device replicates that of a trial insert. Shims can be placed under the device to replicate the thickness of the trial knee system. The sensor relays the loading value of each compartment and the femoral contact point position in real time to a display screen. When the sensor device is in place (Fig. 2b), the loading data are graphically displayed as numbers without decimals and are superimposed on a virtual sensor image (Fig. 2c).
VERASENSE™ intraoperative load sensor system. a VERASENSE™ tibial trial insert is shown, embedded with a micro-force sensor to measure contact force and location. There is a post in the sensor device, due to the use of a NexGen-Legacy posteriorly stabilized prosthesis. Shims can be placed under the device to adjust the thickness of the device. b Intraoperative placement of the VERASENSE™ tibial trial sensor device. c VERASENSE™ display screen is shown, presenting the femorotibial contact points and loading forces (in lbs) of the medial and lateral compartments. The real-time balance status can be confirmed through the loading values on the display screen
Real-time changes in the loading values were observed during soft tissue and bony correction. The intercompartmental loads were checked at 10°, 45°, and 90° of knee flexion with the patella everted. The flexion angle was determined with a sterilized metal goniometer. The loads were reevaluated at the same angles after the patella was relocated in the trochlear groove and fixed with two towel clips [14]. For precise evaluation, the load was measured twice at each flexion angle and patella position. The device was re-zeroed before the second measurement to adjust for plastic deformation, which can affect the load measurements. The test–retest differences were under 3 lbs (1.4 kg) for all knees, and the intraclass correlation coefficient of the test–retest values was greater than 0.8. Additional procedures for appropriate balancing were performed in sensor-assisted TKA patients until the loads of the medial and lateral compartments were < 55 (25.0 kg) and 40 lbs (18.1 kg), respectively, and the difference in load between the two compartments was < 15 lbs (6.8 kg) [13, 23, 28] (Fig. 2c). In group M, the assessment of soft-tissue balancing was subjective, and additional procedures were performed until the senior surgeon judged that the balancing was appropriate.
The postoperative rehabilitation protocols were similar for the two groups [1]. Isometric exercises of the extensor and flexor muscles commenced shortly after the operation. Drains were removed on the second postoperative day, followed by the commencement of active and assisted range of motion (ROM) exercises. Full weight-bearing ambulation commenced at 3 days when the patient’s condition permitted.
Clinical data were recorded before the operation and 6 months after the operation. American Knee Society knee and function scores (KS and FS) and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were used to assess pain and function [11]. Flexion contracture and the maximum flexion angle were measured with a long-armed goniometer. Any complication (arthrofibrosis, instability, aseptic loosening, or periprosthetic joint infection) was noted and investigated.
Pre- and postoperative standing anteroposterior (AP) radiographs, lateral radiographs, and orthoroentgenograms (full-length standing AP radiographs) were used to assess limb alignment and component positioning. All radiographs were taken under weight-bearing conditions. The angle of the mechanical axis was that between the femoral and tibial mechanical axes on the orthoroentgenogram [2]. The positions of all femoral and tibial components were carefully analyzed, and the α, β, γ, and δ angles were calculated by the Knee Society radiographic evaluation method [9].
The preoperative knee condition was evaluated radiographically. The joint line height and convergence angle were evaluated on the AP radiograph. The joint line height was defined as the shortest distance between the fibular head and the lateral femoral condyle [4]. The convergence angle was defined as the angle between the tangent to the subchondral plates of the femoral condyle and the tibial plateau [26]. This reflects the natural laxity of lateral soft tissue under weight-bearing conditions, and influences the thickness of the chosen PE insert.
The posterior tibial slope, the posterior femoral condylar offset, and the length of the AP femoral condyle were evaluated on the lateral radiograph. The posterior tibial slope (PSA) was defined as the angle formed by a line perpendicular to the reference line and the medial tibial plateau [26]. The reference line of the PSA was defined as the line connecting the center of the medullary canal 10 and 20 cm distal to the tibial plateau. The posterior femoral condylar offset was defined as the distance between the posterior femoral cortical margin and the posterior margins of the femoral condyle on a true lateral radiograph [21, 27]. The length of the AP femoral condyle was defined as the distance between the anterior femoral cortical margin and the posterior margins of the femoral condyle on a true lateral radiograph [21, 27]. These parameters reflect the flexion gap and the size of the femoral component.
Measurements were made with a picture archiving and communication system (PACS; Infinitt Healthcare, Seoul, South Korea). The sensitivities were 0.1° and 0.1 mm. To minimize any observation bias, two independent investigators repeated all radiographic measurements. The intra- and interobserver reliabilities of all measurements were assessed with the intraclass correlation coefficient. In this study, the intraclass correlation coefficient values of all the measurements were greater than 0.8 for both intra- and interobserver reliability. Thus, the average values were used for the analysis.
The incidence of additional procedures for appropriate balancing (performed after the initial balancing assessment) was determined. The sizes of the femoral and tibial components were noted, as were the thicknesses of all PE inserts.
In group S, the loads of the medial and lateral compartments were recorded at 10°, 45°, and 90° of knee flexion. They were compared according to the patella position (inverted or everted).
Age, sex, and BMI were similar between the two groups (Table 1). The severity of the preoperative knee condition did not differ between the two groups in terms of degrees of varus deformity, soft-tissue status, or expected implant size (Table 2).
Statistical analysis
Pre- and postoperative clinical and radiographic data were compared through paired t tests, and data obtained 6 months after the operation were compared between the two groups through Student’s t test. The χ2 test or Fisher’s exact test was used to compare categorical variables (rate of postoperative complications, incidence of additional procedures, number of implants of various sizes, and thickness of the PE inserts) between the groups. In group S, the loads of the medial and lateral compartments during inversion and eversion of the patella were compared through a paired t test. All statistical analyses were performed in SPSS ver. 20.0 (Chicago, IL, USA), and p < 0.05 was taken to indicate statistical significance.
A power analysis was performed to determine the minimum sample size affording sufficient power, with clinical results as the primary outcome. Clinically acceptable significant differences in KS, FS, WOMAC score, flexion contracture, and maximum flexion were considered to be 5, 10, 5, 5°, and 10°, respectively. The power analysis indicated that more than 40 cases would be required to ensure sufficient power. Consequently, 50 patients were included in group S, and 50 patients were included in group M. In addition, a post-hoc power analysis was performed on the size of the actual implant, with α of 0.05. A power > 80% was considered sufficient, and the variables that were significantly different met this criterion.
Results
Clinical and radiographic results
The KS, FS, WOMAC scores, and ROM improved significantly in both groups after the operation (p < 0.001). The average postoperative KS was 91.0 in group S and 89.4 in group M (n.s.). The average postoperative FS did not differ significantly between the groups (n.s.; Table 3). The average postoperative WOMAC score was 17.0 in group S and 18.0 in group M (n.s.). The flexion contracture, maximum flexion, and ROM did not differ between the two groups (Table 3). There were no complications (arthrofibrosis, instability, aseptic loosening, or periprosthetic joint infection) in either group. The postoperative mechanical axis did not differ significantly between the two groups (Table 4). There were no significant differences in the positions of components (Table 4).
Comparison of the incidence of additional procedures, the sizes of femoral and tibial components, and the thicknesses of PE inserts
Additional procedures were performed more frequently in group S than in group M (p = 0.035; Table 5). The number of real femoral and tibial components of various sizes did not differ between the groups (n.s., respectively). However, thicker PE inserts were used more frequently in group S than in group M (p < 0.001; Table 6).
Loads by patella position in group S
When the patella was everted, the load of the lateral compartment increased at every knee flexion position (p < 0.001; Table 7), but the average load of the medial compartment did not change significantly (Table 7).
Discussion
The most important finding of this study was that the early postoperative outcomes of sensor-assisted TKA were not better than those of manually balanced TKA when patients were matched for both demographics and preoperative knee condition.
Since Gustke et al. [13] reported the promising short-term clinical outcomes of sensor-assisted TKA, their findings have been cited as representative evidence by proponents of intraoperative load sensors. However, the authors did not compare sensor-assisted and manually balanced TKA, but rather balanced and unbalanced groups after sensor-assisted TKA, similar to Meneghini et al. [23].
Several studies have compared the outcomes of sensor-assisted and manually balanced TKA. Chow and Breslauer [7] reported that the clinical scores and ROM were significantly higher after sensor-assisted TKA than after manually balanced TKA in patients who were matched for demographics and had not undergone radiographic evaluation. Geller et al. [10] reported that the use of the sensor significantly reduced the rate of arthrofibrosis. However, in both of these studies, patients were matched only for general demographics, not for their knee condition, which of course significantly affects clinical outcomes.
In the present study, no significant difference was found in the clinical scores or ROM between sensor-assisted and manually balanced TKA groups with similar demographics and preoperative knee conditions, partly because the factors affecting patient satisfaction and functional performance vary widely; thus, improving only the intercompartmental loads may not significantly affect clinical outcomes. In addition, these results may be due to a ceiling effect in the current clinical scoring system [24]. That is, it is possible that the system could not detect minor differences in the balancing accuracies afforded by sensor-assisted and manually balanced TKA. A further study with long-term follow-up using a sensitive clinical scoring system is needed.
Most orthopedic surgeons seek to make the gap space as small as possible, within reason, because thicker PE inserts are associated with higher failure rates [5]. In a cadaveric study, Meere et al. [22] performed sensor-assisted TKA on one side of the knee and manually balanced TKA on the other. Sensor-assisted TKA significantly reduced the mediolateral gap when varus and valgus stress tests were performed at 20° of flexion, which implies that the gap space was smaller after sensor-assisted TKA than after manually balanced TKA. In our in vivo study, the gap suggested by the sensor was significantly larger than the manually balanced gap; group S usually received thicker PE inserts, because additional procedures for appropriate balancing were more frequently performed during sensor-assisted TKA. In a previous study, Elmallah et al. [8] reported that the loads of manually balanced gaps tended to be higher than those of sensor-assisted gaps at 10°, 45°, and 90° of knee flexion. This indicates that the use of a sensor may require the surgeon to perform more additional procedures, which can increase the risk of creating larger gaps than desired. The surgeon in the present study selected thicker inserts, affording accurate balancing within the appropriate range; many TKA surgeons, fearing that instability may trigger early revision, tend to prefer tighter balancing in such situations [28]. Although no clear consensus has emerged on whether minimal-laxity TKA is associated with more successful outcomes, TKA surgeons need to be aware that the gap may increase when a sensor is used.
The cost–benefit ratio is an important issue when new medical devices are designed. The VERASENSE™ sensor was introduced as a low-cost, high-benefit instrument. This device was expected to improve physical function during the recovery period, promote shorter rehabilitation, and reduce the overall costs for TKA patients [7]. The manufacturer of VERASENSE™ reported multicenter data, showing an almost 75% lower rate of revision TKAs than the US average within 2 years postoperatively, and claimed that this reduction conferred clinical and financial benefits to both patients and providers. However, contrary to the previous results, the present study raises concerns regarding the cost–benefit ratio of the intraoperative load sensor, indicating that further studies on the cost–benefit ratio of the sensor are necessary.
The second important finding of this study was that patellar eversion increased the intraoperative load of the lateral compartment at knee flexion angles of 10°, 45°, and 90°, as was also found in a previous study [29, 32]. Potentially, the everted patella and subsequently lateralized extensor mechanism could act as a lateral tether and thus increase the lateral compartment load [29]. Lateral translation and external rotation of the tibia, as well as increased valgus alignment, are additional reasons for the increase in lateral load during patellar eversion [32]. Accordingly, the surgeon may not assess ligament balance accurately due to the non-physiologic everted patella status. When a sensor is used, balance can be assessed with the patella located on the femoral trochlea, whereas traditional devices for assessing balance require the patella to be lateralized or everted. The advantage of using the sensor device is that gap loadings can be measured without patellar eversion, resulting in a smaller lateral gap and a higher lateral compartment load.
The load of the medial compartment decreased when the patella was dislocated (with or without eversion) in the previous study of Schnaser et al. [29]. However, the load of the medial compartment was not influenced by the position of the patella in the present study (Table 6), perhaps because the sensor device had a central post. Schnaser et al. [29] mentioned that the absence of a central post in the load-measuring device was a limitation of their study and that further work using a sensor with a central post was required to evaluate the PS prosthesis. Sculco et al. [32] reported similar results to those of this study, but did not describe the presence of the post. Other studies comparing sensors with and without posts are necessary.
The last issue of this study is the appropriate range of loads when the sensor is used. In the present study, the average load of the medial compartment was greater than that of the lateral compartment during sensor-assisted TKA (Table 7). The loads of the medial and lateral compartments were balanced to within 10–55 (4.5–24.9 kg) and 10–40 lbs (4.5–18.1 kg), respectively, and the loads of the compartments did not differ by > 15 lbs (> 6.8 kg) [13, 23, 28]. Although VERASENSE™ suggests values of 5–40 lbs (2.3–18.1 kg) as the most appropriate loads, we used an arbitrary upper limit of 55 lbs (24.9 kg) for the load of the medial compartment, given that this compartment is more heavily loaded than the lateral compartment in vivo and that similar values were recommended previously [8, 14]. A slightly greater pressure imparted by the medial compartment and a more compliant lateral gap may facilitate optimal tibiofemoral kinematics [20]. In a previous study by Meneghini et al. [23], the validity of the upper limit for the medial compartment load was also supported; the authors reported that the Knee Society Objective Score remained favorable under high medial compartment loads (> 75 lbs; > 34.0 kg), but decreased significantly under high lateral compartment loads (> 75 lbs; > 34.0 kg).
There are several limitations in the present study. First, the number of patients was small, although the statistical power was adequate. The low numbers were due to the need to match patients for both demographics and preoperative knee conditions. Furthermore, the follow-up period to investigate the complications associated with inadequate knees was short. A large cohort study with long-term follow-up is required to confirm our results and prove the durability of sensor-assisted TKA. Second, because we sought to avoid the bias created by confounding variables, all TKAs were performed by a single surgeon using a standardized technique and a single type of prosthesis. Consequently, the results may not be generalizable to other surgeons, techniques, or prostheses. Third, the patients enrolled in the present study had degenerative osteoarthritis with moderate varus deformities of < 20°, without valgus or severe varus deformities. Valgus deformities were excluded to facilitate preoperative matching, because the incidence of such deformities was lower in our population and could have been an outlier with respect to degenerative osteoarthritis in the general population. Severe preoperative deformities were also excluded for preoperative matching. Fourth, the radiographic parameters for preoperative matching were insufficient to represent the overall knee condition. Other elaborate tests that reveal the knee condition more precisely, such as stress radiographs, bone scans, and magnetic resonance imaging studies, could be conducted in further studies to provide more precise results. Finally, changes in compartment loads caused by patellar eversion and tethering of the extensor mechanism vary according to the quadriceps muscle volume, tourniquet pressure, type of anesthesia induced, and use of muscle relaxants. It must be considered when our findings are extrapolated to other situations.
Conclusion
Sensor-assisted TKA could have the advantage of measuring the medial and lateral compartment loads without patellar eversion, allowing a tight lateral gap and increasing the lateral compartment load. The early clinical and radiographic outcomes did not differ between patients undergoing sensor-assisted and manually balanced TKA. There are concerns about the cost–benefit ratio of the intraoperative load sensor, despite its advantage of more precisely assessing the soft-tissue balance without patellar eversion. A long-term follow-up study with a large cohort is required.
References
Bae DK, Baek JH, Yoon KT, Son HS, Song SJ (2017) Comparison of patellofemoral outcomes after TKA using two prostheses with different patellofemoral design features. Knee Surg Sports Traumatol Arthrosc 25(12):3747–3754
Bae DK, Song SJ, Kim KI, Hur D, Jeong HY (2016) Mid-term survival analysis of closed wedge high tibial osteotomy: a comparative study of computer-assisted and conventional techniques. Knee 23(2):283–288
Bae DK, Song SJ, Kim KI, Hur D, Lee HH (2016) Intraoperative factors affecting conversion from cruciate retaining to cruciate substituting in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 24(10):3247–3253
Bae DK, Song SJ, Yoon KH (2010) Total knee arthroplasty following closed wedge high tibial osteotomy. Int Orthop 34(2):283–287
Berend ME, Davis PJ, Ritter MA, Keating EM, Faris PM, Meding JB et al (2010) “Thicker” polyethylene bearings are associated with higher failure rates in primary total knee arthroplasty. J Arthroplast 25(6 Suppl):17–20
Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP et al (2010) The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468(1):45–51
Chow JC, Breslauer L (2017) The use of intraoperative sensors significantly increases the patient-reported rate of improvement in primary total knee arthroplasty. Orthopedics 40(4):e648–e651
Elmallah RK, Mistry JB, Cherian JJ, Chughtai M, Bhave A, Roche MW et al (2016) Can we really “feel” a balanced total knee arthroplasty? J Arthroplast 31(9 Suppl):102–105
Ewald FC (1989) The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res 248:9–12
Geller JA, Lakra A, Murtaugh T (2017) The use of electronic sensor device to augment ligament balancing leads to a lower rate of arthrofibrosis after total knee arthroplasty. J Arthroplast 32(5):1502–1504
Giesinger JM, Hamilton DF, Jost B, Behrend H, Giesinger K (2015) WOMAC, EQ-5D and knee society score thresholds for treatment success after total knee arthroplasty. J Arthroplast 30(12):2154–2158
Gustke K (2012) Use of smart trials for soft-tissue balancing in total knee replacement surgery. J Bone Jt Surg Br 94(11 Suppl A):147–150
Gustke KA, Golladay GJ, Roche MW, Elson LC, Anderson CR (2014) A new method for defining balance: promising short-term clinical outcomes of sensor-guided TKA. J Arthroplast 29(5):955–960
Gustke KA, Golladay GJ, Roche MW, Elson LC, Anderson CR (2017) A targeted approach to ligament balancing using kinetic sensors. J Arthroplast 32(7):2127–2132
Gustke KA, Golladay GJ, Roche MW, Jerry GJ, Elson LC, Anderson CR (2014) Increased satisfaction after total knee replacement using sensor-guided technology. Bone Jt J 96-B(10):1333–1338
Kamei G, Murakami Y, Kazusa H, Hachisuka S, Inoue H, Nobutou H et al (2011) Is patella eversion during total knee arthroplasty crucial for gap adjustment and soft-tissue balancing? Orthop Traumatol Surg Res 97(3):287–291
Kim CW, Lee CR (2018) Effects of femoral lateral bowing on coronal alignment and component position after total knee arthroplasty: a comparison of conventional and navigation-assisted surgery. Knee Surg Relat Res 30(1):64–73
Kwon KT, Han KY, Lee WS, Kim DH (2017) Full cementation in revision total knee arthroplasty using a constrained condylar knee prosthesis with an average 7-year follow-up. Knee Surg Relat Res 29(4):282–287
Lee DH, Lee SH, Song EK, Seon JK, Lim HA, Yang HY (2017) Causes and clinical outcomes of revision total knee arthroplasty. Knee Surg Relat Res 29(2):104–109
Matsumoto T, Muratsu H, Kubo S, Matsushita T, Kurosaka M, Kuroda R (2011) The influence of preoperative deformity on intraoperative soft tissue balance in posterior-stabilized total knee arthroplasty. J Arthroplast 26(8):1291–1298
Matz J, Howard JL, Morden DJ, MacDonald SJ, Teeter MG, Lanting BA (2017) Do changes in patellofemoral joint offset lead to adverse outcomes in total knee arthroplasty with patellar resurfacing? A radiographic review. J Arthroplast 32(3):783–787 e781
Meere PA, LaMont JG, Baez J, Kang MN, Rasquinha VJ, Anderson CR et al (2015) Surgeon assessment of gapping versus kinetic loading using intraoperative sensors during TKA. Reconstr Rev 5(3):29–34
Meneghini RM, Ziemba-Davis MM, Lovro LR, Ireland PH, Damer BM (2016) Can intraoperative sensors determine the “target” ligament balance? Early outcomes in total knee arthroplasty. J Arthroplast 31(10):2181–2187
Na SE, Ha CW, Lee CH (2012) A new high-flexion knee scoring system to eliminate the ceiling effect. Clin Orthop Relat Res 470(2):584–593
Noble PC, Conditt MA, Cook KF, Mathis KB (2006) The John Insall Award: patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res 452:35–43
Park CH, Bae DK, Kim KI, Lee JW, Song SJ (2017) Serial changes in the joint space width and joint line convergence angle after closed-wedge high tibial osteotomy. Am J Sports Med 45(14):3254–3261
Pierson JL, Ritter MA, Keating EM, Faris PM, Meding JB, Berend ME et al (2007) The effect of stuffing the patellofemoral compartment on the outcome of total knee arthroplasty. J Bone Jt Surg Am 89(10):2195–2203
Risitano S, Karamian B, Indelli PF (2017) Intraoperative load-sensing drives the level of constraint in primary total knee arthroplasty: surgical technique and review of the literature. J Clin Orthop Trauma 8(3):265–269
Schnaser E, Lee YY, Boettner F, Gonzalez Della Valle A (2015) The position of the patella and extensor mechanism affects intraoperative compartmental loads during total knee arthroplasty: a pilot study using intraoperative sensing to guide soft tissue balance. J Arthroplast 30(8):1348–1353.e1343
Scott DF, Smith RR (2014) A prospective, randomized comparison of posterior stabilized versus cruciate-substituting total knee arthroplasty: a preliminary report with minimum 2-year results. J Arthroplast 29(9 Suppl):179–181
Scott WN, Diduch DR, Long WJ (2018) Insall & Scott surgery of the knee, vol 2, 6th edn. Elsevier, Philadelphia, pp 1766–1769
Sculco P, Gruskay J, Nodzo S, Carrol K, Shanaghan K, Haas S et al (2018) The role of the tourniquet and patella position on the compartmental loads during sensor-assisted total knee arthroplasty. J Arthroplast 33(7S):S121–S125
Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J (2014) Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplast 29(9):1774–1778
Yoon JR, Oh KJ, Wang JH, Yang JH (2015) Does patella position influence ligament balancing in total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc 23(7):2012–2018
Yoshino N, Watanabe N, Watanabe Y, Fukuda Y, Takai S (2009) Measurement of joint gap load in patella everted and reset position during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 17(5):484–490
Funding
No external funding was used.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procecures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and national reareach committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of our hospital.
Informed consent
A informed consent form was signed by each patient.
Rights and permissions
About this article
Cite this article
Song, S.J., Kang, S.G., Lee, Y.J. et al. An intraoperative load sensor did not improve the early postoperative results of posterior-stabilized TKA for osteoarthritis with varus deformities. Knee Surg Sports Traumatol Arthrosc 27, 1671–1679 (2019). https://doi.org/10.1007/s00167-018-5314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5314-7