Abstract
Purpose
The primary aim of the study was to examine stability and alignment after total knee arthroplasty (TKA) using patient-specific instrumentation (PSI) and conventional instrumentation (CI). The hypothesis was that stability and alignment would be better using PSI than CI, 12 months postoperatively. The secondary aim included the evaluation of clinical outcomes after TKA.
Methods
In this prospective randomized controlled trial, 42 patients with knee osteoarthritis received a Genesis II PS prosthesis with either PSI or CI. Patients visited the hospital preoperatively and postoperatively after 6 weeks and 3 and 12 months. To evaluate stability, varus–valgus laxity was determined in extension and flexion using stress radiographs 12 months postoperatively. Three months postoperatively, a long-leg radiograph and CT scan were obtained to measure hip–knee–ankle (HKA) alignment and component rotation. Furthermore, frontal and sagittal alignment of the components, the Knee Society Score, VAS Pain, VAS Satisfaction, Knee injury and Osteoarthritis Outcome score, Patella score (Kujala), University of California Los Angeles activity score, anterior–posterior laxity, (serious) adverse device-related events, and intraoperative complications were reported. The clinical outcomes were compared using independent t tests or non-parametric alternatives, and repeated measurements ANOVA with a significance level of p < 0.05.
Results
No significant differences were found between the two groups regarding stability, HKA angle, and rotational alignment. In four patients, the PSI did not fit correctly on the tibia and/or femur requiring intraoperative modifications. Both groups improved significantly over time on all clinical outcomes, with no significant differences between the groups 12 months postoperatively. The PSI group showed less tibial slope than the patients in the CI group [PSI 2.6° versus CI 4.8° (p = 0.02)]. Finally, the PSI group more frequently received a thinner insert size than the CI group (p = 0.03).
Conclusions
Patients operated with PSI did not differ from CI in terms of stability and alignment. However, in the PSI group ligament releases were more often required intraoperatively. Furthermore, the two methods did not show different clinical results. It seems that the preoperative planning for the PSI facilitates more conservative bone cuts than CI, but whether this is clinically relevant should be investigated. Since PSI is more expensive and time consuming than CI, and does not outperform CI with regard to clinical results, we recommend to use CI.
Level of evidence
I.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although operating techniques and prosthesis designs in total knee arthroplasty (TKA) are improving and the survival rate has increased, early implant failure still occurs after TKA [10, 13, 22]. A major cause of disappointing results is incorrect positioning of the tibial or femoral components [5, 18, 19]. To achieve better implant positioning, patient-specific instrumentation (PSI) has been introduced as an alternative for conventional instrumentation (CI). Although this new technique offers interesting opportunities for TKA, there is still no consensus in the literature regarding the effectiveness of PSI in comparison to CI. A recently published review concluded that more studies focusing on rotational alignment are required to determine the effect of PSI on rotational alignment [17].
In addition to correct (rotational) alignment, soft-tissue balancing is essential to achieve stability and proper kinematics after TKA [12, 25, 26]. We currently use a balanced-gap technique with determination of the femur rotation by a tensor in flexion as described by Luyckx et al. [15]. This technique has demonstrated accurate femur component rotation and excellent stability in flexion after TKA [8, 15]. Therefore, the standard TKA procedure with CI in our hospital is a combination of bone-referenced and gap-balancing technique. Although PSI is a bone-referenced method, based on images obtained with an MRI or CT scan, PSI might have a positive effect on knee stability due to the pursued perfect bone cuts of the tibial and femoral components. As a result, PSI might obtain similar or even better stability compared to CI. However, to our knowledge, data concerning the role of PSI in soft-tissue balancing in TKA are not yet available.
Therefore, the primary objective of the present randomized controlled trial was to investigate the stability and alignment of the knee in patients receiving a TKA using PSI compared with CI. Stability was operationalized as the varus–valgus laxity in flexion and extension, and alignment as the hip–knee–ankle (HKA) angle and rotational alignment. The hypothesis was that the PSI group would achieve better alignment and stability than the patients operated with CI. The secondary objective was to compare the radiological, clinical, and functional outcomes between the PSI and CI groups.
Materials and methods
This study was a single-center, patient-blinded, randomized controlled trial performed in the Department of Orthopedics, Sint Maartenskliniek, Nijmegen, The Netherlands. Block randomization lists were computer-generated by an independent researcher; block size was ten with equal allocations to the PSI and CI groups. The randomization sequence was concealed to the patient prior to enrollment until 12 months postoperatively.
The hospital’s institutional review board and the Medical Ethical Review Board of Slotervaart and Reade (NL32953.048.11) approved the study protocol. The trial is registered under the number NTR3585 at the Dutch trial register (The Netherlands trial register; http://www.trialregister.nl). All participants signed written informed consent.
Participants
Patients with non-inflammatory knee osteoarthritis scheduled for a unilateral primary TKA were assessed for eligibility in this study. Patients were included if they were 40–70 years of age at the time of inclusion, had no large deformities (knee flexion < 90°, fixed flexion > 10°, non-correctable varus/valgus > 10°, extension deficits > 30°), and had sufficient femoral and tibial bone stock. Exclusion criteria were as follows: a BMI > 35; rheumatoid arthritis; active, local, or systemic infections; previous hip and/or knee replacement in the last 6 months; sensitive to materials in the implants and/or cutting blocks; and contra-indications for MRI.
An independent research nurse provided eligible patients, who were scheduled for a TKA by one of the three participating surgeons (K.C.D., G.G.v.H., A.B.W.), with verbal information and a patient information sheet about the study. All patients underwent a preoperative MRI and a unilateral AP long-leg radiograph. If the MRI was successfully obtained, the patient was randomized into either having TKA with PSI or CI.
Intervention
The MRIs of the patients allocated to the PSI group were sent to the manufacturer (Smith & Nephew) who developed a preliminary surgical plan. The surgical plan included the level of resection and the alignment and size of the femoral and tibial components according to predetermined default settings. The settings included neutral varus/valgus alignment based on the mechanical axis, standard distal femoral resection and proximal tibial resections for a 9 mm insert thickness, 4.0° external femoral component rotation based on the posterior condyles, and a posterior tibial slope of 3.0°. In eight patients, the MRI could not be used to produce the PSI due to movement artifacts, and these patients were excluded.
Patients received a Genesis II PS (Smith & Nephew, Memphis, Tennessee), posterior-stabilized, fixed-bearing, cemented TKA with patellar resurfacing. Three experienced orthopedic knee surgeons familiar with the Genesis II PS performed all procedures. Depending on randomization, the orthopedic surgeons used either the PSI (Visionaire, Smith & Nephew) or the CI (standard instrumentation Genesis II PS, Smith & Nephew). The CI included intramedullary femoral and extramedullary tibial guides and a ligament tensor. The CI aimed at restoring a neutral mechanical axis of the leg: in extension, the preparation of the tibia and femur was bone-referenced and in flexion the anterior and posterior femoral cuts were ligament-guided using a ligament tensor.
Pre- and postoperative management of the patients with regard to drug treatment, rehabilitation protocol, and discharge was carried out according to the standard practice of the hospital [20].
Outcomes
Demographic characteristics and disease-related data were collected preoperatively. Perioperative data included the operation duration (time from first incision until closing the skin), estimated blood loss, ligament releases, component sizes, and intraoperative complications. Postoperatively, the patients visited the hospital after 6 weeks and 3 and 12 months.
Varus–valgus laxity was determined in extension and flexion on stress radiographs, 12 months postoperatively. Varus and valgus stress were applied in extension with the aid of a Telos device (Fa Telos, Medizinisch-Technische GmbH, Griesheim, Germany; 15 Nm load). When the knee was in 70° flexion, a custom-made apparatus was used [9], also with a 15 Nm load. Preoperatively and 3 months postoperatively, an AP radiograph of the full lower extremity was taken to determine the hip–knee–ankle angle (HKA; mechanical axis). Furthermore, a CT scan to evaluate the femoral and tibial component rotation was obtained 3 months postoperatively. The rotational alignment of the femoral component and the tibial component was measured according to the protocol by Berger [3, 4]. Furthermore, frontal and sagittal alignment of the femoral and tibial components and radiolucency (> 2 mm) were scored using conventional radiographs at 12 months [7]. Radiographs and CT scans were evaluated using IMPAX software (Agfa Healthcare, Mortsel, Belgium), and measurements were made to the nearest 0.1°. The repeatability of varus–valgus laxity measurements was investigated earlier by Heesterbeek et al. [9] and showed values ranging between 0.6° and 0.9°. One researcher, blinded to group allocation, performed all radiological measurements.
Clinical and functional outcomes were scored, preoperatively and at the three follow-up visits, with the Knee Society Score (KSS), VAS pain, VAS Satisfaction, Knee injury and Osteoarthritis Outcome score (KOOS), Patella score (Kujala), and the University of California Los Angeles activity score (UCLA). Furthermore, the anterior–posterior laxity was measured with a rolimeter (Aircast Europe, Neubeuern, Germany) in 20° and 90° knee flexion. All (serious) adverse device events that occurred during the study period were recorded. An independent research nurse performed all clinical assessments.
Statistical analysis
Data were analyzed according to the intention-to-treat principle; the groups were analyzed exactly as randomized. Per-protocol analyses, with patients analyzed as treated, were also performed because four patients did not receive the allocated treatment. Since there were no differences between the intention-to-treat and per-protocol analyses, results based on the intention-to-treat principle were reported unless mentioned otherwise. The varus–valgus laxity, HKA angle, component rotation, baseline and operative characteristics, and radiological and clinical outcomes after 12 months were compared between the two groups using independent t tests, and Mann–Whitney U or Chi2 test if the t test assumptions were violated. The changes in clinical outcomes between the PSI and CI groups, at baseline and postoperative follow-up moments (6 weeks and 3 and 12 months), were assessed by a repeated measures ANOVA. Data analysis was performed with the statistical package STATA 13.0 (StataCorp, College Station, Texas). A p value < 0.05 was considered statistically significant.
The sample size calculation was based on the smallest clinically significant difference in a varus–valgus laxity of 3.0° and a standard deviation of 3.0°. With a two-tailed significance level of 5% and a power of 90%, the required sample size was 42 patients, 21 patients per group.
Results
From 2012 to 2013, the patients were recruited to participate in the study. Figure 1 presents a flow-chart of the allocation of the participants to the two intervention groups.
Baseline and Surgery characteristics
Demographics, disease-related data, and clinical and functional outcomes did not differ significantly between the groups at baseline (Table 1). In four patients, the PSI did not fit correctly on the tibia and/or femur, and intraoperative modifications were needed. The incorrect fits included insufficient resection of tibia and femur, too much varus and slope in the tibia, too much internal rotation in the tibia and incorrect size of the femur, and incorrect size of the tibia.
The mean operative time was 66 ± 15 min for the PSI group and 68 ± 10 min for the CI group (n.s). There were no differences in blood loss and approach (medial parapatellar). The number of ligament releases was higher for the PSI group (n = 7) compared to the CI group (n = 1; p = 0.02). Furthermore, the patient in the PSI group received a thinner insert more often than the patients in the CI group (p = 0.03; Table 2).
Radiological outcomes
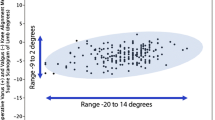
Varus–valgus laxity demonstrated no significant differences between the PSI and CI groups in both flexion and extension (Fig. 2). Additionally, there were no differences between the two groups with respect to the HKA angle and femoral and tibial component rotation (Table 3). Of the measurements taken on the conventional radiographs, sagittal alignment of the tibial component differed between the two groups (p = 0.02). No radiolucency > 2 mm was observed.
Box plot of the measured varus laxity in extension (n.s.), valgus laxity in extension (n.s.), varus laxity in 70° flexion (n.s.) and valgus laxity in 70° flexion (n.s.) 12 months postoperatively for the patient-specific instrumentation (PSI) and conventional instrumentation (CI) groups. The horizontal line in the box indicates the median and the size of the box indicates the 25–75th percentiles, where the whiskers and dots represent the 0–25th and 75–100th percentiles
Clinical and functional outcomes
The two groups significantly improved over time on all clinical and functional outcomes (p < 0.001), but were not different between the groups 12 months postoperatively (Table 3). It appeared that the PSI group scored higher on the KSS clinical subscale than the CI group 6 weeks postoperatively, with a median score of 90 (range 56–95) in the PSI group compared to a score of 65 (range 27–99) in the CI group (p = 0.02). The difference between the two groups occurred in the pain question of the KSS clinical subscale. This difference did not continue, at 3 and 12 months postoperatively (Fig. 3).
Box plot with the Knee Society Score (KSS) clinical subscale at baseline and the three follow-up moments for the patient-specific instrumentation (PSI) and conventional instrumentation (CI) groups. The horizontal line in the box indicates the median, and the size of the box indicates the 25–75th percentiles, where the whiskers and dots represent the 0–25th and 75–100th percentiles. A higher score on the KSS clinical subscale indicates better clinical outcomes
The number and type of (serious) adverse device events were comparable in both groups; three patients had knee flexion problems and underwent manipulation under anesthesia (2 PSI vs. 1 CI), three patients had extension deficits (1 PSI vs. 2 CI), and two patients had prolonged effusion of the knee whereby an infection was excluded (1 PSI vs. 1 CI). No reoperations were performed in the study population.
Discussion
The main finding of this randomized controlled trial was that varus–valgus laxity in flexion and extension, HKA, and rotational alignment after TKA did not differ between PSI and CI. Furthermore, clinical and functional outcomes did not differ 12 months postoperatively between the two groups. The only differences between the groups were less tibial slope and more frequent use of a thinner insert in the PSI group than in the CI group.
Both PSI and CI methods could achieve correct component positioning, as evidenced by comparable laxity and component rotation. In contrast to what one might expect, we did no observe improved stability in flexion with the balanced-gap technique. However, ligament releases were needed more frequently in the PSI than in the CI group to obtain balanced knees. Probably, the ligament releases contributed to the stability of the knees when using a bone-referenced method. The laxity values found in both the PSI and CI groups fell within the previously reported laxity ranges in literature [8].
The tibial slope and insert thickness in the PSI group suggest that the cutting blocks facilitated the preparation of the bone as planned preoperatively. The PSI preoperative surgery plan resulted in more conservative bone cuts compared with the CI bone cuts. Conservative bone cuts might be beneficial in the long term, considering the fact there is more bone available when a revision is required. In their study, Huijbregts et al. reported more outliers in the tibial slope for the PSI than for the CI group [11]. This difference might be explained by the use of an MRI scan in our study vs. the CT scan to fabricate PSI in the study of Huijbregts et al. [1].
As expected, clinical and functional outcomes improved significantly after surgery. Although the sample size in this study is rather low to detect differences in clinical and functional outcomes, the results are in line with those reported in literature [2, 6, 16, 24]. Interestingly, 6 weeks postoperatively the clinical subscale of the KSS was significantly higher in the PSI than in the CI group. This result was driven by less pain reported in the PSI group compared to the CI group. A potential explanation for this short-term benefit of PSI is that no intramedullary rod is used when preparing the femur. The difference in the KSS clinical subscale did not persist at 3 and 12 months.
One of the major advantages of PSI would be a shorter surgical time [23]. Although the per-protocol analysis showed a difference of 7 min in favor of the PSI group, this difference was neither significant nor clinically relevant. The necessary perioperative corrections in the PSI group reduced the mean difference in operative time by 2 min (intention-to-treat analysis). In one out of five patients, PSI was insufficient, which is in line with work by others. This emphasizes the need for improved PSI development procedures to reduce failure of custom-made cutting blocks [14, 21].
Preoperative planning and production of PSI require an MRI of sufficient quality. Sixteen percent of the patients could not be included in the study due to movement artifacts on the MRI image. The preoperative MRI comes at additional costs and effort, since it is not a standard procedure. Another limitation of the use of PSI in clinical practice is the additional time needed to develop and fabricate the PSI. This requires 6 weeks, delaying the planning between TKA indication and surgery. Considering the additional costs and delay in the light of the obtained clinical outcomes in the present study, PSI does not offer advantages above CI in TKA in patients without large deformities.
Certain limitations of this study should be noted. First, the results may represent specific issues with one manufacturer and may not be representative for all PSI technologies. Second, the sample size of the present study was based on the radiological outcomes. Although the statistical power was sufficient to answer the primary research question, the study may have been underpowered to detect differences in clinical and functional outcomes. Finally, due to logistic reasons it was not possible to blind the research nurse, which might have biased the clinical assessments. However, the researcher performing the radiological measurements (primary outcome) was blinded. Nonetheless, the current study is the first randomized controlled trial reporting on stability after TKA using PSI and CI. Results show no clinical and radiological difference and similar stability. PSI therefore does not seem to have a benefit in clinical practice.
Conclusion
PSI does not differ from CI with respect to stability and (rotational) alignment compared with measured resection CI with a gap-balancing technique in flexion. However, in the PSI group ligament releases were more often required intraoperatively. Radiological, clinical, and functional results were not different between the two groups 12 months postoperatively. However, it seems that due to the preoperative planning the PSI facilitated more conservative bone cuts than the CI, reflected by a less tibial slope and thinner inserts.
References
An VVG, Sivakumar BS, Phan K, Levy YD, Bruce WJM (2017) Accuracy of MRI-based vs. CT-based patient-specific instrumentation in total knee arthroplasty: a meta-analysis. J Orthop Sci 22:116–120
Anderl W, Pauzenberger L, Kölblinger R, Kiesselbach G, Brandl G, Laky B, Kriegleder B, Heuberer P, Schwameis E (2014) Patient-specific instrumentation improved mechanical alignment, while early clinical outcome was comparable to conventional instrumentation in TKA. Knee Surg Sports Traumatol Arthrosc 24:102–111
Berger RA, Crossett LS, Jacobs JJ, Rubash HE (1998) Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res 356:144–153
Berger RA, Rubash HE, Seel MJ, Thompson WH, Crossett LS (1993) Determining the rotational alignment of the femoral component in total knee arthroplasty using the epicondylar axis. Clin Orthop Relat Res 286:40–47
Choong PF, Dowsey MM, Stoney JD (2009) Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty 24:560–569
Goyal T, Tripathy SK (2016) Does patient-specific instrumentations improve short-term functional outcomes after total knee arthroplasty? A systematic review and meta-analysis. J Arthroplasty 31:2173–2180
Gromov K, Korchi M, Thomsen MG, Husted H, Troelsen A (2014) What is the optimal alignment of the tibial and femoral components in knee arthroplasty? Acta Orthop 85:480–487
Heesterbeek PJC, Keijsers NLW, Wymenga AB (2010) Ligament releases do not lead to increased postoperative varus–valgus laxity in flexion and extension: a prospective clinical study in 49 TKR patients. Knee Surg Sports Traumatol Arthrosc 18:187–193
Heesterbeek PJC, Verdonschot N, Wymenga AB (2008) In vivo knee laxity in flexion and extension: a radiographic study in 30 older healthy subjects. Knee 15:45–49
Hossain F, Patel S, Haddad FS (2010) Midterm assessment of causes and results of revision total knee arthroplasty. Clin Orthop Relat Res 468:1221–1228
Huijbregts HJTAM, Khan RJK, Fick DP, Hall MJ, Punwar SA, Sorensen E, Reid MJ, Dalle Vedove S, Haebich S (2016) Component alignment and clinical outcome following total knee arthroplasty; a randomised controlled trial comparing an intramedullary alignment system with patient-specific instrumentation. Bone Jt J 98:1043–1049
Insall JN, Binazzi R, Soudry M, Mestriner LA (1985) Total knee arthroplasty. Clin Orthop Relat Res 192:13–22
Le DH, Goodman SB, Maloney WJ, Huddleston JI (2014) Current modes of failure in TKA: infection, instability, and stiffness predominate. Clin Orthop Relat Res 472:2197–2200
Levy YD, An VVG, Shean CJW, Groen FR, Walker PM, Bruce WJM, Walker PM (2016) The accuracy of bony resection from patient-specific guides during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25:1678–1685
Luyckx T, Peeters T, Vandenneucker H, Victor J, Bellemans J (2012) Is adapted measured resection superior to gap-balancing in determining femoral component rotation in total knee replacement? J Bone Jt Surg Br 94:1271–1276
Mannan A, Akinyooye D, Tr F, Hossain F (2016) A meta-analysis of functional outcomes in patient-specific instrumented knee arthroplasty. J Knee Surg 1:2–8
Mannan A, Smith TO, Sagar C, London NJ, Molitor PJA (2015) No demonstrable benefit for coronal alignment outcomes in PSI knee arthroplasty: a systematic review and meta-analysis. Orthop Traumatol Surg Res 101:461–468
Ritter MA, Davis KE, Meding JB, Pierson JL, Berend ME, Malinzak RA (2011) The effect of alignment and BMI on failure of total knee replacement. J Bone Jt Surg Am 93:1588–1596
Ritter MA, Faris PM, Keating M, Meding JB (1994) Postoperative alignment of total knee replacement: its effect on survival. Clin Orthop Relat Res 299:153–156
Schimmel JJP, Defoort KC, Heesterbeek PJC, Wymenga AB, Jacobs WCH, van Hellemondt GG (2014) Bicruciate substituting design does not improve maximal flexion in total knee arthroplasty a randomized controlled trial. J Bone Jt Surg Am 96:1–8
Victor J, Dujardin J, Vandenneucker H, Arnout N, Bellemans J (2014) Patient-specific guides do not improve accuracy in total knee arthroplasty: a prospective randomized controlled trial. Clin Orthop Relat Res 472:263–271
Victor J, Ghijselings S, Tajdar F, Van Damme G, Deprez P, Arnout N, Van Der Straeten C (2014) Total knee arthroplasty at 15–17 years: does implant design affect outcome? Int Orthop 38:235–241
Vide J, Pinto T, Acácio F, Henrique R (2017) Patient-specific instrumentation in total knee arthroplasty: simpler, faster and more accurate than standard instrumentation—a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 25:2616–2621
Vundelinckx BJ, Bruckers L, De Mulder K, De Schepper J, Van Esbroeck G (2013) Functional and radiographic short-term outcome evaluation of the Visionaire system, a patient-matched instrumentation system for total knee arthroplasty. J Arthroplasty 28:964–970
Whiteside LA (2002) Soft tissue balancing: the knee. J Arthroplasty 17:23–27
Zalzal P, Papini M, Petruccelli D, de Beer J, Winemaker MJ (2004) An in vivo biomechanical analysis of the soft-tissue envelope of osteoarthritic knees. J Arthroplasty 19:217–223
Acknowledgements
The authors wish to thank the research nurses Saskia Susan for her effort in patient recruitment and Jolanda Rubrech-van As for data management.
Author information
Authors and Affiliations
Contributions
PH, JS, GvH, AW, and KD created the concept and design of the study. JS managed the acquisition of data. NK and PH analyzed the data and wrote the draft of the full manuscript. All the authors critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The institution received funding from Smith & Nephew to pay for staff and materials. Smith & Nephew had no role in the design or conduct of the study, the collection, management, analyses, and interpretation of the data, or the preparation and review of the manuscript.
Funding
This study was funded by Smith & Nephew.
Ethical approval
The hospital’s investigational review board and the Medical Ethical Review Board of Slotervaart and Reade (NL32953.048.11) approved the study protocol.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kosse, N.M., Heesterbeek, P.J.C., Schimmel, J.J.P. et al. Stability and alignment do not improve by using patient-specific instrumentation in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 26, 1792–1799 (2018). https://doi.org/10.1007/s00167-017-4792-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4792-3