Abstract
In the present study, a hydroponic experiment was conducted to investigate the oxidative stress and the copper (Cu) accumulation in grapevines exposed to three Cu levels (0, 5, and 15 µM) for 1, 2, and 3 days. The results showed that the root elongation was stunted at the highest-exposure concentration. The Cu accumulation in the grapevines increased with increasing Cu treatments, while the other nutrient elements (Ca, Mg and K) absorbed by the grapevines decreased. Most of the Cu taken up by the grapevines was accumulated in the roots. Compared to the data for 1 day after treatment, the Cu-addition significantly decreased the Mg and K concentration in the roots and leaves, yet increased the superoxide dismutase activity in the leaves after 3 days of treatment. For the reactive oxygen species, the malondialdehyde increased with increasing Cu levels in the roots and leaves; however, both the Cu-addition and exposure duration reduced the H2O2 level in the root. Additionally, the Cu-induced accumulation of ·O2− and H2O2 in the grapevine leaves can be observed by the histochemical staining of nitroblue tetrazolium and diaminobenzidine, respectively. In conclusion, the present results indicate that excess Cu results in a change of the root morphology and leads to oxidative stress for the grapevine leaves and roots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Copper (Cu) is a micronutrient to plants; however, excess accumulation of this metal may cause plant toxicity by affecting negatively biochemical and physiological processes (Tiecher et al. 2016; Ambrosini et al. 2018; Ferreira et al. 2018). The background Cu level in natural topsoil is reported to as about 5.0 mg kg−1. In vine-growing areas of the world, however, Cu-based fungicides (e.g., Bordeaux mixture) have been frequently applied for preventing foliar diseases, thus leads to increased Cu level in vineyard soils (Tiecher et al. 2017; Ferreira et al. 2018). For example, it has been reported that the vineyard soils can contain as high as 3200 mg kg−1 of Cu in Southern Brazil, which surpasses the background level by 640 times (Mirlean et al. 2007). At the individual level, high Cu concentration in grapevine may result in growth inhibition, reduction of root elongation, increased root diameter, darkening and thickening of the roots, root-tip swelling and abnormal branching, and leaf chlorosis (Juang et al. 2012; Tiecher et al. 2016, 2018; Ambrosini et al. 2018). At the cellular level, the excess of Cu may cause damage to the cell membranes and oxidative stress of grapevines (Miotto et al. 2014; Tiecher et al. 2016, 2018). The bioavailability of metals in soils may be influenced by several geochemistry parameters including soil pH, texture, water content, dissolved organic matter, and cations and anions concentrations in soils. In our published studies, Cu accumulation and translocation in grapevines was significantly affected by the coexisting cations such as magnesium (Mg2+) (Juang et al. 2014) and calcium (Ca2+) (Chen et al. 2013). The competition of these cations with Cu on biotic ligands in roots was reported to alleviate the phytotoxicity of Cu to grapevines. For a better understanding of ecotoxicological effects of Cu, therefore, it is necessary to investigate not only the fate and transport of Cu, but also the geochemistry parameters in soil-grapevine ecosystems.
Owing to direct contact with soils, plant roots have generally been recognized as the bioindicator of metal phytotoxicity. Several previous studies thus employed root elongation as endpoints while constructing the dose–response relationship of grapevines exposed to Cu (Juang et al. 2011, 2012; Cambrolle et al. 2015). In practice, however, a direct observation of root elongation is quite difficult. In recent years, some studies tried to relate the excess formation of reactive oxygen species (ROS) in leaves, such as superoxide (O2−) and hydrogen peroxide (H2O2), to the oxidative stress of Cu in grapevines (Tiecher et al. 2017, 2018; Ferreira et al. 2018). An increase of ROS in plants may result in the activation of the antioxidant enzyme system; therefore, the production of protective enzymes, such as superoxide dismutase (SOD) and catalase, was also utilized as biomarkers for the Cu toxicity of grapevines.
From the microscopic point of view, the cytotoxic effects of Cu on plant roots have also been researched in several previous studies. Observed previously for grass plants exposed to excessive Cu, the main histological changes in root tissues include strong cell vacuolization, damage in epidermal cells, plasmolysis (a phenomenon whereby the cells among the cortex were obviously enlarged), and cell rupturing of the rhizodermis and outer cortex (Liu and Kottke 2004; Kopittke et al. 2008; Rossini Oliva et al. 2010). In our published work (Juang et al. 2012), histological changes in the grapevine rhizodermal cells were also found when exposed to excessive Cu. For a better understanding of intracellular fate and the transport of Cu in grapevine cells, however, it is necessary to add ultrastructural study and micro-morphometric analysis in the evaluation of the phytotoxic effects of Cu, especially in regions of the grapevine not showing any visible symptoms.
Considering these factors, the aim of this study was to conduct a hydroponic experiment to investigate the root morphology, copper accumulation, and oxidative stress in tissue-cultured grapevine seedlings exposed to elevated Cu levels (0, 5, and 15 µM) for 1, 2, and 3 days. These concentrations were used because some toxicity signs such as increased vacuolization and plasmolysis occurred at an exposure Cu concentration greater than 10 µM (Juang et al. 2012). The nitroblue tetrazolium (NBT) and 3′,3′-diaminobenzidine (DAB) were employed as chromogenic substrates to observe the formation of superoxide and hydrogen peroxide in leaves treated with different Cu levels.
Materials and Methods
The annual shoots of Kyoho grapevines (Vitis vinifera L.) were collected from vine-growing areas in central Taiwan and transferred to the laboratory. Each shoot was divided into several cuttings so that each cutting contained three nodes and spurs. One end of each grapevine cutting was placed in distilled water for 30 days until the spurs had enough leaves, and the axillary buds were utilized as tissue cultures. When the axillary bud explants are rooting and shooting in vivo, they can be transplanted into a peat moss mixture (Plantaflor® Blocking Substrate) and acclimated in vitro for 30 days. The main nutrients composition of the mixture included about 205 mg L−1 N, 240 mg L−1 P2O5, and 255 mg L−1 K2O.
The young plants with three new leaves were used for the experiment. Grapevine roots were pruned to 5 cm and transplanted into a 0.7 L plastic cup filled with 10% modified Hoagland solution for 2 days. The plants were exposed to three Cu levels (0, 5, and 15 μM) for 1, 2, and 3 days. Treatment levels of 5 and 15 μM Cu are equal to relative Cu concentrations of 0.32 and 0.96 µg L−1 in solution, respectively. The treatment solution was renewed every day. One cutting was placed in a cup containing the treatment solution. Each experimental unit consisted of six plants as replicates per treatment. The experiment was conducted in growth chambers with fixed temperatures and relative humidity. The light cycle was 16:8 light:dark. The test media were aerated throughout the experiment.
The grapevine roots from each test-solution-set were photographed after the experiment and transferred into electronic files. Then, the roots, stems, and leaves of the grapevine plants were separately harvested and thoroughly washed with distilled water. The tissue samples were oven-dried at 75°C for 72 h, and the dry-weights of the plant tissues were recorded between 0.01 and 0.2 g. Plants were ground and digested with HNO3/HClO4 (4:1 v/v), and the Ca, Mg, K, and Cu concentrations both in the plants and in the hydroponic medium were then determined with a flame atomic absorption spectrophotometer (Thermo scientific iCE 3000). All chemical analyses were performed in duplicates. Analytical quality control was achieved by digesting and analyzing identical amounts of standard reference material of tomato leaves (SRM 1573a). Recovery was then determined; the method detection limits for Ca, Mg, K, and Cu were 10.6, 0.05, 2.90, and 0.21 mg kg−1, respectively.
For the extraction of antioxidant enzymes SOD and H2O2, 0.05 g of grapevine root and leaf samples from the hydroponic experiment were homogenized with 3 mL of a 50 mM sodium phosphate (Na2–PO4) buffer (pH 7.0) in a pre-chilled mortar and pestle. The homogenate was centrifuged at 15,000 rpm for 30 min at 4°C to collect supernatant for the estimation of SOD and H2O2.
The SOD (EC. 1.15.1.1) activity was assayed according to Beauchamp and Fridovich (1971). The assay mixture consisted of a total volume of 0.75 mL, containing 0.1 M potassium phosphate, a 7.8 pH, 1 mM Na2–EDTA, 130 mM methionine, 0.63 mM NBT, 7.5 μM riboflavin, and a sample. The illumination of the reaction mixtures caused the formation of the blue formazan which increased absorbance at 560 nm. One unit of SOD is defined as the amount of enzyme that inhibited the reduction of NBT by 50% at 560 nm.
The H2O2 content of the grapevines was determined according to Jana and Choudhuri (1981). The reaction mixture contained 0.1% (v/v) titanium chloride dissolving 20% (v/v) H2SO4 and the supernatant at a proportion of 1:2 at 1000 rpm for 15 min to mix homogeneity. The content was evaluated by comparing its absorbance at 410 nm with a standard calibration curve.
The level of lipid peroxidation products was estimated following the method of Heath and Packer (1968) by measuring the concentration of malondialdehyde (MDA) as an end-product of lipid peroxidation through reaction with thiobarbituric acid (TBA). Root and leaf samples were homogenized with 2 mL of 5% (w/v) trichloroacetic acid (TCA) in a prechilled mortar and pestle and centrifuged at 10,000 rpm for 5 min at 4°C. One millilitre of the supernatant and 2 mL of 20% TCA containing 0.5% TBA were added. The mixture was heated at 95°C for 30 min and quickly cooled in an ice bath for 15 min. After centrifugation at 3000 rpm for 10 min, the absorbance of the supernatant was recorded at 532 nm and corrected by measurement at 410 nm and 600 nm.
The leaf discs were randomly mixed and rinsed with distilled water and then wiped dry. In situ localization of O2− and H2O2 was performed as described by Liu et al. (2014). Compared to the data for 3 days after treatment, the leaf discs were infiltrated using 0.5 mg mL−1 NBT with 10 mM NaN3 in a 50 mM HEPES–NaOH buffer (pH 7.6) for O2− histochemical localization or in a 0.5 mg mL−1 DAB-phosphate buffer (50 mM, pH 5.8) for the histochemical detection of H2O2. Infiltration was conducted under vacuum for 30 min and centrifuged at 3000 rpm until the discs were below the solution. The leaf discs were then held at room temperature until a blue or brown color became visible. Chlorophylls in leaves stained with a blue (O2−–NBT formazan precipitates) or deep-brown (H2O2–DAB polymerization) color were removed using a 95% ethanol solution 2–3 times. We used stereo-zoom microscopes (Motic, SMZ-171 series) to observe the stained leaf discs and took images with a moticam connected to a computer.
Results and Discussion
There were some morphological changes such as darkening of the roots and less root-hair formation of grapevine cuttings when treated with the highest Cu concentration (15 μM). The elongation of the roots seemed to be stunted at that concentration. No obvious difference was observed between the control and the lowest Cu concentration (5 μM). Additionally, no temporal effect was observed between the 1 and 3 days treatments (Fig. 1).
The phytotoxic effects of excess Cu on grapevines have been widely studied in recent years. Briefly, high Cu level in the root growth environment may cause auxin homeostasis disorder, thus result in the reduction of lateral root-hair numbers and root elongation (Tiecher et al. 2017, 2018; Ambrosini et al. 2018). These morphological alterations may further affect water and nutrient uptake and eventually stunt the growth of grapevines. The symptoms observed in the present study are similar to previously published works. On the other hand, the threshold of Cu level that causes toxic effects may be varied under different situations. Kopittke et al. (2010) indicated that the median toxic concentration of Cu to plants mainly ranged from 0.9 to 20 μM. Based on our results, the concentration that inhibited the root growth of the grapevines (15 μM) fell well within the range proposed by Kopittke et al. (2010). However, Cambrolle et al. (2015) studied the toxic effect of Cu on wild grapevine and reported that the median toxic concentration was higher than 23 mM. These variations may mainly be attributed to the difference of plant species and geochemical properties. In addition, the growth rate was stimulated at an exposure concentration of 5 μM of the present study, indicating that a hormesis phenomenon, a phenomenon that toxicants can stimulate the organism’s response at low concentrations, exists for grapevine roots exposed to lower-Cu-concentrations. This phenomenon was also observed in many other studies and our published work of grapevine cuttings exposed to waterborne Cu (Juang et al. 2012).
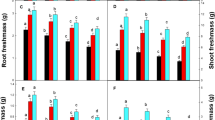
In the current findings, increases in Cu-levels and the duration of treatment led to the increase of the Cu-concentration in grapevine seedlings. On the contrary, the addition of Cu generally decreased cations (K, Mg, and Ca) accumulation compared to the control (Fig. 2). As shown in Fig. 3, the mean Cu-concentrations were much higher in roots than those in leaves and stems, revealing that the Cu absorbed by grapevines remain mainly in the roots. The highest Cu-concentration was 3300 ± 572 mg kg−1 in the roots at the Cu exposure level of 15 μM after 3 days exposure; however, the Cu-concentration was only 119 ± 22 and 60 ± 21 mg kg−1, respectively, for stems and leaves under the same Cu exposure concentration and duration. On the other hand, the Ca and Mg accumulation in the plant parts were decreased in this order: root ≈ leaf > stem; still, the K-level remained relatively constant in different grapevine parts (Fig. 4). Generally, the translocation of Ca, Mg, and K from the roots to above-ground parts was far higher than that of Cu.
Results of the present study showed that Cu accumulation in grapevines occurred mainly in the roots, with low translocation to the above-ground parts. This result aligned with many previous studies regarding Cu distribution in grapevines (Juang et al. 2012; Cambrolle et al. 2015; Tiecher et al. 2017). Recently, Ambrosini et al. (2018) studied Cu translocation in ‘Red Niagara’ (Vitis labrusca), an important grape varieties in Brazil, and indicated that grapevines cope with Cu-stress by accumulation of Cu in apoplast and reducing its translocation to the shoots. However, leaf Cu level may increase when grapevine was exposed to extremely high Cu content (Cambrolle et al. 2015). In addition, it generally has been recognized that the Cu levels of between 20 and 100 mg kg−1 in leaves may cause toxic effects to plants (Cambrolle et al. 2015). In this study, mean Cu concentration in grapevine leaf was 37 and 60 mg kg−1 after Cu treatment of 15 μM for 1 and 3 days, respectively, thus indicating a toxic effect on growth inhibition. For Mg, Ca, and K, the present result aligned with some previous findings which indicated that the maintenance of adequate micronutrients levels in leaves is critical for grapevines (Perez-de-los-Reyes et al. 2013; Cambrolle et al. 2015; Ambrosini et al. 2018). Furthermore, these micronutrients concentrations in grapevine seedlings were reduced especially at the highest external Cu level (15 μM). This may be due to the impairment of nutrient uptake, or the alteration of membrane permeability when exposing grapevines to higher Cu level (Ambrosini et al. 2018).
The antioxidant enzymes are important components in preventing oxidative stress in plants (Thounaojam et al. 2012). In this study, however, the increase in the SOD activity within the leaves was significant only at a Cu exposure concentration of 50 μM for 3 days. No obvious dose–effect relationship was found between the SOD activity in the roots and the Cu exposure concentration for all other treatments (Fig. 5).
It was generally recognized that SOD constitutes the first line-of-defense against ROS. Induction of SOD was associated with a strategy to overcome Cu-induced stress. In the present study, a Cu-induced increase in SOD activities was observed for grapevine leaves when exposed to 15 μM Cu after 3 days (Fig. 5). This result aligned with several previous studies (Thounaojam et al. 2012, 2014). Under severe oxidative stress, however, a decline of SOD activity may happen in preventing cellular damage (Mostofa et al. 2014). The decline of Cu-induced SOD activity after long-term exposure to higher levels of Cu was also observed for rice seedlings (Thounaojam et al. 2014).
In the case of heavy metal stress, the production of H2O2 and MDA generally increases in plants (Gill and Tuteja 2010). In the present results, the addition of Cu significantly increased H2O2 content in leaves after a 1 day treatment; however, compared with the control, no obvious increase was observed for H2O2 content in the roots. On the other hand, both in the grapevine leaves and roots, the MDA content treated with 15 μM of Cu increased significantly compared with the control after 1 day and 3 days treatment (Fig. 5).
In this study, Cu-stress increased the production of H2O2 and MDA in grapevine leaves after a 1 day treatment with 15 μM of Cu. The increase of ROS production such as H2O2 can disturb metabolic pathways through oxidative damage to the cells. MDA is a product of lipid peroxidation and generally increases with the increase of ROS contents (Mostofa et al. 2014; Thounaojam et al. 2014). A higher MDA level in plants indicates severe cell-membrane damage. The present results show that Cu-exposure causes oxidative stress to grapevine leaves. However, the reason for the decline of H2O2 content in roots after long-term exposure needs further investigation.
In the present study, Cu-induced accumulation of O2− and H2O2 in grapevine leaves was detected by histochemical staining with NBT and DAB (Fig. 6). Distinctive amounts of O2− and H2O2 were respectively observed as dark blue spots and as reddish-brown spots in the leaves treated with 15 μM of Cu. These results further confirm the Cu-induced oxidative stress in grapevine leaves from the microscopic viewpoint. Based on the present results, therefore, it is possible to employ the histochemical changes in leaves as a biomarker for monitoring the phytotoxicity of Cu in grapevines. In conclusion, this study focused on the toxic effects of Cu and coexisting cations on grapevine seedlings. The results indicated that excess Cu may cause morphological changes in roots, reduce nutrients uptake, and result in oxidative stress to the leaves of grapevine seedlings. Therefore, the present findings can not only provide information for a better understanding of phytotoxic effects of Cu, but also the influence of cations supplementation on Cu-induced oxidative stress in grapevine seedlings.
References
Ambrosini VG, Rosa DJ, de Melo GWB, Zalamena J, Cella C, Simao DG, da Silva LS, dos Santos HP, Toselli M, Tiecher TL, Brunetto G (2018) High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol Biochem 128:89–98
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Cambrolle J, Garcia JL, Figueroa ME, Cantos M (2015) Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 120:171–178
Chen PY, Lee YI, Chen BC, Juang KW (2013) Effects of calcium on rhizotoxicity and the accumulation and translocation of copper by grapevines. Plant Physiol Biochem 73:375–382
Ferreira PAA, Marchezan C, Ceretta CA, Tarouco CP, Lourenzi CR, Silva LS, Soriani HH, Nicoloso FT, Cesco S, Mimmo T, Brunetto G (2018) Soil amendment as a strategy for the growth of young vines when replanting vineyards in soils with high copper content. Plant Physiol Biochem 126:152–162
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidants machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Heath RL, Packer L (1968) Photoperoxidation in isolate chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Juang KW, Lai HY, Chen BC (2011) Coupling bioaccumulation and phytotoxicity to predict copper removal by switchgrass grown hydroponically. Ecotoxicology 20:827–835
Juang KW, Lee YI, Lai HY, Wang CH, Chen BC (2012) Copper accumulation, translocation, and toxic effects in grapevine cuttings. Environ Sci Pollut Res 19:1315–1322
Juang KW, Lee YI, Lai HY, Chen BC (2014) Influence of magnesium on copper phytotoxicity to and accumulation and translocation in grapevines. Ecotoxicol Environ Saf 104:36–42
Kopittke PM, Blamey FPC, Menzies NW (2008) Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 303:217–227
Kopittke PM, Blamey FPC, Asher CJ, Menzies NW (2010) Trace metal phytotoxicity in solution culture: a review. J Exp Bot 61:945–954
Liu D, Kottke I (2004) Subcellular localization of copper in the root cells of Allium sativum by electron energy loss spectroscopy (EELS). Bioresour Technol 94:153–158
Liu D, Chen J, Mahmood Q, Li S, Wu J, Ye Z, Peng D, Yan W, Lu K (2014) Effects of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ Sci Pollut Res 21:13615–13624
Miotto A, Ceretta CA, Brunetto G, Nicoloso FT, Girotto E, Farias JG, Tiecher TL, de Conti L, Trentin G (2014) Copper uptake, accumulation and physiological changes in adult grapevines in response to excess copper in soil. Plant Soil 374:593–610
Mirlean N, Roisenberg A, Chies JO (2007) Metal contamination of vineyard soils in wet subtropics (Southern Brazil). Environ Pollut 149:10–17
Mostofa MG, Seraj ZI, Fujita M (2014) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251:1373–1386
Perez-de-los-Reyes C, Ortiz-Villajos JAA, Navarro FJG, Martin-Consuegra SB, Ballesta RJ (2013) Grapevine leaf uptake of mineral elements influenced by sugar foam amendment of an acidic soil. Vitis 52:157–164
Rossini Oliva S, Mingorance MD, Valdes B, Leidi EO (2010) Uptake, localisation and physiological changes in response to cooper excess in Erica andevalensis. Plant Soil 328:411–420
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Panda SK (2012) Excess copper induced oxidative stress and responses of antioxidants in rice. Plant Physiol Biochem 53:33–39
Thounaojam TC, Panda P, Choudhury S, Patra HK, Panda SK (2014) Zinc ameliorates copper-induced oxidative stress in developing rice (Oryza sativa L.) seedlings. Protoplasma 251:61–69
Tiecher TL, Ceretta CA, Ferreira PAA, Lourenzi CR, Tiecher T, Girotto E, Nicoloso FT, Soriani HH, de Conti L, Mimmo T, Cesco S, Brunetto G (2016) The potential of Zea mays L. in remediating copper and zinc contaminated soils for grapevine production. Geoderma 262:52–61
Tiecher TL, Tiecher T, Ceretta CA, Ferreira PAA, Nicoloso FT, Soriani HH, de Conti L, Kulmann MSS, Schneider RO, Brunetto G (2017) Tolerance and translocation of heavy metals in young grapevine (Vitis vinifera) grown in sandy acidic soil with interaction of high doses of copper and zinc. Sci Hortic 222:203–212
Tiecher TL, Soriani HH, Tiecher T, Ceretta CA, Nicoloso FT, Tarouco CP, Clasen BE, De Conti L, Tassinari A, Melo GWB, Brunetto G (2018) The interaction of high copper and zinc doses in acid soil changes the physiological state and development of the root system in young grapevines (Vitis vinifera). Ecotoxicol Environ Saf 148:985–994
Acknowledgments
This study was financed by the Ministry of Science and Technology, Taiwan under Grant Nos. MOST 105-2313-B-343-001 and MOST 106-2313-B-343-001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juang, KW., Lo, YC., Chen, TH. et al. Effects of Copper on Root Morphology, Cations Accumulation, and Oxidative Stress of Grapevine Seedlings. Bull Environ Contam Toxicol 102, 873–879 (2019). https://doi.org/10.1007/s00128-019-02616-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02616-y